Keywords
HSP701, BiP, segmental vitiligo, non-segmental vitiligo
This article is included in the Cell & Molecular Biology gateway.
HSP701, BiP, segmental vitiligo, non-segmental vitiligo
Skin depigmentation that occurs over time as a result of cutaneous melanocytes is a characteristic of vitiligo, where the classification of vitiligo is divided into segmental vitiligo (SV) and non-segmental vitiligo (NSV), as well as mixed and unclassified vitiligo.1 Prevalence of the disease is 0.5–2% of the worldwide population.2 In Indonesia, 187 of the 2700 patients who visited the outpatient Dermatology and Venereology clinic of Kepanjen Hospital in South Malang between 2015 and 2019 had vitiligo, amounting to a prevalence of 7%. The peak occurrence of vitiligo is usually between the first and third decade of life and it has a psychological impact on the sufferer, although vitiligo is non-life threatening.3,4 The diagnosis of vitiligo can be made from clinical examination and additional laboratory tests are usually needed only for confirmation.5
The pathogenesis of SV, including neuropeptide, somatic mosaicism, and microvascular skin horning, which eventually cause melanocyte damage, is better known than that of NSV.6–8 Otherwise, NSV pathogenesis is still multifactorial and polygenic. The dominant factors in the NSV pathogenesis are oxidative stress and autoimmunity.6,9,10 However, the mechanisms involved in the onset and progression of vitiligo remain unclear. Several previous studies have linked endoplasmic reticulum (ER) stress, which has a relationship with oxidative and immune stress in the pathogenesis of vitiligo.11 Oxidative stress disrupts the cellular redox potential that extends to the ER, so that protein will fail to fold and its accumulation will activate a response called the unfolded protein response (UPR).11–13
Binding immunoglobulin protein (BiP), an ER chaperone in the accumulation of unfolded protein, preferentially binds to unfolded protein peptides and releases the ER stress sensor to enable its spontaneous dimerization and activation.14,15 Inducible heat shock protein 70 (HSP70i) can also be secreted from the stressed live cells as a chaperone. Intracellular HSP70i will bind native protein in response to stress, to prevent misfolding dan cellular apoptosis. Some experimental animal models have confirmed the action and overexpression of HSP70i in progressive depigmentation.16–18 Thus, the presence of UPR is related to the extent and severity of vitiligo because it will eventually cause damage to and destruction of melanocyte cells.6,9,10,19 The progressive depigmentation of NSV and its unpredictable disease course makes it an important subject for research. Therefore, this study aims to compare BiP and HSP70i associated with melanocyte cell death in SV and NSV patients.
This research has been declared to be ethically feasible by the Health Research Ethics Committee, Faculty of Medicine, Brawijaya University, dated July 28th, 2021, with approval number 218/EC/KEPK-S3/07/2021.
The subjects were composed of 64 patients diagnosed with vitiligo, who were split into two groups: 33 patients in the NSV group and 31 patients in the SV group. The diagnosis of vitiligo was established by anamnesis, clinical examination, and skin biopsy. Anamnesis identifies the presence of milky white patches. In terms of clinical examination, SV is acute and appears rapidly within a few weeks and stabilizes within 1–2 years.9 On the other hand, NSV is characterized by the expansion of lesions on both sides of the body, and the disease progression is chronic and lifelong. The skin biopsy identifies if there are melanocytes at the lesion area in the epidermis.14
All the recruited patients had attended the outpatient Dermatology and Venereology clinic at Kanjuruan Kepanjen General Hospital, Malang, East Java during the period of August 2021 to January 2022. All patients’ vitiligo was established by anamnesis, clinical examination and skin biopsy. The patients had no prior keloid history and ranged in age from 18 and 60. Exclusion criteria included pregnant women and patients with diabetes mellitus, HIV, Cushing syndrome, active infection, trauma, and malignancy, as well as smokers and those with too much sun exposure. Subjects who met the criteria gave written informed consent as research subjects, and we recorded demographic history, clinical disease history, and family history of vitiligo.
