Keywords
Periodontitis, Periodontal disease, Oral health, Dental Hygiene, Lycium Barbarum, Goji berry, Mouthwash, Chlorhexidine, Herbal, Antioxidants
This article is included in the Manipal Academy of Higher Education gateway.
Removal of the microbial deposits (plaque and calculus) by performing effective scaling and root planing (SRP) is the fundamental step for managing periodontal disease (gingivitis and periodontitis). Various adjuncts in the form of mouthwash, gels, and toothpaste are also being used as adjuncts to SRP for managing periodontitis. Recently, Lycium barbarum (L. barbarum), commonly known as goji berry or wolfberry, has gained popularity for managing chronic inflammatory and infectious diseases. However, its efficacy in managing periodontitis has never been explored. Hence the present study aims to evaluate the efficacy of goji berry mouthwash compared to chlorhexidine mouthwash for managing periodontitis.
60 adult participants were divided randomly using computer-generated random sequences into two groups (case group: L. barbarum mouthwash (Males: 16; Female: 14); control group: 0.2% chlorhexidine gluconate mouthwash (Males: 14; Females: 16)). The changes in the gingival index (Gi), plaque index (Pi), bleeding on probing (BOP), clinical attachment loss (CAL), probing pocket depth (PPD), microbial load, and antioxidant levels (protein thiol) in saliva were noted at the baseline, at 15 days and one month.
A significant reduction in the mean PPD, Pi, and Gi was seen from baseline to one month in both the control (P-value=0.006, 0.027, and 0.036 respectively) and test groups (P-value=0.035, 0.000, and 0.000 respectively). CAL was reduced significantly only in the control group. However, the antioxidant levels (protein thiol) in saliva were significantly increased only in the test group.
Goji berry mouthwash along with SRP reduced the Gi, Pi, BOP, and PPD in patients with periodontitis. However, no statistically significant difference was noted between the use of goji berry and chlorhexidine mouthwash. Goji berry mouthwash was more effective than chlorhexidine in increasing the antioxidant levels in saliva.
Periodontitis, Periodontal disease, Oral health, Dental Hygiene, Lycium Barbarum, Goji berry, Mouthwash, Chlorhexidine, Herbal, Antioxidants
The title has been updated to: Efficacy of Lycium barbarum (Goji berry) mouthwash is a viable alternative to 0.2% chlorhexidine gluconate for managing chronic periodontitis: a randomized clinical trial. The word ‘chronic periodontitis’ is changed to periodontitis and word efficacy has been added as suggested by the reviewers. The author's name Shravya S Mangalore has been changed to Mangalore S. Shravya as requested by the Author. In the abstract results and conclusion have been modified. The methodology and result sections have been corrected as per the recommendation made by the reviewers as follows: The participant flow diagram revised; mention of allocation concealment; method to use the mouthwash mentioned; participant details providing age and gender distribution, loss to follow-up mentioned. The discussion has been modified to newer references as suggested by reviewers along with the limitations of the study. The conclusion has been revised. The reference list has been and six new references have been added. Figure 3 has been removed as suggested by the reviewers. The grammatical and typographical error have corrected
See the authors' detailed response to the review by Francisco Wilker Mustafa Gomes Muniz
See the authors' detailed response to the review by Paulo José Lima Juiz
See the authors' detailed response to the review by Rajinder K. Sharma and Deepti Anand
Periodontitis is defined as a chronic immuno-inflammatory multifactorial disease that affects the soft tissue around the teeth.1 Periodontitis is considered the 6th most common disease, affecting approximately 20–50% of the adult population worldwide.2 It is more prevalent in males compared to females. It is primarily caused by the interaction of the gingival and periodontal tissues with the microorganisms in the oral biofilm. This host-microbial interaction triggers a massive influx of various pro-inflammatory mediators, microbial by-products, proteolytic enzymes, and free reactive oxygen species (ROS) leading to periodontal tissue destruction and inflammation.3–6 Due to increased ROS during periodontal disease, it is also referred to as free radical-mediated tissue injury.7 Some of the common putative periodontal microbes associated with periodontitis are Porphyromonas gingivalis, Tanerella forsythia, Treponema denticola, Campylobacter rectus, and Fusobacterium nucleatum.7 Apart from the microbial etiology, other risk factors such as smoking, diabetes mellitus, HIV, nutritional deficiency, medications, poor oral hygiene, and genetics influence the severity of periodontal inflammation.8
