Keywords
Secretome, Mesenchymal stem cells, Immunomodulator, In vivo, Safety
Secretome, Mesenchymal stem cells, Immunomodulator, In vivo, Safety
Mesenchymal stem cells (MSC) have been a potential candidate therapeutic agent for many degenerative diseases due to their ability to modulate many processes in the body, such as angiogenesis, inflammation, apoptosis, wound healing, and the immune response.1–3 Their pharmacological activities have been suggested to be the result of their ability to differentiate into cells that can perform repairs in problematic areas in the body.4 In addition, MSCs secrete substances that can repair damage in the body and maintain body homeostasis. Some of the substances produced by MSCs include growth factors, extracellular vesicles, and other soluble proteins.2,5 These materials are often called secretomes, and since they exist in the MSC medium, the medium is called “conditioned medium”.
Secretomes are becoming a very attractive agent in drug development because of their several advantages over MSCs. Among them are their safety, easy administration, and easy storage. Secretomes are considered safer than MSC because they don’t contain cells, have no risk of causing an embolism, have no risk of tumor formation due to wrong cell differentiation, do not need cryopreservation, and have a low risk of carrying infectious agents as well. In addition to their advantages over MSCs, secretomes also have a pharmacological activity, including anti-inflammatory, anti-fibrosis, wound healing, and immunomodulatory effect.6
Studies have shown the immunomodulatory effect of MSCs.7 However, there is a lack of information regarding the effect of secretomes derived from human umbilical cord MSCs (UCMSCs) in vivo. Therefore, this study aimed to investigate the immunomodulatory activity of secretomes. We also aimed to evaluate the safety of the secretome by conducting an acute toxicity assay.
The experimental protocol was approved by the Medical and Health Research Ethics Committee (MHREC) Faculty of Medicine Universitas Gadjah Mada (KE/0108/01/2022) on February 23rd 2022. The collection of secretomes was approved by PT Tristem Medika Indonesia (UC-2-RMD-3) on June 15th 2021. Participants provided informed written and verbal consent prior to their participation.
PT Tristem Medika Indonesia provided the MSC secretome. The MSC secretome was produced in four batches, on 17th September 2021 (Batch I), 27th January 2022 (Batch II), 10th April 2022 (Batch III), and 5th July 2022 (Batch IV), respectively. The MSC secretome production procedure followed the PT Tristem Medika Indonesia method. Briefly, the umbilical cord tissue was collected from eligible consenting donors following caesarean delivery and transported to the laboratory using a sterile bottle with DPBS (Gibco) with 2% penicillin-streptomycin (Sigma Aldrich, Germany). The tissue was rinsed and cleaned from blood using a similar solution that was used for transport. The blood vessel was removed and cut transversally into 1-cm segments. Then, it was cut longitudinally into halves. The tissues were rinsed using DPBS (Gibco, Thermo Fisher Scientific, USA), minced into 2-5 mm small pieces, and explanted to a 100 mm cell dish (Nunc, Thermo Fisher Sceintific, USA) in MEM Alpha Glutamax (Gibco) supplemented with 10% UltraGro (AventaCell Biomedical, USA) and 1% penicillin-streptomycin. The explants were incubated at 37°C, 5% CO2 and 2-8% O2. The medium was replaced every four days, and the tissues were removed once the cells were migrated. After 14 days, the cells were harvested and subcultured using the antibiotic-free complete medium with a seeding density of 8000 cells/cm2. The UCMSCs were further expanded until passage four to create a two-tier cell bank, master cell bank (passage two), and working cell bank (passage four). The working cell banks were thawed and cultured to passage six. After cells reached 90% confluency (day three), the culture medium was replaced with MEM Alpha without phenol red (Gibco) and further cultured for another three days. After three days, the conditioned medium was harvested, filtered using a 0.22 μm membrane (Nunc), and filled into cryovials (Corning) with 1.5 mL volume. The secretome products were stored at -20°C.
