Keywords
Uniprot ID Q8N474, SFRP1, Secreted frizzled-related protein 1, antibody characterization, antibody validation, Western Blot, immunoprecipitation
This article is included in the Cell & Molecular Biology gateway.
This article is included in the YCharOS (Antibody Characterization through Open Science) gateway.
Uniprot ID Q8N474, SFRP1, Secreted frizzled-related protein 1, antibody characterization, antibody validation, Western Blot, immunoprecipitation
SFRP-1 is a secreted protein belonging to the secreted frizzled-related protein (SFRP) family.1 Having an amino acid terminal cysteine-rich domain homologous to the putative Wnt-binding domain of frizzled receptors, sFRP-1 functions as a modulator of the Wnt/β-catenin signalling pathway that is essential to multiple cellular processes.2,3
Dysregulated Wnt/β-catenin activity levels play a role in various disease processes.4 Epigenetic silencing of SFRP1 elevates Wnt/β-catenin activity, which has been linked to cancer.2,4 Conversely, high concentrations of sFRP-1 suppresses Wnt/β-catenin activity, which is predicted to have implications in neurological diseases, such as Alzheimer’s disease (AD).1,3–5 Targeting sFRP-1 to restore the Wnt/β-catenin signalling pathway is of current interest in AD research to discover novel therapies.3 Mechanistic studies would be greatly facilitated with the availability of high-quality antibodies.
Here, we compared the performance of a range of commercially available antibodies for sFRP-1 and identified high-performing antibodies for Western Blot and immunoprecipitation, enabling biochemical and cellular assessment of sFRP-1 properties and function.
Our standard protocol involves comparing readouts from wild-type and knockout (KO) cells.6,7 The first step is to identify a cell line(s) that expresses sufficient levels of a given protein to generate a measurable signal. To this end, we examined the DepMap transcriptomics database to identify all cell lines that express the target at levels greater than 2.5 log2 (transcripts per million “TPM” +1), which we have found to be a suitable cut-off (Cancer Dependency Map Portal, RRID:SCR_017655). Commercially available A549 cells expressed the sFRP-1 transcript at RNA levels above the average range of cancer cells analyzed. Parental and SFRP1 knockout A549 cells were obtained from Abcam (Table 1).
| Institution | Catalog number | RRID (Cellosaurus) | Cell line | Genotype |