The expression of the UPR marker was observed by skin biopsy and then an immunofluorescence examination to identify expression of BiP and HSP70i. In this research, all samples were taken from skin biopsy in the perilesional zone. Skin biopsy was taken the following way. Perilesional vitiligo was disinfected with 70% alcohol, and 0.25 ml lidocaine was applied as local anesthesia to the area to be biopsied. In this area, skin biopsy was performed using the punch biopsy method with a diameter of 3 mm, and the skin tissue was included in an Eppendorf. After completion, the wound was cleaned with 0.9% NaCl solution, had topical fucidic acid applied, and was closed using sterile gauze. All skin biopsies were performed at the Dermatology and Venereology outpatient clinic at Kanjuruhan Kepanjen General Hospital, and the samples were given to the clinical pathology department, Faculty of Medicine, Universitas Brawijaya Malang, for further processing.
The tissue was cut on a microtome (Leica RM2245) to a thickness of 2–3 mm then given a code according to the researcher’s gross code, with the aim that tissue samples were not exchanged between patients before the sample was inserted into a cassette. After that, it was processed with the Automatic Tissue Tex Processor (Thermo, STP 121-2977-1902) tool for 90 min, according to the standards of the Anatomical Pathology Laboratory of the Faculty of Medicine, Universitas Brawijaya Malang, until an alarm sounded completion. The process of blocking and cutting the tissue was performed, with the epidermis removed from the Tissue Tex Processor and the tissue blocked with paraffin according to the researcher’s gross code. The tissue was cut with a microtome tool with a thickness of 3–5 microns. Next came the deparaffination process in which the tissue was cut to a thickness of 3–5 microns and placed into four tubes. These were put in an oven for 3 min at a temperature of 70–80°C. The tissue was then placed into two tubes of xylol solution for 20 min each. After that, the tissue was put into four alcohol tubes, each for 3 min (hydration), and placed in running water for 15 min. The process and cutting of the skin biopsy tissue with a microtome and hematoxylin eosin (HE) staining was carried out at the Anatomical Pathology Laboratory. All slides were viewed using an Olympus IX71 fluorescence microscope under 40× magnification.
All slides that had been prepared were then stained with the direct immunofluorescence process. The staining process for BiP used the anti-BiP antibody kit (Abcam, rabbit polyclonal to GRP78 BiP, concentration μg at 1 mg/ml, catalog number Ab21685). Immunofluoresence processes were conducted in the biomedical laboratory at the Faculty of Medicine, Universitas Brawijaya. The slides were heated at 60°C for 60 min. Then the slides were immersed in the following solutions in sequence: first they were soaked in xylol solution for 2 × 10 min, then the slide was put into absolute ethanol for 2 × 10 min, then 90% ethanol for 1 × 5 min, then 80% ethanol for 1 × 5 min, then 70% ethanol for 1 × 5 min, and a last soak in the sterile Aquadest for 3 × 5 min.
The antigen retrieval process was carried out with citrate buffer. In the first step, the slide was immersed in a chamber containing citrate buffer at pH 6.0. Then the slide was placed in a water bath at 95oC for 20 mins. The slide was removed from the water bath after 20 mins, then allowed to cool to room temperature for about 20 mins. The slides were then cleaned three times for five mins with phosphate-buffered saline (PBS). The slides were then washed five times for one min with PBS Triton-X 100 0.2% (Sigma-Aldrich). The slide was incubated for 30 mins at room temperature with 3% Bovine Serum Albumin (BSA) andthe BSA solution was discarded. Subsequently, the slide with BiP antibody was incubated for an overnight period at 4°C. The slides with PBS were washed three times for 5 mins after an overnight incubation. Then the slide was incubated with 4’,6-diamidino-2-phenylindole (DAPI) staining solution (Abcam) 1:1000 for 5 mins. Next, the slides were washed with PBS for 3 x 5 mins. The slides were then closed with mounting medium and a cover glass. An immunofluorescence microscope (Olympus IX71 fluorescence microscope under 40× magnification) was used to view and document the expression of BiP. The next process was to analyze expression using ImageJ software version 1.53k (Wayne Rasband and contributors, National Institutes of Health, USA; RRID:SCR_003070), and the results were compared.