To control periodontal inflammation the primary and most vital step is the removal of oral biofilm formed around the teeth by performing effective mechanical debridement with either hand or machine-driven instruments.9,10 Effective scaling and root planning (SRP) can reduce gingival inflammation, thereby preventing disease progression and restoring gingival health.11 However, studies have shown that complete debridement of calculus is technically demanding, as many times removal of hard and soft tissue deposits from deep pockets (>5 mm) and interdental regions becomes challenging.11,12 Additionally, it is difficult to completely remove the smooth or burnished calculus from deep and circuitous periodontal pockets, furcation areas, root concavities, and irregular roots owing to a lack of good visibility and accessibility to such areas.12,13 Hence for the management of biofilm from deep and tortuous pockets additional periodontal therapy and use of adjuncts are needed.12–15
It is also noted that the efficacy of SRP is dependent on the patient’s compliance and motivation to maintain a meticulous oral care regime and effective plaque control at home.16 SRP alone may not be sufficient to maintain the required plaque control if patient compliance is poor and the patient does not effectively follow oral hygiene instructions. Additionally, studies have shown that even after a good plaque control regime; posterior, palatal, and lingual surfaces of the teeth retain some amount of plaque. Thus for many patients, adjuncts such as mouthwash and gels along with regular toothbrushing are indicated.11,17–24 Various chemical plaque control agents with antimicrobial and anti-inflammatory are being used in the form of mouthwash, gels, gum paints, fibers, varnishes, microspheres, chips, tablets, powder, and capsules for managing gingivitis and periodontitis. Among these agents, chlorhexidine gluconate is the most popular and routinely used agent for managing periodontitis. Additionally, recent reports have shown no additional benefits of using chlorhexidine and the development of antimicrobial resistance among many oral bacteria to chlorhexidine molecules.25–29 Prolonged use of chlorhexidine is contraindicated owing to various side effects like altered taste sensation, staining of the teeth and soft tissues such as tongue and mucosa, increased calculus formation, and parotid gland swellings.25 Chlorhexidine is also known to have cytotoxic effects on the gingival fibroblasts,26 periodontal ligament,27 and osteoblastic cells.28
Thus, there is an emerging trend to use natural and herbal extracts with antioxidant and antimicrobial properties to treat periodontal diseases. Herbal extracts from neem, Tulsi, guava, green tea, turmeric, curcumin, pomegranate, and many more plants have been tried to effectively treat gingivitis and periodontitis.30–34 Since periodontal disease causes a massive release of ROS and increases oxidative stress, adjuncts with antioxidant potential are often used with SRP to control the oxidative stress present locally in the gingival and periodontal tissues. Recently, goji berry, commonly known as Wolfberry, Himalayan goji, or Tibetan goji, has gained a lot of popularity due to its strong antioxidant and anti-inflammatory properties.35–46
Goji berry, scientifically known as Lycium barbarum (L. barbarum), is a fruit native to southeast Europe, China, and Asia.35 The fruit belongs to the family of Solanaceae and is consumed either in both fresh and dried form. It has shown powerful antioxidant, antimicrobial, immuno-modulating, and anticancer properties.36–46 Soesanto et al. (2021) showed that ethanolic extract of L. barbarum is effective against oral bacteria (Streptococcus mutans and P. gingivalis) at 100 μg/mL.47 Previous in-vitro studies have also reported that the minimal inhibitory concentration (MIC) of L. barbarum was comparable to chlorhexidine, however, its efficacy was less as compared to the antibiotic doxycycline. At 50 μg/mL, ethanolic extract of goji berry could inhibit most of the periodontal pathogens.48,49 However, no clinical study has yet assessed the effectiveness of goji berry mouthwash as an adjunct to SRP for the management of periodontitis. Therefore, this clinical study aims to evaluate the efficacy of L. barbarum mouthwash along with SRP for patients with periodontitis compared to chlorhexidine for the first time.