|---|---|---|---|---|
| Abcam | ab275463 | CVCL_0023 | A549 | WT |
| Abcam | ab277906 | CVCL_B2Q1 | A549 | SFRP1 KO |
SFRP-1 is predicted to be a secreted protein. Accordingly, we collected concentrated culture media from both wild-type and SFRP1 KO cells and used the conditioned media to probe the performance of the antibodies (Table 2) side-by-side by Western blot and immunoprecipitation. The profiles of each of the antibodies are shown in Figures 1 and 2. The datasets can be found as Underlying data.10,11
| Company | Catalog number | Lot number | RRID (Antibody Registry) | Clonality | Clone ID | Host | Concentration (μg/μl) | Vendors recommended applications |
|---|---|---|---|---|---|---|---|---|
| Thermo Fisher Scientific | MA5-32675** | WJ3417745 | AB_2809952 | recombinant-mono | JA11-68 | rabbit | 1.00 | Wb |
| Thermo Fisher Scientific | MA5-38193** | WJ3417799B | AB_2898110 | recombinant-mono | ARC1683 | rabbit | 0.88 | Wb |
| GeneTex | GTX24193 | 822102161 | AB_370619 | polyclonal | - | rabbit | 1.00 | Wb, IF |
| GeneTex | GTX102371 | 39911 | AB_1951886 | polyclonal | - | rabbit | 0.33 | Wb |
| ABclonal | A9656** | 4000001683 | AB_2863750 | recombinant-mono | ARC1683 | rabbit | 0.88 | Wb |
| ABclonal | A2911 | 31570101 | AB_2764730 | polyclonal | - | rabbit | 2.68 | Wb |
| Bio-Techne | AF1384 | IRQ1020021 | AB_2285831 | polyclonal | - | goat | 0.20 | Wb, IF |
| Abcam | ab4193 | GR3345186-4 | AB_304357 | polyclonal | - | rabbit | 1.00 | Wb, IF |
| Abcam | ab126613** | GR3350102-3 | AB_11128257 | recombinant-mono | EPR7003 | rabbit | 0.41 | Wb |
| Abcam | ab267466** | GR3321068-3 | AB_2904616 | recombinant-mono | EPR23092-253 | rabbit | 0.46 | Wb, IP |
| Abcam | ab84003 | GR42188-1 | AB_10670402 | polyclonal | - | rabbit | 1.00 | Wb |
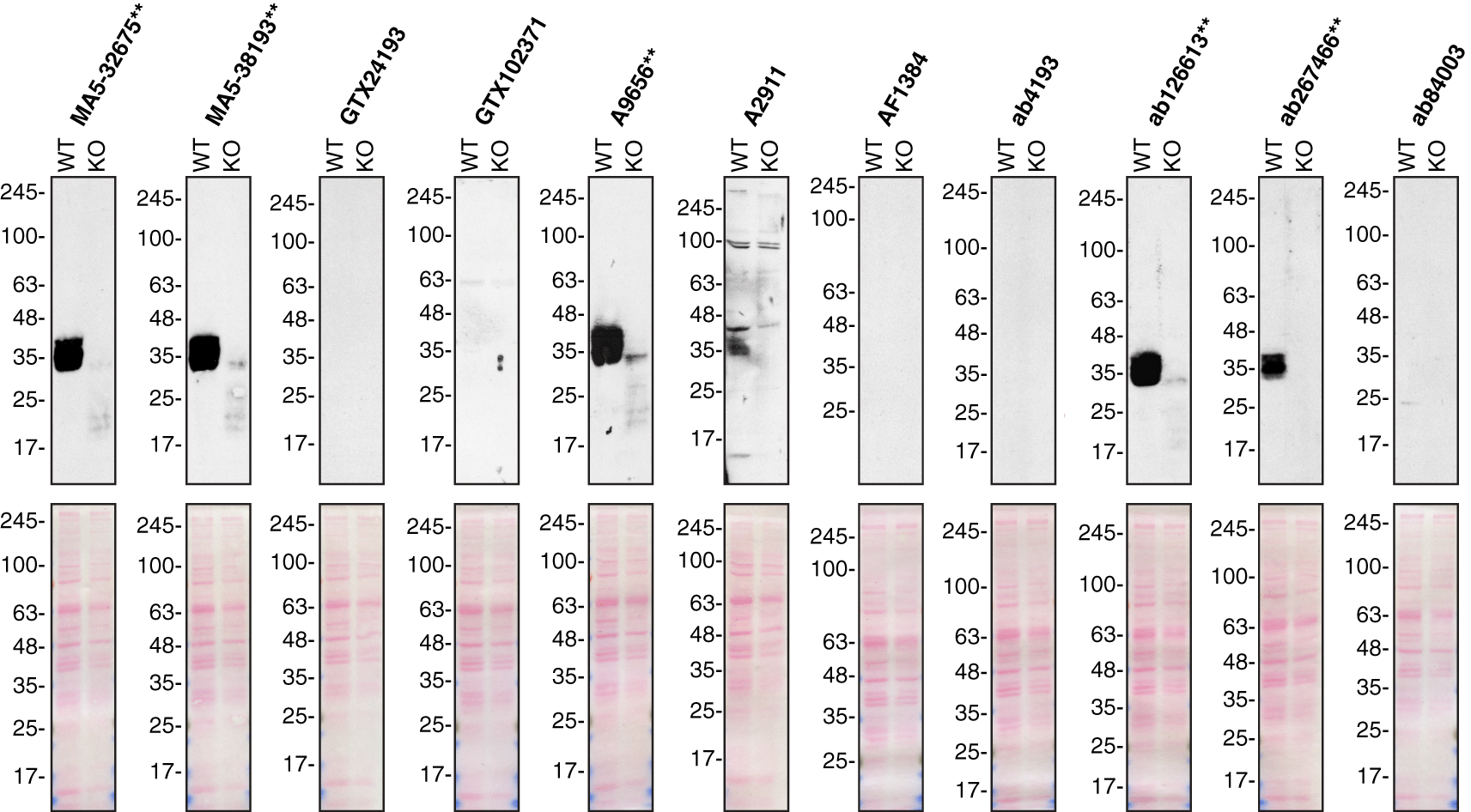
A549 (WT and SFRP1 KO) were treated with Brefeldin A at 3.0 μg/ml for 18 hrs. 50 μg of protein from concentrated culture media were processed for Western Blot with the indicated sFRP-1 antibodies. The Ponceau stained transfers of each blot are shown. Antibody dilutions were chosen according to the recommendations of the antibody supplier. Antibody dilution used: MA5-32675** at 1/500; MA5-38193** at 1/500; GTX24193 at 1/1000; GTX102371 at 1/1000; A9656** at 1/1000; A2911 at 1/1000; AF1384 at 1/500; ab4193 at 1/500; ab126613** at 1/1000; ab267466** at 1/1000; ab84003 at 1/1000. Predicted band size: 35 kDa. **Recombinant antibody.
