Keywords
Salivary Orosomucoid 1, Hepatocellular Carcinoma, Early Diagnostic, Prognostic, Biomarker
This article is included in the Oncology gateway.
Salivary Orosomucoid 1, Hepatocellular Carcinoma, Early Diagnostic, Prognostic, Biomarker
Salivary orosomucoid 1 (ORM1) or alpha-1-acid glycoprotein (AGP) is an acute phase protein of about 1-3% of all plasma proteins. ORM1 is normally produced by hepatocyte cells but can also be produced and found in extrahepatic tissues such as endothelial cells and some tumor cells.1 ORM1 is expressed in inflammatory injuries, infections, and stress conditions.2 ORM1 is predominantly synthesized in the liver so liver disease, especially hepatocellular carcinoma (HCC) induced by hepatitis B can affect changes in its concentration or expression more in saliva as well as serum. The expression pattern of ORM1 shows a rapid increase in saliva and serum at the beginning of HCC induced by hepatitis B because a characteristic of this disease is that liver cell destruction happens from very early stages.2,3 Even though ORM1 is not only used to detect HCC related to hepatitis B, HCC related to hepatitis B is the only disease that has a specific behavior as mentioned above.2,4
Orosomucoid is produced by hepatocyte cells and it increases when there is inflammation in the body, especially the liver as the place of production. When inflammation occurs, hepatocyte cells will secrete ORM1 as an inflammatory expression agent that can be detected through saliva, urine, and serum.1 HCC related to hepatitis B is associated with a massive inflammatory response that leads to the production of a huge amount of ORM1. It indicates that ORM1 is suitable for early diagnosis of HCC related to hepatitis B.2 Malignant cells in hepatocytes can affect the function of the liver in the body. The liver is the place of albumin synthesis in the human body, and if HCC is detected, it will be followed by hypoalbuminemia in laboratory tests.5 So hypoalbuminemia is one of the early diagnostic parameters that can be assessed besides ORM1.
Modalities of early detection of HCC are usually looking at biomarkers. Alpha-fetoprotein (AFP) is one of the biomarkers that is often used in detecting the presence of hepatic cancer. However, AFP has the disadvantage of having low specificity and cannot detect early-stage hepatic cancer compared to ORM1.6
Saliva is a molecule rich in protein, DNA, RNA, and microorganisms, and can be considered a library of biomarkers. Cancer biomarkers present in the blood can also be found in saliva, and changes in their concentration can be used as biomarkers to detect cancer early and monitor response to treatment management. Saliva is a biomarker that is non-invasive, simple and does not require professional medical personnel, and has the ability to last longer than blood and urine. Screening methods using saliva can be used to detect the incidence of cancer.2
Gani et al. (2015) found ORM1 to have significantly higher expression in the saliva of patients with HCC compared to the non-HCC group (p < 0.001) and ORM1 expression in saliva liver cancer was significantly higher than adjacent normal tissue (p < 0.001).7 ORM1 expression differs in each response to a disease condition, be it infection, drug sensitivity or cancer. However, its role in HCC remains largely unknown. Therefore, ORM1 may become an enhanced diagnostic tool for HCC. In addition, in a study by Higuchi et al. (2020) it was found that ORM1 was expressed differently in HCC tumors and non-tumor tissues using a combination of in vitro, in silico, and clinical sample immunohistochemistry methods. Thus, ORM1 can be considered as a potential target for the development of future therapies against HCC.8
This article aims to fulfil two objectives. First, we try to investigate the role of salivary ORM1 as an early diagnostic biomarker of HCC related to hepatitis B, including sensitivity and specificity. Second, we try to identify the general prognosis of the patient with HCC induced by hepatitis B, i.e., whether the outcome will be favorable or unfavorable, using levels of salivary ORM1 as a biomarker.
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).9 This review was registered with Open Science Framework (OSF) (March 16, 2023).