1. To evaluate the effect of L. barbarum (goji berry) mouthwash on the Plaque index (Pi), Gingival index (Gi), Bleeding on probing (BOP), probing pocket depth (PPD), and clinical attachment loss (CAL) at baseline, 15 days and one month compared to 0.2% chlorhexidine gluconate mouthwash.
2. To evaluate and compare the change in the protein thiol levels in saliva at 15 days, and one month compared to baseline in participants using goji berry and chlorhexidine mouthwash.
3. To evaluate and compare the reduction in the microbial count at the end of one month compared to baseline in participants using L. barbarum mouthwash compared to chlorhexidine mouthwash.
The study was designed as a randomized, double-blind single-centered parallel arm study with an allocation ratio of 1:1. The study was conducted at Manipal’ from 2019 to 2020 following the “Helsinki Declaration of 1975 (as revised in 2000)”. The trial was initiated after receiving ethical clearance from the Kasturba Medical Hospital Institutional Ethics Committee with IEC no 117/2019. The trial has been registered at the ‘Clinical Trial Registry (CTRI/2019/05/019042)’ and was done following the CONSORT and SAGER guidelines (Figures 1 and 2).48 The steps in the clinical trials are explained as follows:
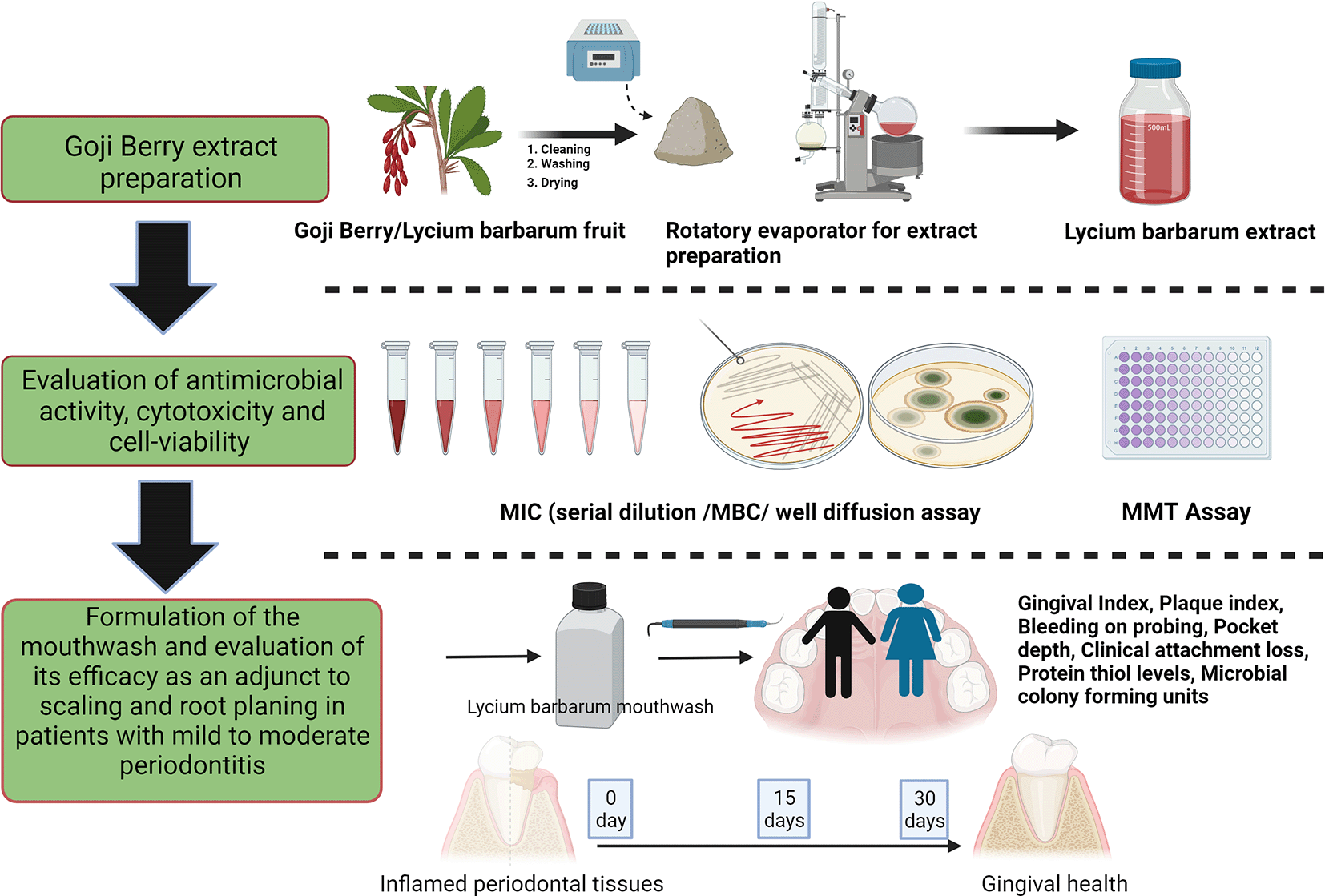
The mouthwash was prepared at the “Manipal College of Pharmaceutical Sciences, Manipal”. L. barbarum mouthwash was prepared by preparing an ethanolic extract of dried goji berry using Soxhlet’s method and dissolving the 50 ug/ml of weighed goji berry extract for formulating the mouthwash as described previously.49 A concentration of 50 ug/ml was mixed in 1.15 of 100% alcohol and then homogenized using 15% glycerol. To this around 15% propylene glycol and 1% Tween solution was added to formulate the mouthwash. 0.1% menthol was added to adjust the taste of the mouthwash. The volume was adjusted to 100% by using distilled water. 100% alcohol was added to serve both as a preservative and a dissolvent.