The staining process for HSP70 used anti-HSP70i antibody kit (Santa Cruz, catalog number Sc-32239, mouse monoclonal IgG1 κ HSP70 antibody, with concentration 200 μg/ml). Immunofluoresence processes were conducted in the biomedical laboratory at the Faculty of Medicine, Universitas Brawijaya. The slides were heated at 60°C for 60 min. Then the slides were immersed in the following solutions in sequence: first they were soaked in xylol solution for two × 10 min, then the slides were put into absolute ethanol for two × 10 min, then 90% ethanol for one × 5 min, then 80% ethanol for one × 5 min, then 70% ethanol for one × 5 min, and the last soak was in sterile Aquadest for three × 5 min.
Furthermore, the antigen retrieval process was carried out with citrate buffer. The first step involved immersing the slides in a chamber containing citrate buffer at pH 6.0. Then the slides were placed in a water bath at 95oC for 20 mins. The slides were removed from the water bath after 20 mins, then allowed to cool to room temperature for 20 mins. The slides were then cleaned three times for five mins with PBS. They were then washed five times for one min with PBS Triton-X 100 0.2%. The slides were then incubated for 30 mins at room temperature with 3% Bovine Serum Albumin (BSA) and the BSA solution was then discarded. The slides were then incubated with the HSP antibody for an overnight period at 4°C. The slides were washed with PBS three times in five mins after the overnight incubation. Then the slide was incubated with DAPI 1:1000 for 5 mins. Next, the slides were washed with PBS for 3 x 5 mins. Then the slides were closed with mounting medium and cover glass. An immunofluorescence microscope was used to view and document the expression of BiP. The next process was to analyze expression using ImageJ software, and the results were compared.
All research data processing techniques were analyzed using the Statistical Product and Service Solution software, IBM SPSS Statistics 20 (RRID:SCR_019096). Descriptive statistics were used to examine the data and determine percentages, mean values, and standard deviation. We used the non-parametric Mann-Whitney U test since the data were not regularly distributed. To examine the variance between the two groups, the student’s t-test was used. Statistical significance was defined as a p-value 0.05.
This study was composed of 64 vitiligo patients, of whom 33 had NSV and 31 had SV. The mean age was 22.32 ± 9.20 years in the SV group and 44.79 ± 11.24 years in the NSV group. Table 1 lists the characteristics of the subjects.15
Median UPR-HSP70i and UPR-BiP expression in the NSV group was found to be 14.79 ± 14.72 and 12.55 ± 11.85 respectively, with a p-value of 0.001 (<α = 0.05). Thus, it can be stated that there were differences in UPR expression when using the HSP70i and BiP markers in NSV patients. UPR expression using the HSP70i marker was higher compared with the BiP marker in the NSV group. For the SV group, we found the median UPR-HSP70i and UPR-BiP expression to be 3.85 ± 4.92 and 2.66 ± 3.07 respectively.15 Graphics of median UPR-BiP expression in NSV and SV, as well as fluorescence of UPR-BiP expression in NSV and SV are shown in Figure 1. Graphics of median UPR-HSP70i expression in NSV and SV, as well as fluorescence of UPR-HSP70i expression in NSV and SV are shown in Figure 2.