We included original articles published in 2013 until 2023 (last date searched January 2023). The study comprised original research articles or research reports that used observational cohort or diagnostic studies as the inclusion criteria. Scientific posters, study protocols, narrative reviews, systematic reviews, meta-analyses, non-comparative research, in silico studies, in vitro studies, in vivo studies, technical reports, editor responses, and conference abstracts were all disqualified. Additionally, non-English, non-full-text, and irrelevant articles that were not related to either salivary ORM1, or HCC, or hepatitis B, or kidney failure were also excluded. The eligibility criteria for articles were as follows: i) Population, adults with cancer or hepatitis B or kidney failure; ii) Intervention, measuring salivary ORM1; iii) Comparison, healthy patient (control); and iv) Outcomes, level of ORM1.
Outcome measure
ORM1 is the parameter assessed as the outcome measure in this systematic review. This parameter is synthesized by hepatocytes and has a normal plasma concentration between 0.6–1.2 mg/mL (1–3% plasma protein). ORM1 can be found in several types of fluid, including saliva, urine, and plasma. Since it’s produced by hepatocytes, the level of this parameter can increase in conditions that affect hepatocytes’ activity and damage its product (albumin), such as hepatitis, cancer, and kidney failure.
Index test
Studies that provided the data of evaluations of ORM1 are included in this systematic review.
Reference standard
Reference standards are professional research using observational cohorts or diagnostic studies to assess the transition of ORM1 serum levels.
Research for this study were gathered through ProQuest (RRID:SCR_006093), Google Scholar (RRID:SCR_008878), Springer, and Science Direct database searches. Boolean operators were utilized among the keywords. In Table 1, the keywords used in each database are displayed.
The filters used for each database included year (from 2013 until January 2023), type of documents from ProQuest was filtered by scholarly journal, any type of article was used in Google Scholar, content type article was used as the Springer database filter, and research article type was used for Science Direct database filter. The last date the databases were searched was 10 January 2023. The database search gave a total result of 2,482 studies (324 ProQuest, 1,683 Google Scholar, 221 Springer, and 254 Science Direct). Once the aforementioned filters were added, 712 studies were then imported to the Mendeley Group Reference Manager in the authors’ library before the selection process.
After using the search terms listed in Table 1, four independent reviewers (KCT, AA, NADR, and INSS) and one validator (EKSL) merged the results from four databases. They then screened the abstracts and full text to eliminate the irrelevant articles and keep the relevant articles and expelled non-cohort and non-diagnostic articles. From this process, 655 studies were excluded, then the 57 studies left were checked for whether the full text could be retrieved. As a result, only 17 full text articles could be retrieved. Following this, 12 articles were excluded due to duplication, as found using the title, year of publication, and DOIs. A total of five included studied were assessed for eligibility using the Risk Of Bias In Non-randomized Studies - of Interventions (ROBINS-I) tool. From this procedure, all the five included studies passed the assessment bias check. The research selection processes were recorded in the PRISMA flow chart.
After the final screening, the pertinent information from the studies was retrieved and entered into a Google Spreadsheet. Data were recorded and validated by all five authors into columns as follows: i) First, author and year; ii) second, country; iii) third, study design; iv) fourth, sample size; v) fifth, gender; vi) sixth, mean age; vii) seventh, intervention name; viii) eighth, intervention length; ix) ninth, comparison; and x) tenth, outcome consisting of ORM1 expression levels.
The main outcome of interest was the concentration of ORM1, sensitivity, and specificity in detecting HCC related to hepatitis B. Expression of ORM1 related genes, correlation between ORM1 and prognosis or other medical conditions were decided as secondary outcomes.
The ROBINS-I tool was used by five independent reviewers (KCT, AA, NADR, INSS, and EKSL) to evaluate each study that was included in this analysis. Later, the disagreements were discussed and settled between the reviewers, and we decided to exclude article with critical overall bias from ROBINS-I assessment.
According to the ROBINS-I assessment bias conducted by KCT, AA, NADR, INSS, and EKSL, All the five included articles did not show any critical overall bias as mentioned in Figure 1 so we did not remove any articles.
Overall, 2,482 studies were produced by doing a literature search using four databases: ProQuest, Google Scholar, Springer, and ScienceDirect. In order to exclude non-cohort studies, automation techniques from each database were employed, which led to the exclusion of 1,770 publications. A total of 655 irrelevant topic articles were removed after authors evaluated every last article from the title and abstract for relevance. Then, 40 non-retrieved articles were excluded due to the inability to access the full text document. In total, 12 duplicate study papers were then removed. The complete texts of five articles were then obtained. Last but not least, the author determined the eligibility of each study and all five articles passed the eligibility criteria. Five papers were included in this systematic review. Our study selection process is presented in the PRISMA diagram flow chart in Figure 2.