The shelf life of the mouthwash was measured as per the ICH guidelines and the samples were tested for three months under three different conditions: “35°±2% C with a relative humidity of 60% ± 5%; 25°C± 2°C and relative humidity: 60% ± 5%; and 40°C ± 2°C with a relative humidity of 75% ± 5%”. The mouthwash was kept in opaque plastic bottles and one of the bottles was kept in the stability compartment (Thermo lab, India). The samples were analysed at baseline, one, two, and three months by visual observation and UV spectrum analysis (Shimadzu UV-1601PC, Japan) and analysed for its physical parameters and stability. The mouthwash showed no change in color, odor, consistency, or no phase separation at three months. The sample showed no change when observed under the ultraviolet analysis at 274 nm. This indicated that the mouthwash was stable till the end of three months (Table 1).
Ninety subjects aged 20 to 50 years (both male/females) visiting the outpatient department were screened for the presence of localized/generalized periodontitis according to the 2017 classification of periodontal disease and selected based on the following exclusion and inclusion criteria:
Inclusion criteria:
1. Participants in the age group of 20-50 years diagnosed with stage I to stage II localized/generalized periodontitis (Grades A to B).
2. Participants with a minimum of 24 functional teeth.
Exclusion criteria:
1. Individuals with severe periodontitis (localized or generalized stage III and stage IV) were excluded.
2. Participants with any allergic reactions to chlorhexidine.
3. Participants with any systemic diseases such as hypertension, diabetes mellitus, cardiovascular, renal, and neurological diseases.
4. Participants who were on any anti-inflammatory, antibiotics, or analgesics in the last six months were excluded.
5. Pregnant or lactating mothers.
6. Participants with any oral abusive habits such as smoking, alcohol, betel nut chewing, gutka, paan, supari, areca nut.
7. Participants who had undergone any periodontal treatment/surgery in the last six months.
8. Participants undergoing orthodontic treatment.
9. Participants using any other oral hygiene agents (mouthwash or gels) were excluded to remove confounding bias arising due to the difference in plaque control measures.
All participants who satisfied the above-mentioned criteria were recruited after obtaining both oral and written informed consent.