Standard ELISA procedures using EliKineTM Human IL-10 ELISA KIT (Abbkine, China) were used to evaluate the IL-10 concentration in the secretome. The measurements of IL-10 in secretome were conducted based on the protocol provided by the kit manufacturer. The absorbance was read at 450 nm using an iMar Microplate reader (Bio-Rad, UK). The ELISA data were analyzed using Microplate Manager Software (Bio-Rad).
Exosomes were isolated from MSC secretome using Total Exosome Isolation from biofluids (Invitrogen, Thermo Fisher Scientific, USA). The starting volume of the secretome used was 10 mL and prepared according to the manufacturer’s instructions. The isolated exosomes were then diluted using PBS as much as 1 mL. Diluted exosomes were stored for further characterization.
The size distribution and concentration of isolated exosomes were examined using NTA. The NTA (ViewSizer 3000 – Horiba) instrumentation was equipped with multi-Laser; Blue-445 nm, Green-520 nm, and Red-635 nm, and a fluorescent detector with 450, 550, and 650 nm filters so that analysis could be done using a fluorescent marker. The volume required for the measurement process was as much as 500 μL exosome in PBS, and the observed particle sizes were limited to a 30 to 150 nm range.
For TEM analysis, as much as 200 μL of isolated exosomes were diluted using PBS to reach a volume of 1 mL exosome in PBS. Exosomes were dripped onto the 400 mesh Cu coated grid, allowed to absorb, and dried using filter paper. Negative staining was applied, using 1% uranyl acetate. The negative staining was dripped on the grid and allowed to be absorbed, then dried with filter paper. TEM visualizations were performed using JEOL 1400 Transmission Electron Microscope at 120 kV, and images were captured using a fully integrated high resolution camera system (8 million pixel CCD camera). Exosome particle size measurements were carried out using ImageJ software version 1.53K.
The miRNA profiling started by isolating the exosome using exoRNeasy Midi Kit (Qiagen) based on the manufacturer’s protocols. The total RNA calculation was done using a NanoDrop (Thermo Scientific) and the miRNA profiling was conducted using the Nanostring OID NGS-036 (NanoString Technology, USA). Then, the miRNA data analysis was conducted using nSolver Analysis Software 4.0, with which the ten highest normalized expressions of the miRNA were obtained. The endogenous housekeeping gene was beta-actin. The threshold for reading background subtraction was determined using negative control read.
The prediction of the top 10 miRNA biological activity was done using free software DIANA miRPath v3.0 tools available From DIANA Lab, supported by experimental interaction from DNA-TarBase v.7.0. TarBase v7.0 is a combination of half a million miRNAs derived from gene interactions from hundreds of publications and more than 150 CLIP-Seq libraries.8
Male Swiss albino mice, 6-8 weeks old, weighing 15 to 25 g, were used in the study. The animal’s room temperature is set to 22° ± 3°C, with a relative humidity of 30–70%, 12 hours of light, and 12 hours of darkness. Animals are fed according to laboratory standards and given indefinitely (ad libitum). The animals were maintained under standard conditions according to Regulation of the Head of National Agency of Drug and Food Control (BPOM) No. 7 of 2014 concerning guidelines for in vivo tests.8 The person conducting the animal handling was not blinded to the treatment group. However, the person conducting the measurement of the carbon clearance assay was blinded to the treatment group.
After the test was complete, all experimental animals were sacrificed using ketamine at a dose of 80 mg/kg BW. Ketamine is injected intraperitoneally. The experimental protocol was approved by the Medical and Health Research Ethics Committee (MHREC) Faculty of Medicine Universitas Gadjah Mada (KE/0108/01/2022).