In total, 533 people participated in the five studies included in this systematic review. Three studies were conducted in China such as Dalian city, while two studies were conducted in Japan, including Maebasi City, and Sapporo City. The complete study characteristics, including the PICO of each study, are stated in Table 2.
| No. | Author; Year | Title | Location | Study design | Population | Intervention & Comparison | Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size, n | Mean age, years (Mean ± SD) | Intervention | Comparison | ||||||||||||
| 1 | Yazawa et al., 2016.10 | Fucosylated glycans in alpha-1 acid glycoprotein for monitoring treatment outcomes and prognosis of patients with cancer | Japan | Cohort | 30 cancer patients (9 gastric, 8 colon, 5 lung, 4 esophagus, 2 liver, 1 rectum, 1 pancreas), and 21 healthy patients | N/A | AGP levels in serum samples were measured by ELISA, then AGP was purified from 0.5 ml serum, then used for preparation of labeled N-glycans followed by spectrophotometric analysis. | Cancer patients vs. healthy patients | FUCAGP in cancer patients (Mean ± SD) = 32.25 ± 6.89; FUCAGP in healthy patients (Mean ± SD) = 20.70 ± 6.61; P<0.05 | Serum AGP concentration in cancer patients (Mean ± SD) = 2507.33 ± 1093.14; Serum AGP concentration in healthy patients (Mean ± SD) = 1119.75 ± 456.65.14; P<0.05 | In post-operative period the FUCAGP and AGP levels showed no significant difference in association with their prognostic status, even though elevated levels were found in cancer patients compared with healthy patients. | Notes: quantitative changes in AGP is associated with inflammation, pregnancy, estrogen treatment, cancer, liver disease and autoimmune diseases (RA). | |||
| 2 | Gu et al., 2022.11 | ORM1 as a biomarker of increased vascular invasion and decreased sorafenib sensitivity in hepatocellular carcinoma | China | Cohort | 15 pairs of HCC samples with adjacent non-tumor tissues and 5 HCC samples with microvascular invasion were collected from patients who underwent surgical resection. | N/A | Cells were cultured and total RNA was isolated and the quality and concentration were evaluated with spectrophotometer. Cell proliferation was evaluated with cell counting kit, and immunohistochemistry was used to evaluate ORM1 expression in HCC and adjacent non-tumor samples. | GSE45114 & GSE45267 dataset (cancer & tumor) vs. noncancer and nontumor dataset | 919 DEGs were identified, 354 were upregulated and remaining downregulated, functional analysis using gene ontology and gene set enrichment analysis analyses showed DEGs were associated with immune response, cell adhesion, and cell metabolism associated with carcinogenesis. | Expression of ORM1 was significantly downregulated in HCC in both datasets compared to non-tumor. IHC was later used with results indicating ORM1 was downregulated in HCC compared to noncancer, although ORM1 had higher levels in stroma of HCC tissues. | ORM1 was significantly upregulated in the microvascular invasion samples (P=0.042) | ORM1 expression levels did not correlate with overall survival, or distant, or lymphatic metastasis, but strongly correlated with the tumor stage and grade of HCC. | Correlation between ORM1 expression and sorafenib resistance in HCC: GSE93595 showed lower in resistant but there was no significant difference (p=0.333), GSE 75620 showed ORM1 was higher in sorafenib-treated cell but there was no significant difference (p=0.421) between short-term treated and long-term resistant cells. | CCK-8 assay revealed that ORM1 could promote tumor growth, and its knock-down could suppress cancer cell growth. | |