After screening the participants, 60 participants were recruited after obtaining oral and written informed consent. The participants were randomly allocated to the test and control group using the computer-generated random sequence as follows: test group (L. barbarum, n = 30) or the control group (0.2% chlorhexidine gluconate mouthwash, n = 30). This sample size was based on p-value <0.05, alpha value = 0.05 two-tailed, power = 0.8, and the effect size = 0.7 for CAL. The allocation of participants into two groups was done by an investigator who was not part of either sample collection or analysis, periodontal therapy, or periodontal examination. The allocation concealment was done by dispensing both the mouthwash in similar-sized opaque amber-colored bottles. The investigators giving the bottles to the patients did not know the content and nature of the mouthwash. Following recruitment, all participants were assigned to another investigator who was blinded about the randomization and grouping. The participants were also blinded about their group about the content of the mouthwash, although the taste of the mouthwash could not be matched. The investigator and statistician analyzing the clinical data were not aware of the group. Following grouping, the following biological samples were collected at baseline:
a. Microbiology plaque sample collection
The plaque from the subgingival region was collected from the pocket with the maximum probing depth in each quadrant using a sterile site-specific Gracey curette. After collection, the curette was gently submerged in a reduced transport medium (Thioglycolate bath) for testing the total colony Forming Units (CFU). The number of colonies were calculated on the plated blood agar and then converted to CFU/mL using the following formula: CFU/mL = (no. of colonies × dilution factor)/volume of the culture plate.
b. Saliva Collection
Following plaque collection, a stringent method for saliva collection was followed. All participants were requested to sit comfortably in an upright position. Following this, around 2ml of saliva was collected by the ‘spitting method’ and without any stimulation.50 All participants were requested to spit saliva into an Eppendorf vial. The collected saliva was then stored immediately in a refrigerator at -80 degrees Celsius. The Eppendorf tubes used to store saliva samples were numerically marked according to the participant number. The saliva collected was sent for biochemical analysis for evaluation of the protein thiol levels using Ellman's Reagent. The absorbance of the agent was measured after incubation at room temperature at 412 nm for around five minutes and the concentration of protein thiol was determined with the standard curve of glutathione.
c. Examination of the clinical periodontal parameters
Following saliva sample collection, a periodontal examination was done for all the sextants. The following periodontal parameters were recorded: Gi by Loe & Silness, 1963; percentage of sites with BOP; PI by Silness & Loe 1964; PD and CAL. All the clinical evaluation was done by the investigator who was blinded about the patient’s grouping. The BOP, PPD, and CAL were recorded by the Williams periodontal probe (Hu-Friedy, USA). The sites with BOP were checked by noting the presence and absence of bleeding on all four surfaces (buccal, lingual, mesial, and distal) for all the teeth. The percentage of sites with BOP was considered by the percentage of the teeth with BOP to the total teeth present. The PPD and CAL were calculated at the buccal, mesial, distal, and lingual sides. At the interdental region (mesial and distal), probing was done from both the buccal and lingual sides. The deepest pocket depth at each surface was recorded. The average of each surface was considered as the reading for that tooth. The mean PPD and CAL were assessed by adding the reading from each tooth and dividing it by the total number of teeth.
Following periodontal examination and sample collection, a thorough SRP was initiated for all the participants. A single trained investigator examined the baseline and follow-up visits for all the patients. The scaling was assessed by the supervisor to ensure the complete removal of plaque and calculus was done. All patients were educated to brush their/teeth in modified bass technique for two minutes twice daily, to nullify any confounding effects arising due to differences in the oral hygiene measures. All participants were given the opaque amber color bottles which were coded (AX or BX). This was done to blind the patients and investigators regarding the type of mouthwash given to the participants. All patients were instructed to use 10 ml mouthwash diluted with 10 ml of water for 30-45 seconds twice a day for a month. Patients were recalled after 15 days and one month for revaluation. At each recall visit Pi, Gi, % of sites with BOP, CAL, and PPD were noted. The plaque from the same site and saliva samples were collected at each recall visit. The investigation for each participant, at each recall visit, was done by the same investigator.