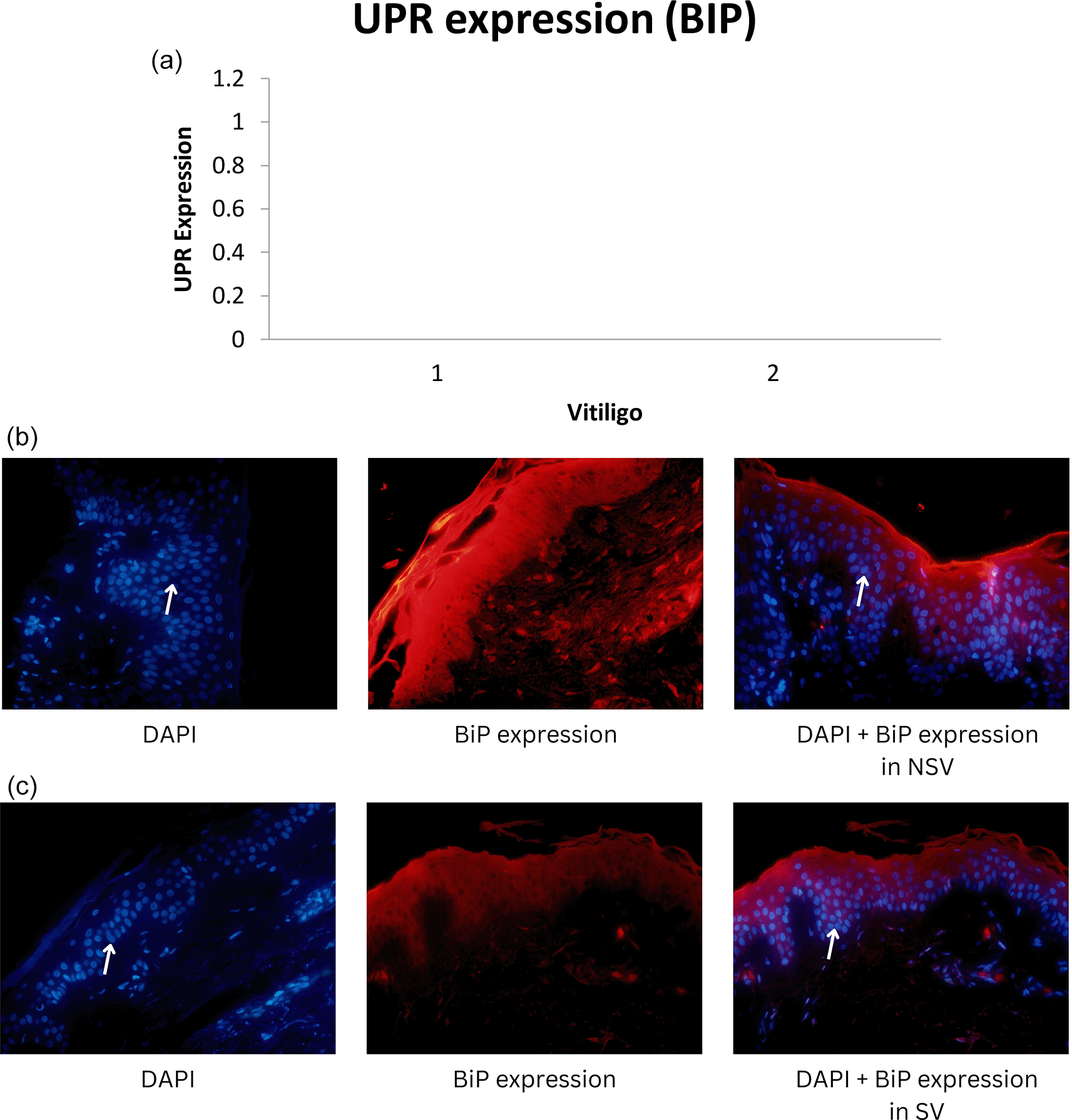
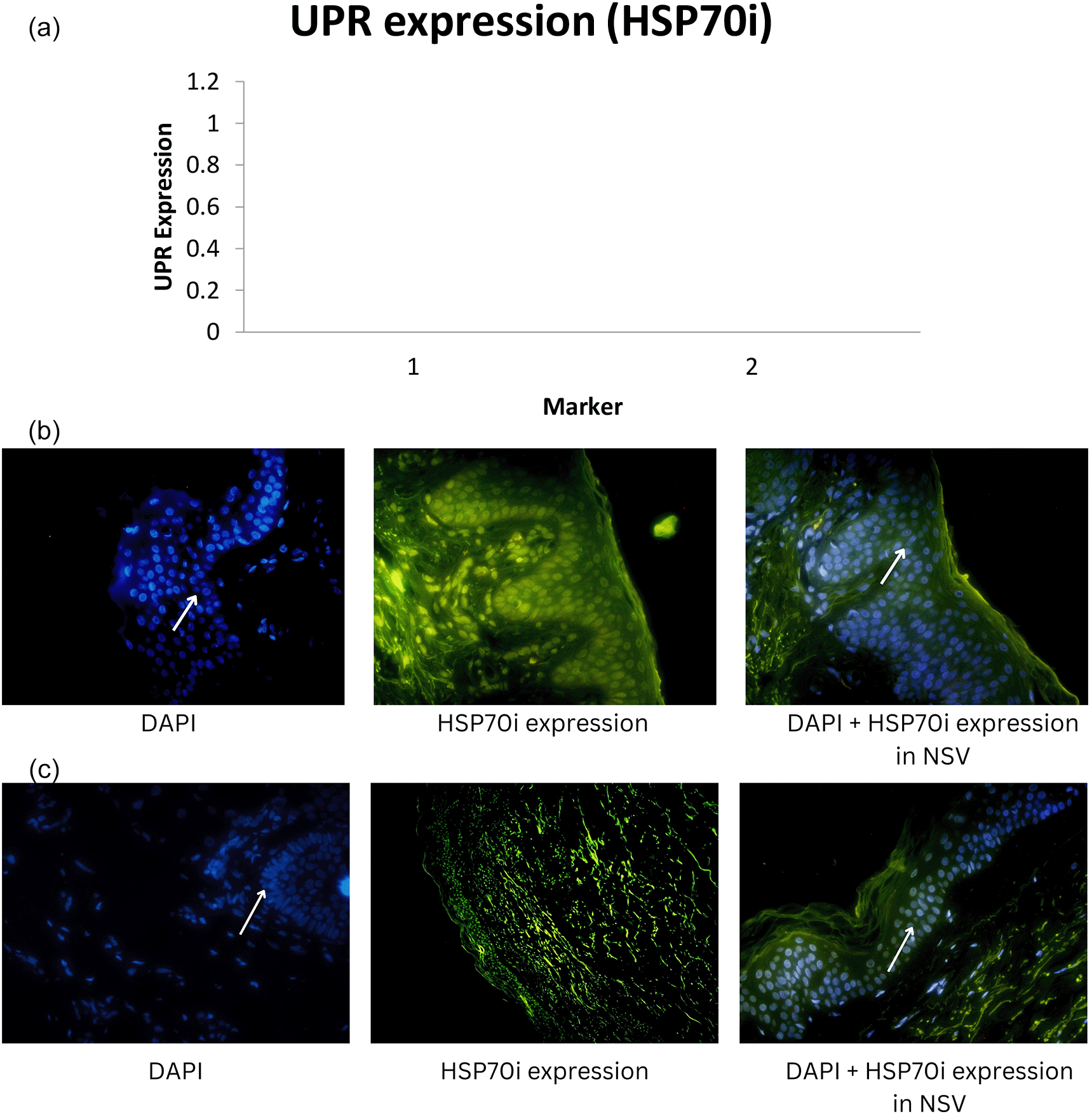
Mean UPR-HSP70i and UPR-BiP expression in the NSV group was found to be 14.79 ± 14.72 and 12.55 ± 11.85 respectively, with a p-value of 0.001 (<α = 0.05). Thus, it can be stated that there were differences in UPR expression when using the HSP70i and BiP markers in NSV patients. UPR expression using the HSP70i marker was higher compared with the BiP marker in the NSV group. In the SV group, median UPR-HSP70i and UPR-BiP expression were found to be 3.85 ± 4.92 and 2.66 ± 3.07, respectively, with a p-value of 0.001 (<α = 0.05). Thus, it might be concluded that there were differences in UPR expression when using the HSP70i and BiP markers in SV patients.15
The ER in eukaryotic cells has an important role in synthesis, maturing protein, lipid metabolism, and intracellular calcium homeostasis.1,12 Physiologically, the ER ensures that proteins are folded, always oxidized around the folds, and filled with calcium. Subtoxic levels of ER-stress-induced UPR in the skin are required for normal cellular function, including differentiation.16 Melanocyte are more susceptible to oxidative stress in patients with vitiligo than in healthy individuals and melanocytes release reactive oxygen species (ROS) as a respond to stress.20 The over-production of ROS and it's accumulation triggers protein oxidation and fragmentation, lipid peroxidation, and DNA damage, which compromises cellular function.10,21 In addition, ROS also interfere with the work of the folding machinery of the ER, resulting in UPR.10 This situation leads to chronic and continual ER stress that induces UPR, which has a deleterious effect on cells, and the UPR in turn becomes mechanism of a cell death.15 The accumulation of UPR also disturbs transcription, translation, and mRNA translation in protein synthesis.22–24
The ER stress response or the so-called UPR has been widely recognized in autoimmune and inflammatory diseases, where the UPR contributes to the course of autoimmune disease through the formation of antigens during the degradation of misfolded proteins, secreting neo-antigens by apoptotic cells or impairing immune tolerance.4,25 The UPR can also be activated through an increase in unfolded protein that occurs as a result of the production of large amounts of protein during melanin synthesis.10 Intracellular oxidative emphasise that outcomes from outside shows vulnerability to chemicals and harsh environmental factors, besides failure of the intracellular systems to overcome stress, and induces targeted melanocytic destruction.26 Production of pro-inflammatory cytokines and signals that activate the innate immune system result from the ROS- and UPR-mediated death of keratinocytes and melanocytes. Then, pattern recognition receptors (PRR) and nucleic acid receptors through danger-associated molecular patterns (DAMP) recognize host-derived self-DNA/RNA from damaged cells.27