| 3 | Higuchi et al., 2020.8 | Orosomucoid 1 is involved in the development of chronic allograft rejection after kidney transplantation | Japan | Cohort | 17 CAAMR, 30 IFTA, 25 CNI-T, 17 Normal | 46.0 (CAAMR), 44.5 (IFTA), 43 (CNI=T), 38 (Normal) | Serum and urine samples collected from kidney transplant patients. Then, samples were centrifuged and the supernatant of the urine and blood were analyzed. | CAAMR vs. interstitial IFTA vs. CNI-T vs. normal | Serum Creatinine mg/dl (CAAMR, IFTA, CNI-T, Normal) = 1.47, 1.14, 1.15, 1.05. P<0.05 for CNI-T and normal vs. CAAMR | CRP mg/dl (CAAMR, IFTA, CNI-T, Normal) = 0.02, 0.03, 0.03, 0.03 with P > 0.05 | Urinary TP/Creatinine g/g Cre (CAAMR, IFTA, CNI-T, Normal) = 0.53, 0.05, 0.03, 0.02. with P < 0.05 for CNI-T and IFTA, N VS CAAMR. | ||||
| 4 | He et al., 2022.2 | Salivary orosomucoid 1 as a biomarker of hepatitis B associated hepatocellular carcinoma | China | Cohort | Discovery Cohort: 10 HCC, 10 LC, 10 CHB, 10 NC, Validation Cohort: 80 HCC, 40 LC, 40 CHB, 40 NC | HCC: 52.47 ± 8.65, LC: 51.93 ± 12.18, CHB: 47.88 ± 9.36, NC: 48.2 ± 10.76 | Saliva were collected and mixed in each groups then underwent through protein extraction and labeled with iTRAQ marker reagent. Samples later went to peptide fractionation, high performance liquid chromatography, mass spectrometry detection, target protein determination, and analyzed with western blot, immunohistochemistry, ELISA, and statistical analyses. | HCC patients vs. LC patients vs. CHB) patients vs. NC | Serum ALT IU/ml (HCC, LC, CHB, NC) = 54,5 ± 13.533, 35 ± 11,1212, 428.5 ± 10.2466, 25 ± 11.41. P>0.05 for HCC vs. LC, P<0.05 for HCC vs. CHB, HCC vs. NC. | Serum ALT IU/ml (HCC, LC, CHB, NC) = 81.95 ± 20.558, 49 ± 15,1011, 190 ± 17.2134, 25.5 ± 13.38. P>0.05 for HCC vs. LC, P<0.05 for HCC vs. CHB, HCC vs. NC. | Serum AFP ug/L (HCC, LC, CHB, NC) = 301.7 ± 1.51,8890, 11.78 ± 1.63,358.4, 13.52 ± 1.55,600.6, NA. P<0.05 for HCC vs. LC, HCC vs. CHB | HBV-DNA log IU/ml (HCC, LC, CHB, NC) = 3.11 ± 1.7,7.43, 1.7 ± 1.7,7.21, 5.24 ± 1.7,9.52, NA. P<0.05 for HCC vs. LC, HCC vs. CHB | HBeAg (Positive/negative) (n) (HCC, LC, CHB, NC) = 23/57, 9/31, 21/19, 0/40. P>0.05for HCC vs. LC, P<0.05 for HCC vs. CHB, HCC vs. NC. | Early HCC (n)/advanced HCC (n) (HCC) = 40/40 | The AUC of salivary ORM1 was 0.8499 (95% CI: 0.7724–0.9075), with a sensitivity of 77.5%, a specificity of 81.67%, when the cut-off point was set to 1,277 ng/ml; the AUC of salivary AFP+ORM1 combination was 0.9207 (95% CI: 0.8825–0.9590), with a sensitivity of 95%, a specificity of 74.17%. Salivary ORMI may be a potential biomarker for diagnosis or screening of HCC, and performed much better when combined with salivary AFP. |
| 5 | Li et al., 2016.1 | The increased excretion of urinary orosomucoid 1 as a useful biomarker for bladder cancer | China | Cohort | 112 patients with bladder cancer and 21 cases with benign bladder damage | 2- Dimensional Electrophoresis: Healthy controls (67.64 ± 8.34), Bladder cancer (69.23 ± 14.11); Western blotting: Healthy controls (63.12 ± 7.54), Bladder cancer (67.89 ± 17.21); ELISA: Healthy controls (65.33 ± 10.57), Benign Damage (61.27 ± 9.77), Bladder Cancer (64.85 ± 18.85). | Urine samples were collected and underwent through 2-DE analysis, Spectrometric analysis, and western blot analysis. | Bladder cancer vs. NC (2-De and Western Blot), bladder cancer vs. benign damage vs. NC (ELISA) | urinary ORM1 was significantly (P<0.05) upregulated in bladder cancer cases