20 healthy male mice were randomly divided into four groups. Each group consisted of five mice, and all received an intramuscular injection of secretome or MEM-α basal medium at 0.025 mL in each thigh. The first group received 0.00625 mL of secretome intramuscularly in each thigh (dose 1). The second group received 0.0125 mL of secretome intramuscularly in each thigh (dose 2). The third group received 0.025 mL of secretome intramuscularly in each thigh (dose 3). The control group received 0.025 mL of sterile MEM-α basal medium in each thigh (negative control). After three days of treatment, all the groups received 0.1 mL of carbon ink suspension through the tail vein. Blood was collected at an interval of 0 and 15 min immediately after injection of carbon suspension. Measurement of carbon in the blood was determined using a spectrophotometer at 675 nm. The phagocytic index was calculated based on optical densities value with the following formula.
Note:
OD1 is the optical density at 0 minutes after carbon injection
OD2 is the optical density at 15 minutes after carbon clearance injection
t2 is 15 minutes
t1 is 0 minutes
Female Swiss albino mice were used in this test. Healthy female mice at 8-10 weeks old were used because, generally, female animals are more sensitive to toxic substances than male animals.8 The method for conducting the acute toxicity test was modified fixed dose according to Regulation of the Head of National Agency of Drug and Food Control (BPOM) No. 7 of 2014.8 There are two parts to the fixed-dose methods: preliminary and main parts. In this preliminary part, we used two mice divided into two groups, the treatment group, and the control group. The mice received secretome or MEM-α basal medium intramuscularly with the highest possible volume that can be given intramuscularly to mice, which was 0.05 mL in each thigh or 0.1 mL per mouse. The mice were randomly assigned to receive treatment or control. Observations of their behavior and clinical signs were conducted in