Data obtained was analysed by the ‘SPSS version 26.0, IBM’. For descriptive data frequency and for categorical data mean and standard deviation for all the numerical data was analysed using the ‘Kolmogorov–Smirnov test’. The normality of the distribution was checked for all variables. The inter-group comparisons of all the assessed outcomes were done using an ‘independent sample t-test’. The comparisons between the goji berry mouthwash and chlorhexidine mouthwash were done to measure any significant increase/reduction from baseline to 15 days and one month using Repeated measures ANOVA with Geisser correction’ followed by a ‘post-hoc analysis with Bonferroni adjustment’. Inter-group comparison of reduction of all the variables at follow-up was done using ANCOVA after adjusting the respective baseline scores. The p-value of less than 0.05 was considered to be significant.
Of the 90 participants assessed for eligibility, 30 participants were excluded as they did not meet the eligibility criteria. The rest 60 participants were allocated into test (n=30) and control (n=30) groups. The mean age of participants in the test group was 35.42 ± 11.79 years and in the controls was 32.12 ± 12.85. The gender-wise distribution in the test group was males: 16; females: 14 and the control group was males: 14; females: 16 (Table 2). Out of the 30 patients in each group, 14 patients in the test group and 16 patients in the control did not come for the follow-up visit due to the sudden lockdown imposed by COVID-19. Additionally, one patient in test group reported bitter taste of the goji berry mouthwash and discontinued the mouthwash. Thus, for analysis 15 patients in the test and 14 patients in the control were included in the analysis. The comparison between goji berry mouthwash and chlorhexidine mouthwash showed no significant differences in the mean values for Pi (p = 0.470), Gi (p = 0.239), BOP (p = 0.450), PPD (p = 0.216), CAL (p = 0.220), and Microbial level (p = 0.251) (Table 3).
| Groups | Goji berry group (case group) | Chlorhexidine group (Control group) | p-value |
|---|---|---|---|
| Age (in Years) | 35.42 ± 11.79 | 32.12 ± 12.85 | 0.29a |
| Male-Female | Male-Female | ||
| Gender | 16-14 | 13-14 | 0.11b |
| Group | Baseline | 15 days | 1 month | P-value ¥ | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Baseline vs 15 days | Baseline vs 1 month | 15 days vs 1 month | ||
| Plaque index | Control | 1.45 ± 0.42 | 1.03 ± 0.50 | 0.92 ± 0.43 | 0.104 | 0.027 | 0.334 |
| Test | 1.6 ± 0.38 | 1.04 ± 0.36 | 0.89 ± 0.17 | 0.000 | 0.000 | 0.076 | |
| P-value # | 0.470 | ||||||
| Gingival index | Control | 1.4 ± 0.64 | 0.87 ± 0.47 | 0.81 ± 0.43 | 0.001 | 0.036 | 0.214 |
| Test | 1.35 ± 0.38 | 0.85 ± 0.31 | 0.75 ± 0.24 | 0.000 | 0.000 | 0.092 | |
| P-value # | 0.239 | ||||||
| Percentage of sites with bleeding on probing | Control | 80.7 ± 0.42 | 42.7 ± 0.49 | 10.7 ± 0.42 | 0.001 | 0.034 | 0.210 |
| Test | 84.8± 0.32 | 40.8± 0.29 | 09.0± 0.32 | 0.000 | 0.000 | 0.090 | |
| P-value# | 0.450 | ||||||
| Probing pocket depth | Control | 2.35 ± 0.56 | 1.62 ± 0.56 | 1.78 ± 0.35 | 0.004 | 0.006 | 0.186 |
| Test | 2.76 ± 1.06 | 1.73 ± 0.45 | 2.15 ± 0.68 | 0.002 | 0.035 | 0.327 | |
| P-value # | 0.216 | ||||||
| Clinical attachment level | Control | 2.03 ±0.90 | 1.52 ± 0.65 | 1.33 ± 0.43 | 0.020 | 0.005 | 0.174 |
| Test | 2.62 ± 1.16 | 2.29 ± 0.99 | 2.02 ± 0.61 | 0.103 | 0.248 | 0.260 | |
| P-value # | 0.220 | ||||||
| Biochemical analysis | Control | 233.06 ± 144.49 | 235.48 ± 103.39 | 225.06 ± 72.11 | 0.874 | 0.899 | 0.760 |
| Test | 132.68 ± 56.17 | 227.05 ± 72.21 | 248.30 ± 68.31 | 0.000 | 0.000 | 0.272 | |
| P-value # | 0.021 | ||||||
| Microbial level | Control | 7.44 ± 0.43 | 7.35 ± 0.584 | 7.33 ± 0.567 | 0.183 | 0.130 | 0.756 |
| Test | 7.57 ± 0.77 | 7.19 ± 0.74 | 7.04 ± 0.85 | 0.222 | 0.564 | 0.264 | |
| P-value # | 0.251 | ||||||