The three ER transmembrane sensor proteins control UPR, namely inositol-requiring enzyme 1 alpha (IRE1a), double-stranded RNA-dependent protein kinase-like ER kinase (PERK) and activating transcription factor (ATF) 6.16 All three sensors are primarily bound with BiP, which helps to maintain their inactive state, in non-stress conditions. The interplay between these three major arms of the UPR signaling pathways determines whether stressed cells survive or die.11 The protein is known as a binding immunoglobulin protein because it is found in the ER and is bound to secreted Ig heavy chains.28 The UPR pathway is activated when the calcium concentration in the ER is reduced, which causes a rapid buildup of unfolded/misfolded proteins and the promotion of dissociated BiP from three ER transmembrane sensor proteins: IRE1a, PERK, and ATF6.16 In addition, BiP/GRP78 levels are very high in various human cancers.29
In response to cellular stress and UPR activation, melanocytes secrete HSP70i, as many other cells do.27 HSP70i binds native proteins to prevent misfolding and cellular apoptosis. In experimental animal models, the role of HSP70i overexpression in gradual depigmentation has been demonstrated.24,30,31 The effector T cells are attracted to the dendritic cells by increasing signaling, which is induced by HSP70i.27 Under stress, DAMPs that convey danger signals cause the innate immune system to become more activated by way of pattern recognition receptors (PRR) that include TLRs, RIG-I similar receptors, and NLRs. By producing cytokines, chemokines, and antigen-presenting molecules, this mechanism has the potential to both negatively destroy melanocytes and spark adaptive immunity.18 Furthermore, it activates dendritic cells, and the activated DAMP and CD8+ T cells stimulate the immune response to again destroy melanocytes.27,32,33 Melanocyte-specific, cytotoxic CD8+ T lymphocytes are crucial for the melanocyte’s eventual targeted demise.34–36 It has been found that the severity of vitiligo disease is related to the intensity of the CD8+ reaction.25,37,38