the first 30 minutes and every 4 hours for 24 hours. After 24 hours, the mice were observed daily until day 15. Since there was no mortality in the preliminary parts of the acute toxicity test, the main test was carried out with the same dose as the dose used in the preliminary part. In the main test, we used four mice in the secretome group and one mouse in the control group. The mice were observed in the first 30 minutes after injection, every 4 hours during 24 hours, and every 24 hours until day 15. On day 15, all mice were killed, and all vital organs were examined.
The IL-10 concentration measured by ELISA showed that the concentration of IL-10 was 0.272-1.951 pg/mL (Figure 1).
The mean particle size in secretome and water was 117 nm and 104 nm, respectively. The particles in size 30-150 nm in secretome and water were 3.1×108 and 1.5×106, respectively. Figure 2 presents the particle examination result using NTA.
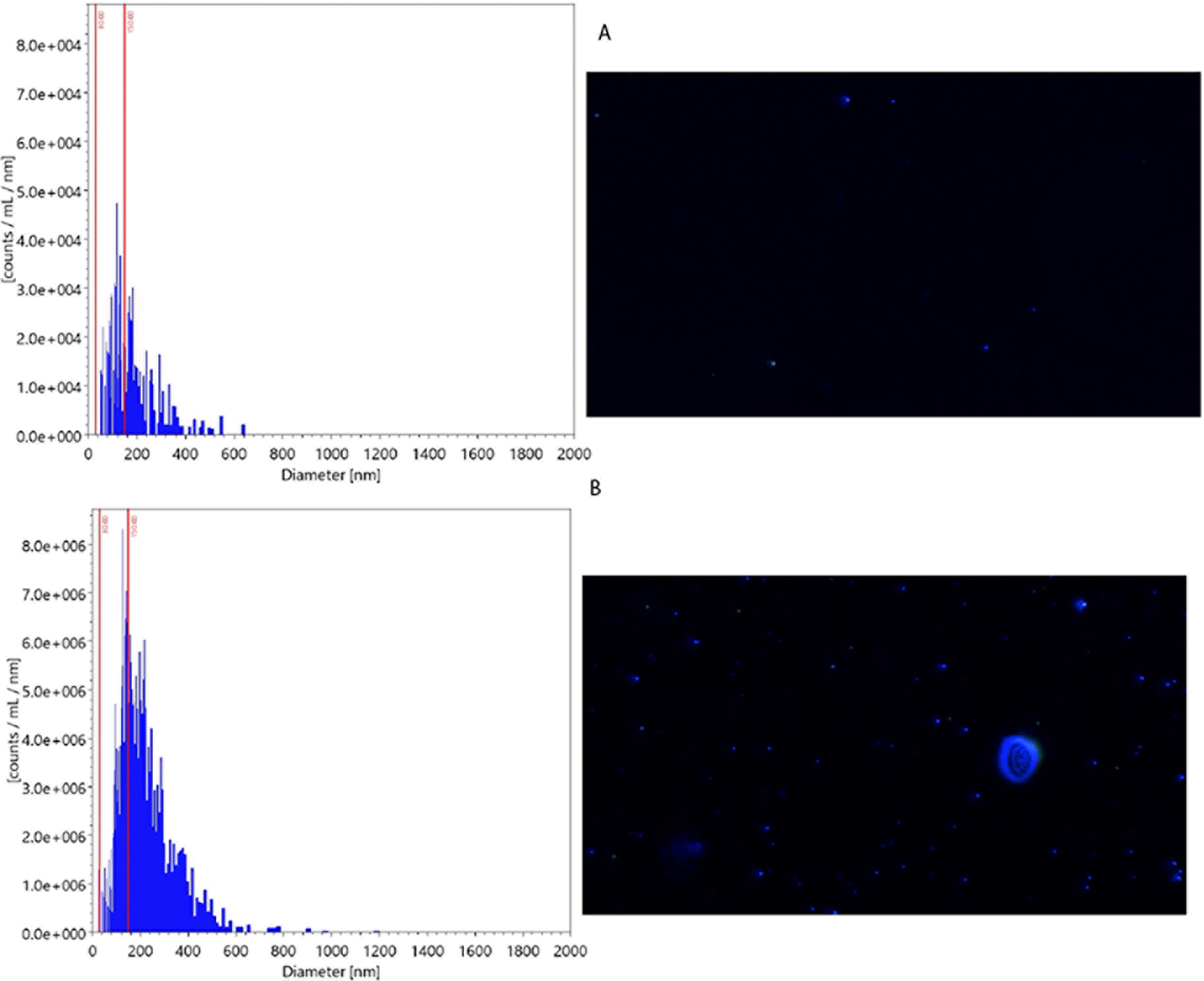
The graph on the left side shows the particle distribution across the different sizes. The picture on the right side shows the particle picture in measured samples. The mean particle size (x-axis) in the secretome was larger than those in water (117 nm (B) vs104 nm (A), respectively). The count of particles (y-axis) in size 30-150 nm was also greater in secretome than in water (3.1×108 (B) vs 1.5×106 (A), respectively).
The visualization using TEM revealed that exosomes had been successfully isolated within the expected size range (30-100 nm). The analyzed particles’ morphology (Figure 3) was consistent with NTA results.
The 10 highest reads of miRNA normalized by beta actine are presented in Table 1. The 10 miRNA with highest read were hsa-miR-23a-3p, hsa-miR-4454 + miR-7975, hsa-miR-1915-3p, hsa-miR-4488, hsa-miR-125b-5p, hsa-miR-21-5p, hsa-miR-130a-3p, hsa-miR-320e, hsa-miR-494-3p, and hsa-miR-4516.
Prediction of miRNA biological activity
The predicted biological activity of the top 10 miRNA in the MSC are presented in Figure 4.