than that of the control group. | the urinary ORM1 was markedly elevated in bladder cancer patients than in controls and benign cases (7,172.23±3,049.67 vs. 2,243.16±969.01, 2,493.48±830.37 ng/ml, P<0.0001). After division of the patients into groups by tumor classification, the urinary ORM1 concentrations were 5,313.35±1341.39, 5,892.76±1,943.94 and 1,0376.49±2,677.76 ng/mg for bladder cancer with low grade non-invasive papillary urothelial carcinoma, high grade noninvasive papillary urothelial carcinoma and infiltrating urothelial carcinoma. | Spearman rank correlation = 0.712 (P<0.0001). There was no significant association between the urinary ORM1 expression and other clinical features of bladder cancer, except ORM1 content was associated with urinary inflammation (OR: 1.841, 95% CI: 1.123–2.565, P=0.024) and the classification of tumor pathology in bladder cancer (OR: 2.432, 95% CI:1.253–3.589, P<0.001). | ROC curve analysis showed cut-off value of 3,912.97 ng/mg corresponding to 91.96% sensitivity and 94.34% specificity with AUC 0.965 (95%CI: 0.923–0.987) for utility of urinary ORM1 in early diagnosis and surveillance of bladder cancer. Also, a cut-off value with 7,351.28 ng/mg corresponding to 91.89% sensitivity and 90.67% specificity. The AUC was 0.921 (95% CI 0.853–0.963) for utilization of ORM1 to distinguish infiltrating urothelial carcinoma from bladder cancer. | |||
With the use of the ROBINS-I risk-of-bias tool for observational studies, the quality of each study was rigorously evaluated. No bias was indicated in five of the studies. Figure 1 provides a summary of the bias risk assessment.
The journal selection method that has been carried out produced five studies that were used in the systematic review process. The five studies were conducted by Yazawa et al. (2016),10 Gu et al. (2022),11 Higuchi et al. (2020),8 He et al. (2022)2 and Li et al. (2016).1 Brief profiles of the five studies are shown in Table 2.
This systematic review confirms the evidence of salivary ORM1 as a possible early diagnostic and prognostic biomarker for HCC related to hepatitis B. From the studies included, we can generally find how ORM1 has a role in the early stage of HCC induced by hepatitis B and could be a potential early stage biomarker and determine the correlation of ORM1 level related to patient prognosis.
The methodical approach to the identification, selection, and extraction of data using a causality framework that offers a framework for the assessment of varied sources of evidence and a substantial number of review questions are the study’s strengths. To our knowledge, this is the first systematic review that assesses the levels of ORM1 in patients with cancer along with the diagnostic and prognostic evidence. The main limitation of the traditional systematic review was high workload and time necessary to maintain it. Another disadvantage was the time required to settle inter-reviewer variations in the interpretation of qualifying requirements. This might have led to subjectivity in judgments about inclusion in the review. Although a second reviewer examined all extractions, changes in the review team might cause inconsistency. We utilized case definitions as given by authors in individual articles, as we did in the baseline review.
Even though this systematic review can qualitatively analyze the evidence of the included studies, the limited number of original studies with homogenous PICO is our limitation. In the future with more original related studies, it will be possible to conduct quantitative analyses.