In our study, HSP70i and BiP expression were found to be relatively higher in NSV than in SV, indicating a higher response to oxidative stress that can interfere with transcription, translation, and translation of mRNA in protein synthesis, which is related to the extent and severity of vitiligo in NSV. Thus, dissimilar to SV, melanocyte destruction in NSV is mediated over diverse, intricate, and interconnected mechanisms.26
Our study showed that expression using HSP70i was higher than with BiP, both in SV and NSV. Stressed vitiligo melanocytes secrete HSP70i that induce dendritic cells to increase the expression of CD80 and CD86, that stimulate immune responses to melanocytes. In a previous study using a mouse model, adding HSP70i alone could worsen vitiligo, perhaps causing over activation of dendritic cells in the skin. Meanwhile, in a mouse model lacking HSP70i expression, there was no depigmentation in the experimental study, which indicates the role of HSP70i in vitiligo. These dendritic cells can be stimulated to express more coactivation markers like CD80 and CD86, which will amplify the immune response against melanocytes.4 As a result, HSP70i, which is overexpressed in lesional vitiligo epidermis compared to normal skin, might cause an autoimmune reaction in response to environmental stressors such oxidative stresses.4,35 Thus, HSP70i is preferred because it can serve as a link between the innate and adaptive immune systems.27
The existence of this UPR is seen as crucial because if it is not immediately addressed, it will disrupt homeostasis and protein, lipid, and DNA synthesis, which will then activate the apoptotic signaling pathway, resulting in melanocyte apoptosis. Furthermore, the apoptosis produces neo antigens that will stimulate the immune system.14,16 Melanocyte apoptosis is a programmed cell death mechanism involving endonuclease enzymes, characterized by shriveled melanocyte cells that have the characteristic pattern of a step ladder and eventually become fragmented and release apoptosis bodies.38 Precisely, CD8+ T lymphocytes play a variety of roles in mediating melanocyte death.39 The majority of melanocyte death is caused by immune cells breaching the self-tolerance as a result of melanocyte apoptosis, antigen exposure, and an inflammatory microenvironment.18 In our investigation, the SV group had a relatively lower expression of melanocyte cell apoptosis than the NSV group. Although both SV and NSV exhibit melanocyte damage, it is unknown whether the loss of melanocytes in SV is brought on by an autoimmune reaction or a cellular defect that is already present.18 However, our study is consistent with other studies showing an elevated anti-melanocyte antibody titer in NSV patients. This can be used as an important basis in the vitiligo pathogenesis.40