The intra-group comparison showed a significant reduction in the mean PPD, Pi, and Gi from baseline to one month in both the control and test groups. The CAL reduced significantly only in the chlorhexidine group compared to the goji berry group (Table 3, Table 4). A significant difference was noted in the antioxidant levels (protein thiol) in saliva in the goji berry group at the end of one month. No change in the salivary antioxidant level was noted in the chlorhexidine group. No significant differences were reported in the log-transformed microbial CFU counts in both groups at any given time. In the case of the test group, the mean Pi reduced from 1.6 ± 0.38 at baseline and was 0.89 ± 0.17 at one month. However, in the case of the goji berry mouthwash, the mean PPD was reduced during the 15-day follow-up (1.73 ± 0.45) compared to the baseline (2.76 ± 1.06).
Intergroup comparison at one-month follow-up (15 days and one month) was done using ANCOVA after adjusting the respective baseline scores. There were no differences seen in the mean values for Pi (p = 0.791), Gi (p = 0.594), PPD (p = 0.134), protein thiol levels (p = 0.211), and microbial levels (p = 0.188) between the two groups (Table 4). No harms were reported by any patient.
The present study is the first clinical trial that aimed to evaluate the role of goji berry mouthwash for the treatment of periodontitis. The study assessed the efficacy of goji berry mouthwash compared to 0.2% chlorhexidine mouthwash in controlling the gingival inflammation, plaque formation, BOP, PPD, CAL, microbial profile, and antioxidant levels (protein thiols) in the saliva. We found that the mean reduction in Gi, Pi, BOP, and PPD with goji berry mouthwash was comparable to chlorhexidine mouthwash. The reduction in CAL was better for chlorhexidine mouthwash compared to goji berry mouthwash. However, we did not find any statistically significant difference between the goji berry mouth and chlorhexidine mouthwash for Gi, Pi, BOP, and PPD at the end of one month. Since goji berry mouthwash showed comparable results to chlorhexidine, it can be used along with SRP for managing periodontitis, especially in the current times when oral bacteria are becoming resistant to chlorhexidine.50–53 Additionally, since prolonged use of chlorhexidine is not advised to prevent the onset of various side effects such as alterations in taste, staining, and increased calculus, alternatives to chlorhexidine becomes important. Costa et al. (2017) in a systematic review and meta-analysis also concluded that adjunctive use of chlorhexidine with SRP provides only a minor PPD reduction compared to SRP alone. Therefore, clinicians should consider the small additional gain in PPD reduction, negligible effect on CAL, and potential for adverse effects when prescribing chlorhexidine to their patients.52
In such situations, the use of herbal mouthwash like goji berry would be a viable alternative.51 The present study also found that goji berry mouthwash increased the protein thiol levels in saliva, unlike chlorhexidine. This is a positive finding that goji berry would be advantageous compared to chlorhexidine in increasing the antioxidant levels in periodontal disease. Since periodontitis is linked with increased oxidative stress and free radical-mediated tissue injury, use of compounds with antioxidant properties are often used. The increase in antioxidant levels in saliva upon use of goji berry mouthwash would help to reduce and control the inflammation. The antioxidant properties of goji berry are attributed to the presence of constituents like Lycium barbarum polysaccharides (LBPs), catechin, epicatechin, quercetin, chlorogenic acid, citric acid, coumaric acid, scopoletin, linoleic acid, kaempferol, and coumaric acid.50–57 The flavonoids in goji berries are proven to have good antimicrobial effects and this justifies the reduction in the PI and control of gingival inflammation with goji berry mouthwash.58 The reduction in gingival inflammation can also be attributed to the ability of goji berry to inhibit proinflammatory cytokines, radical scavenging activities, and interactions with other antioxidants. Studies have shown that goji berries can improve fibroblast healing, increase collagen formation, and reduce oxidative stress.41,59–62