Orosomucoid is a component of plasma protein that has numerous roles in body physiology, including capillary barrier regulation, metabolism, and the immune system.12 ORM1 or alpha-1-acid glycoprotein (AGP) is a class of proteins with carbohydrate content up to 45% and protein pI 2.8-3.8, which often appears during the acute phase of the disease.6 ORM is formed by the liver and finally secreted throughout the body with a normal value of 0.4–1.2 mg/ml plasma in the human body. If the amount increases, it can be a sign of an unusual condition in the body, such as pregnancy, drug use, or certain diseases. The alpha (1)-acid glycoprotein glycan structure is shown in Figure 3.10
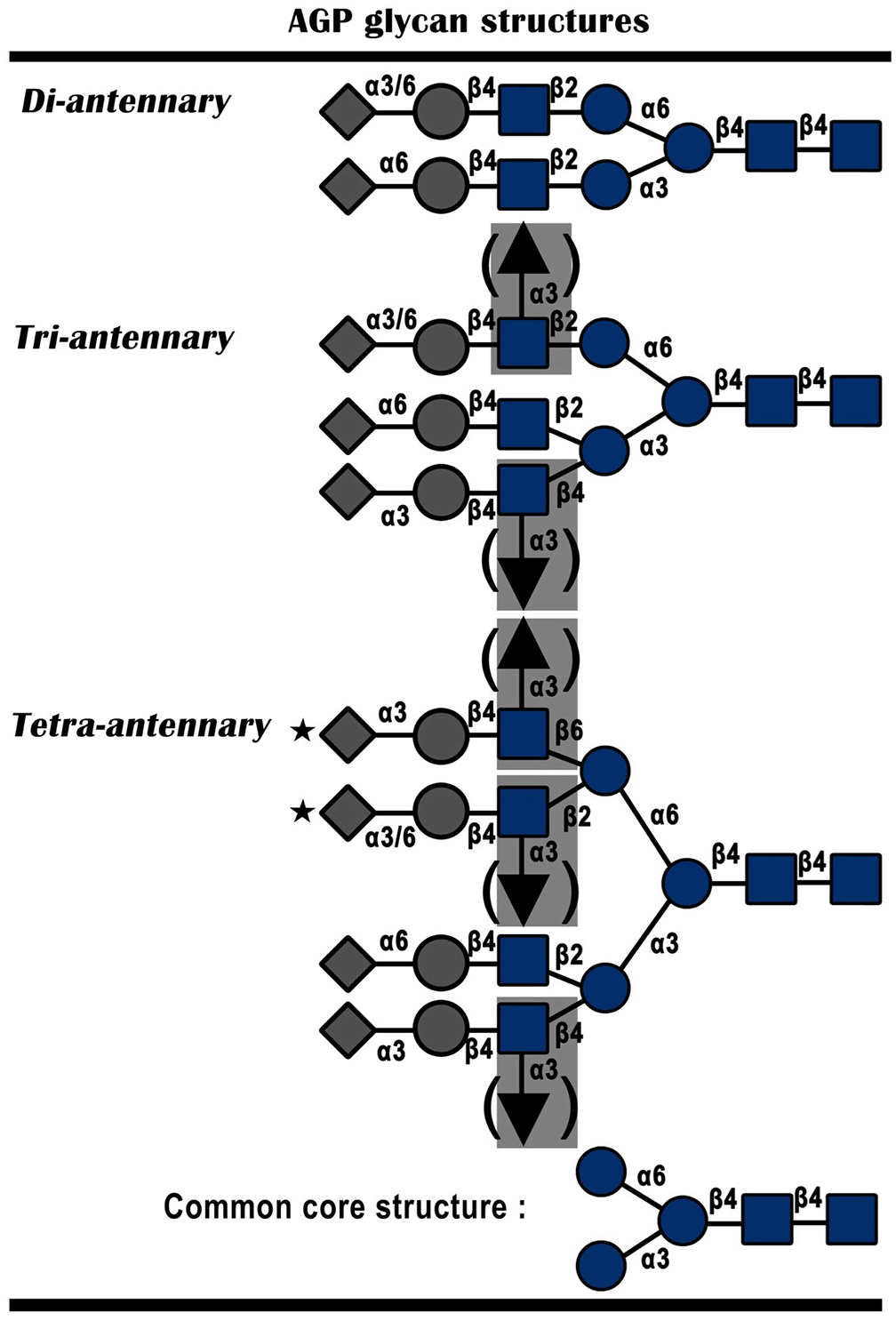
This figure is reproduced from Yazawa et al., 2016.10
The human ORM is located on the long arm of chromosome 9 in the AGP-A, AGP-B, and AGP-B′ clusters.11,13 α-1 acid glycoprotein (AGP) or ORM is a class of plasma glycoproteins consisting of many N-linked glycan branches and a total molecular weight of 41–43 kD.10 The ORM structure consists of 201 polypeptide chain residues, of which 22 residues distinguish ORM1 and ORM2. Structural analysis of ORM1 shows a combination of N-linked glycan chains associated with neutral hexagons, fructose, sialic acid, hexosamine (N-acetylglucosamine), mannose, and galactose.4 Theoretically, each of the five N-linked glycans can join branches with different degrees, express other glycan chains, and form more than 105 other glycoforms of ORMs.