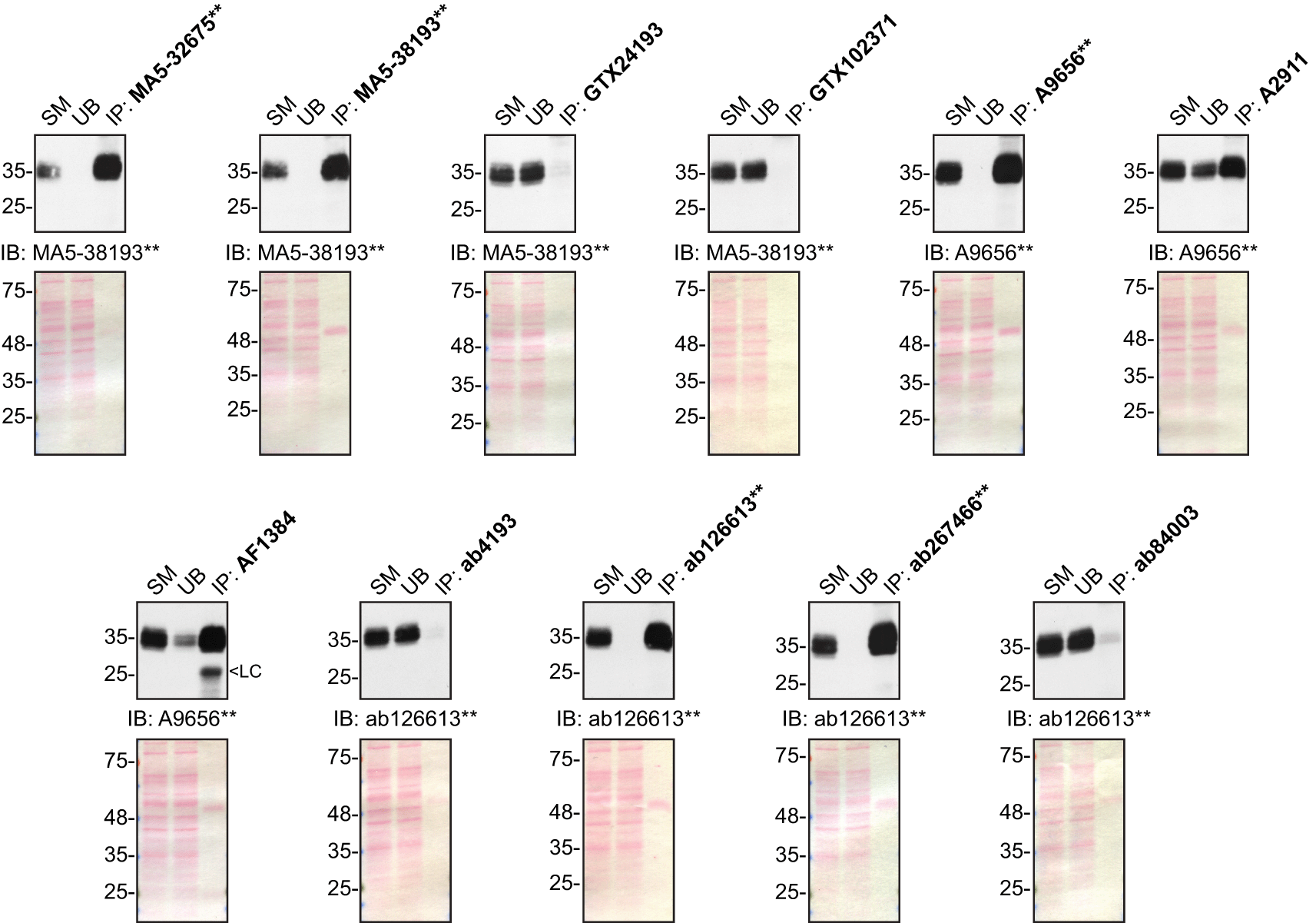
Immunoprecipitation was performed on concentrate culture media using 1.0 μg of the indicated sFRP-1 antibodies pre-coupled to either protein A or protein G magnetic beads. Samples were washed and processed for Western Blot with the indicated sFRP-1 antibody. For Western Blot, MA5-38193** was used at 1/500, A9656** at 1/1000 and ab126613** at 1/1000. The Ponceau stained transfers of each blot are shown for similar reasons as in Figure 1. SM = 10% starting material; UB = 10% unbound fraction; IP = immunoprecipitated; LC = light chain. **Recombinant antibody.
In conclusion, we have screened sFRP-1 commercial antibodies by Western Blot and immunoprecipitation and identified several high-performing antibodies under our standardized experimental conditions.