Sanghavi et al. (2022) evaluated the antimicrobial, anti-adhesion, anti-biofilm, and cytotoxic properties of L. barbarum against five potential periodontal pathogens (P. gingivalis, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, Prevotella intermedia, Tanerella forsythia) and found that goji berry extract could inhibit the growth of periodontal pathogens, however, the zone of inhibition of was less when compared to doxycycline and chlorhexidine. The authors also reported that goji berry was compatible with gingival fibroblast and oral keratinocytes at a concentration of 1 mg/mL. The study also found that goji berry has good anti-adhesion property against P. gingivalis. The anti-adhesion properties of LBE (96%) were comparable compared to chlorhexidine (96.3%). However, the anti-biofilm activity of chlorhexidine (96%) was found to be slightly better than that of goji berry extract (91.6%).49 The ability of goji berry mouthwash to reduce the PI and GI can also be explaind by it ability to modulate the immune cells. Studies by Du et al. (2014) and Ren et al. (2012) also found that goji berry supplementation enhances the maturation and increases the recruitment of neutrophils and monocytes to sites of infection.63,64 These properties of LBP would be beneficial in lowering the microbial load and controlling periodontal inflammation. Lai et al. (2022) conducted an in-vitro and animal study to assess the efficacy of alkaline phosphatase activity and osteogenic potential of human periodontal ligament stem cells upon treatment with goji berry and found that goji berry can enhance the proliferation and migration of stem cells. This could facilitate superior healing and control of gingival inflammation. The study also showed a reduction in alveolar bone resorption and confirmed the positive effect of L. barbarum for managing periodontitis.65
Hence based on our results and existing evidence, it can be stated that goji berry has a promising role inmanaging periodontitis. However, one should also note that the present study evaluated the role of goji berry for only a short recall time for one month and prolonged exposure to chlorhexidine mouthwash was avoided to prevent side effects. One should also note that although the patients were blinded by using coded amber-coded colored bottles to mask the color of the mouthwash, the taste of each mouthwash was different. The study was conducted on patients with mild-moderateperiodontitis, therefore future studies should assess the efficacy of goji berry mouthwash on severe cases of periodontitis with longer follow-up. Future studies should also evaluate the effect of goji berry on specific periodontal pathogens or local and systemic inflammatory markers for periodontitis.
Goji berry mouthwash along with SRP was effective in reducing the Gi Pi, BOP, and PPD in patients with periodontitis. No statistically significant difference was noted between the use of goji berry mouthwash and chlorhexidine mouthwash. However, goji berry mouthwash was more effective than chlorhexidine in increasing the antioxidant levels in saliva.
Figshare.: Data on study titled: Lycium barbarum (Goji berry) mouthwash is a viable alternative to 0.2% chlorhexidine gluconate for managing chronic periodontitis: a randomized clinical trial; doi: 10.6084/m9.figshare.21834939.66
This project contains the following data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The datasets related to our study is also available with the corresponding author and can be shared on reasonable request via email to aditi.chopra@manipal.edu.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Periodontology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
References
1. Sanz M, Herrera D, Kebschull M, Chapple I, et al.: Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline.J Clin Periodontol. 2020; 47 Suppl 22 (Suppl 22): 4-60 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Periodontology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Periodontitis, periodontal medicine
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
|
Version 4 (revision) 26 Nov 24 |
read | read | read | ||
|
Version 3 (revision) 05 Jul 24 |
read | read | |||
|
Version 2 (revision) 16 Nov 23 |
read | ||||
|
Version 1 20 Mar 23 |
read | read | |||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)