As aforementioned, if ORM1 is produced in hepatocyte cells in the liver, it will then be distributed to the peripheral circulation, even in saliva, and it will increase its value if the tissue is inflamed or malignant.4 Serum is the gold standard in the detection of ORM levels, but the potential of saliva and urine for a similar role is currently being developed. It has been investigated whether there are differences between those three media, and which results showed that the correlation between the reference method and assay of ORM1 serum was 0.97 (p < 0.001). Meanwhile, the saliva also gave a similar result as serum, that is 0.97 (p < 0.001) higher than in media that showed 0.93 (p < 0.001).12
ORM1 is synthesized by hepatocytes and parenchymal cells of the liver. Orosomucoid synthesis is upregulated by inflammatory cytokines and then released into the blood where it is distributed in body fluids, such as saliva, urine, plasma, and mucus.14–16 ORM1 expression is regulated by TGF-β and mediated by the TGF-β/Smad signaling pathway.2 ORM1 concentration can elevate 1–10 times during pathological conditions depending on the severity of the disease. Inflammation level and degree of injury are also factors that can increase the concentration of ORM1. ORM gene expression in liver cells is related to inflammatory mediators, such as glucocorticoids, cytokines, interleukins, and TNF-alpha. Hepatocytes and the liver are the primary sources of ORM synthesis.17–20
ORM1 has various biological functions, including being a biomarker for disease, immunomodulatory effects, and roles in important drug-binding processes, transporting proteins in the serum with albumin and lipoproteins, maintaining capillary barrier function, and various metabolisms. ORM1 serum and saliva concentration can change due to stressful stimuli, such as physical trauma, bacterial infection, and unspecific inflammatory stimuli.4 Due to its immunomodulatory effects, Higuchi et al., stated that ORM1 can be used as a possible therapeutic molecular target. ORM1 concentration was found to be significantly higher after kidney transplant due to cytokine mediated NFxB and STAT3 activation in primary kidney tubular cells.8 ORM1 could also be used as a potential biomarker for cancers in the pancreas, bladder, and liver cancer by determining the level of ORM1 in the serum of body fluids, saliva, urine, and blood.10 Damage in the hepatocytes due to liver cancer may cause a change of ORM1 serum levels. He et al., showed that salivary ORM1 is significantly increased in HCC related to hepatitis B virus.2 Gu et al., also stated that the expression levels of ORM1 were strongly correlated with the tumor stage and grade of HCC.11 From these studies, we can conclude that even if salivary ORM1 is significant in several other cases, the highest increased level compared to normal controls happened in HCC related to hepatitis B, which is 4.02 times higher than the normal level.2,8,10
ORM1 functions as a transport protein in the bloodstream. ORM1 plays an important role in modulating immune system activity during acute phase inflammation.20 A previous study showed that serum ORM levels are increased in inflammatory and lymphoproliferative disorders and cancers such as liver, lung, breast, and ovarian cancer.1 ORM1 has a down-regulating effect on the inflammatory cascade, thereby protecting against tissue damage due to excessive inflammation and malignancy.
ORM1 increases in types of cancer such as invasive breast carcinoma and colon adenocarcinoma. ORM1 is also differentially expressed between tumoral and non-tumor tissues. ORM1 expression was found to be higher in embolic cancer than in surrounding tumor cells, indicating that ORM1 expression is associated with vascular invasion thereby reducing the overall prognosis of patients with HCC.11
ORM1 is predominantly synthesized in the liver, therefore liver disease has more influence on its expression, ORM1 can be induced by injury in the liver and can activate the liver cell cycle to achieve liver regeneration. He et al., reported that ORM1 expression was highly correlated with tumor growth according to tumor grade and stage.2 This is consistent with the finding that salivary ORM1 increased significantly in patients with advanced liver cancer compared to patients with early-stage liver cancer, that is advanced HCC ORM level is 9.39 times higher than the normal level, while early-stage HCC is only 4.02 times higher than the normal level.2 Elevated salivary ORM1 may be a risk factor for poor prognosis in patients with HCC.