All sFRP-1 antibodies are listed in Table 2. Peroxidase-conjugated goat anti-rabbit and donkey anti-goat antibodies are from Thermo Fisher Scientific (cat. number 65-6120 and A15999).
A549 WT and SFRP1 KO used are listed in Table 1. Cells were cultured in DMEM high-glucose (GE Healthcare cat. number SH30081.01) containing 10% fetal bovine serum (Wisent, cat. number 080450), 2 mM L-glutamate (Wisent cat. number 609065), 100 IU penicillin and 100 μg/ml streptomycin (Wisent cat. number 450201). Cells were starved in DMEM high-glucose containing L-glutamate and penicillin/streptomycin.
A549 cells (WT and SFRP1 KO) were washed 3× with PBS and starved for ~18 hrs. Culture media were collected and centrifuged for 10 min at 500 × g to eliminate cells and larger contaminants, then for 10 min at 4500 × g to eliminate smaller contaminants. Culture media were concentrated by centrifuging at 4000 × g for 10 min using Amicon Ultra-15 Centrifugal Filter Units with a membrane NMWL of 10 kDa (MilliporeSigma cat. number UFC901024).
Western Blots were performed as described in our standard operating procedure.8 Western Blots were performed with large 8-16% gradient polyacrylamide gels and transferred on nitrocellulose membranes. Proteins on the blots were visualized with Ponceau staining which is scanned to show together with individual Western blot. Blots were blocked with 5% milk for 1 hr, and antibodies were incubated overnight at 4°C with 5% bovine serum albumin in TBS with 0,1% Tween 20 (TBST). Following three washes with TBST, the peroxidase conjugated secondary antibody was incubated at a dilution of ~0.2 μg/ml in TBST with 5% milk for 1 hr at room temperature followed by three more washes with TBST. Membranes were incubated with ECL from Pierce (cat. number 32106) prior to detection with HyBlot CL autoradiography films from Denville (cat. number 1159T41).
Immunoprecipitation was performed as described in our standard operating procedure.9 Antibody-bead conjugates were prepared by adding 1 μg of antibody to 500 ul of Pierce IP Buffer from Thermo Fisher Scientific (cat. number 87788) in a microcentrifuge tube, together with 30 μl of Dynabeads protein A - (for rabbit antibodies) or protein G - (for goat antibodies) from Thermo Fisher Scientific (cat. number 10002D and 10004D, respectively). Pierce IP Lysis Buffer (25 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40 and 5% glycerol) was supplemented with the Halt Protease Inhibitor Cocktail 100X from Thermo Fisher Scientific (cat. number 78446) at a final concentration of 1×. Tubes were rocked for ~2 hrs at 4°C followed by two washes to remove unbound antibodies.
Starved A549 WT culture media were concentrated as described above. 1 ml aliquots at 0.5 mg/ml of lysate were incubated with an antibody-bead conjugate for ~2 hrs at 4°C. Following centrifugation, the unbound fractions were collected, and beads were subsequently washed three times with 1.0 ml of IP Lysis Buffer and processed for SDS-PAGE and Western Blot on 8-16% polyacrylamide gels. Prot-A: HRP (MilliporeSigma, cat. number P8651) was used as a secondary detection system at a dilution of 0.4 μg/ml.
Zenodo: Antibody Characterization Report for Secreted frizzled-related protein 1, https://doi.org/10.5281/zenodo.6370454. 10
Zenodo: Dataset for the Secreted frizzled-related protein 1 antibody screening study, https://doi.org/10.5281/zenodo.7571170. 11
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We’d like to thank the NeuroSGC/YCharOS collaborative group for their important contribution to the creation of an open scientific ecosystem of antibody manufacturers and knockout cell line suppliers as well as the development of community-agreed protocols. Members of the group can be found below.
NeuroSGC/YCharOS collaborative group: Riham Ayoubi, Aled M. Edwards, Carl Laflamme, Peter S. McPherson, Chetan Raina and Kathleen Southern.
An earlier version of this article can be found on Zenodo (doi: 10.5281/zenodo.6370454).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for creating the dataset(s) clearly described?
Yes
Are the protocols appropriate and is the work technically sound?
Partly
Are sufficient details of methods and materials provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biomarkers, antibody generation/characterization and validation, antigen design, recombinant protein expression, genetic engineering, assay development.
Is the rationale for creating the dataset(s) clearly described?
Yes
Are the protocols appropriate and is the work technically sound?
Yes
Are sufficient details of methods and materials provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunochemistry, metabolomics, cancer, oxidative stress.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 02 Apr 24 |
read | read | |
|
Version 1 16 Mar 23 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)