In conclusion, the appearance of UPR and oxidative stress affect the progression of vitiligo, especially in NSV. Both HSP70i and BiP expression can be UPR markers, although HSP70i is superior in expressing UPR, compared with BiP, because it represents a link between the adaptive and innate immune systems; thus, HSP70i is recommended for use in future studies. The existence of this UPR is crucial because it can activate the apoptotic signaling pathway resulting in melanocyte apoptosis. Additional studies are necessary to investigate if UPR can be used as a new target in the treatment of vitiligo.
Written informed consent for publication of their clinical details and clinical images was obtained from the patients.
Zenodo: Underlying data for ‘Comparison of BiP and HSP70i as markers of unfolded protein response (UPR) in segmental and nonsegmental vitiligo’. https://doi.org/10.5281/zenodo.7228247. 15
This project contains the following underlying data:
• Data file 1: Data and Demographic Journal 2.xlsx [demographic data of subjects]
• Data file 2: Journal 2-Statistical Data.docx [analysis data results]
• Data file 3: UPR expression of HSP70i and BiP.docx [Figure of UPR expression]
• Supplementary file 1: Ethical Approval Letter.pdf
• Supplementary file 2: BiP-NSV.jpeg
• Supplementary file 3: BiP-SV.png
• Supplementary file 4: DAPI BiP in NSV.jpg
• Supplementary file 5: DAPI BiP in SV.jpg
• Supplementary file 6: DAPI HSP70i NSV.jpg
• Supplementary file 7: DAPI HSP70i SV.jpg
• Supplementary file 8: HSP70i-NSV.png
• Supplementary file 9: HSP70i-SV.png
• Supplementary file 10: DAPI only (BiP NSV).jpg
• Supplementary file 11: DAPI only (BiP SV).jpg
• Supplementary file 12: DAPI only (HSP70i NSV).jpg
• Supplementary file 13: DAPI only (HSP70i SV).jpg
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0)
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cell biology and biochemistry (vitiligo pathophysiology, tight junction barrier regulation, and regulation of hyaluronan metabolism in the skin)
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Giri PS, Bharti AH, Begum R, Dwivedi M: Calcium controlled NFATc1 activation enhances suppressive capacity of regulatory T cells isolated from generalized vitiligo patients.Immunology. 2022; 167 (3): 314-327 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Autoimmunity; Immunogenetics; Skin Immunity
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 28 Mar 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)