As shown in the study conducted by He et al. (2022) salivary ORM1 and AFP are significantly higher in other liver diseases. In the same study, it was found that ORM1 had significantly higher expression in the saliva of patients with HCC compared to the non-HCC group (p < 0.001) and ORM1 expression in liver cancer tissue was significantly higher than in adjacent normal tissues.2 There are different levels of salivary AFP expression and salivary ORM1 expression in total HCC, liver cirrhosis (LC), chronic hepatitis B (CHB), and normal control (NC) groups. On the same finding, salivary ORM1 showed good specificity and sensitivity for detecting HCC, especially in HCC associated with hepatitis B.7 The sensitivity and specificity of salivary ORM1 reached 81.67% and 77.5%.
As an acute-phase protein, AGP has been studied for its potential physiological significance, and both quantitative and qualitative changes to the molecule’s glycan structure have been examined in relation to inflammation and malignancy of the liver.2,21–23 The comparison value of liver disease related to hepatitis B shows in a sequence of normal liver, LC, CHB, early HCC, HCC, and advanced HCC is 429, 657, 1,073, 1,723, 2,143 and 4,029 ng/ml, respectively.2
The first rapidly increased level of ORM1 shown in early HCC (four times higher than normal) indicates that ORM1 can detect HCC in the early stage with high sensitivity and specificity. This early diagnostic biomarker has a sensitivity of 77.5% and a specificity of 81.67%.2
According to the study conducted by Yazawa et al. (2016) the reason ORM1 can be used as an early diagnostic biomarker is that it is exclusively synthesized in the liver by the encoded FUT6 gene, then this enzyme acts as a catalyzer for many reactions related to L-fucose addition to the receptor in order to protect liver cells from damage caused by HCC related to hepatitis B.10 Moreover, this biomarker is also able to detect the prognosis of the disease with or without chemotherapy, this is because the higher the level of ORM1, the more liver damage occurs that leads to a poor prognosis.12
A combination of salivary ORM1 and AFP is specific and sensitive in detecting HCC as written in a study conducted by He et al. (2022). Thus, salivary ORM1, α 1,3 fucosyltransferase, and AFP are going to be a sensitive and specific combination in detecting HCC related hepatitis B as well as prognosis, microvascular invasion (MVI), and sorafenib drug sensitivity. This method of using salivary ORM1, α 1,3 fucosyltransferase, and AFP is also a promising and less invasive method, so it has a high potential to be commercialized.2
Salivary ORM1 is a potential early diagnostic biomarker of HCC related to hepatitis B, as well as a biomarker of disease prognosis. This biomarker has a sensitivity of 81.67% and a specificity of 77.5%.
In order to increase the sensitivity and specificity of ORM1 as an early diagnostic biomarker, a combination of other biomarkers may be a good topic for further research and application. The sensitivity and specificity of combined salivary AFP and salivary ORM1 tend to be 95% and 74.17%, respectively. In order to overcome the lack of specificity of the aforementioned biomarker combination, hypoalbuminemia detection from albumin assessment as an additional sign of poor liver function is suggested. Therefore, the combination of salivary ORM1, salivary AFP and albumin assessment may be able to provide high sensitivity and specificity in detecting HCC related to hepatitis B.
All data underlying the results are available as part of the article and no additional source data are required.
Mendeley Data: PRISMA checklist for ‘The role of salivary orosomucoid 1 as an early diagnostic and prognostic biomarker of hepatocellular carcinoma related to Hepatitis B: A systematic review’. https://doi.org/10.17632/ykr5jtj6sx.1. 9
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Partly
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Not applicable
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Nursing, cancer, general medicine, systematic review, and meta-analysis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 06 Jun 23 |
read | read |
|
Version 1 14 Apr 23 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)