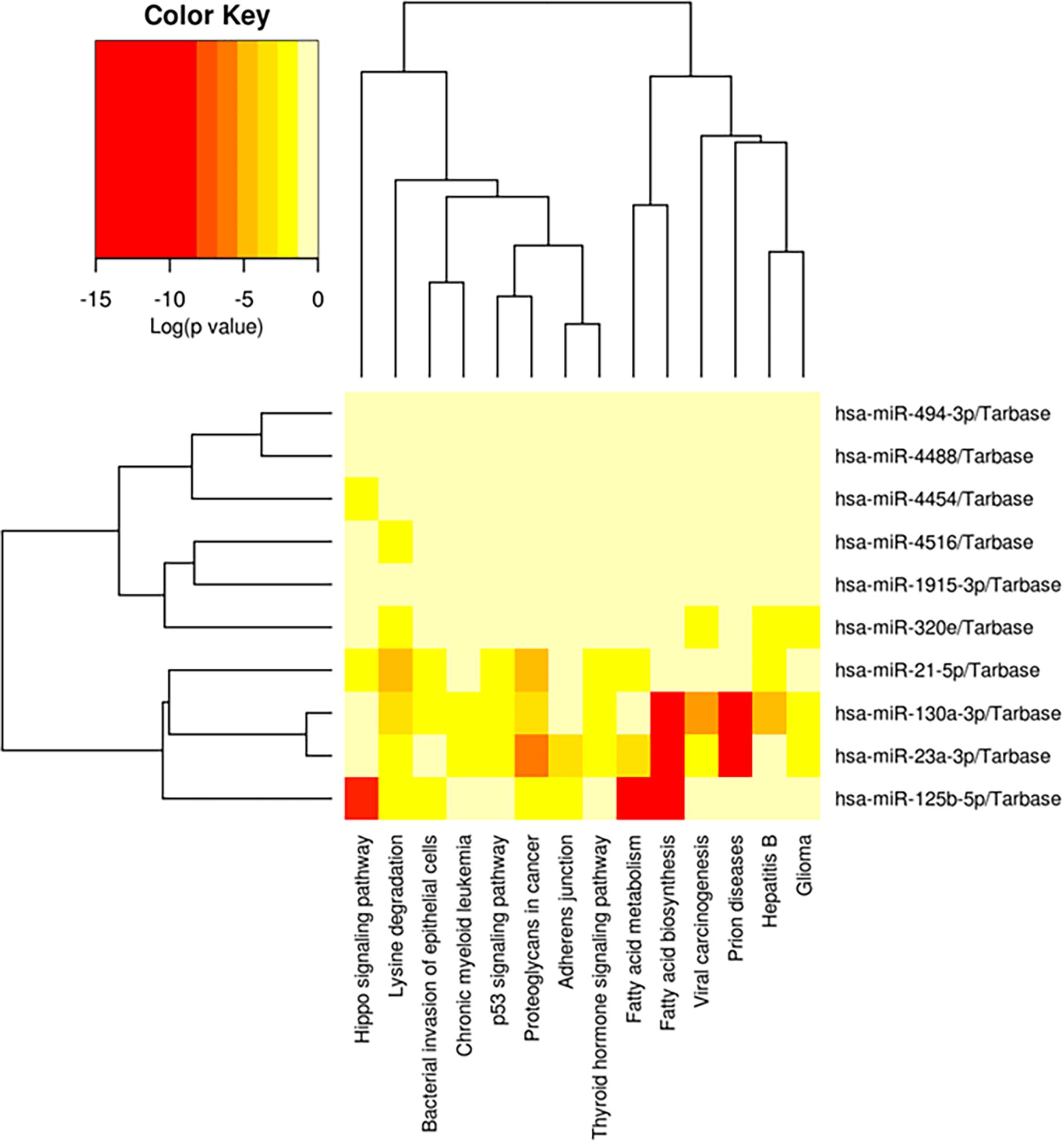
The prediction of related biological activity of the 10 highest miRNA in the secretome MSC was fatty acid biosynthesis, fatty acid metabolism, prion diseases, and hippo signalling pathway (red color).
The biological activity that were most related to the miRNA in the secretome MSCs were fatty acid biosynthesis, fatty acid metabolism, prion diseases, and hippo signalling pathway.
The phagocytic index was significantly different between those in the control and 0.025 mL group (dose 3). Figure 5 shows the phagocytic index calculation of every group.
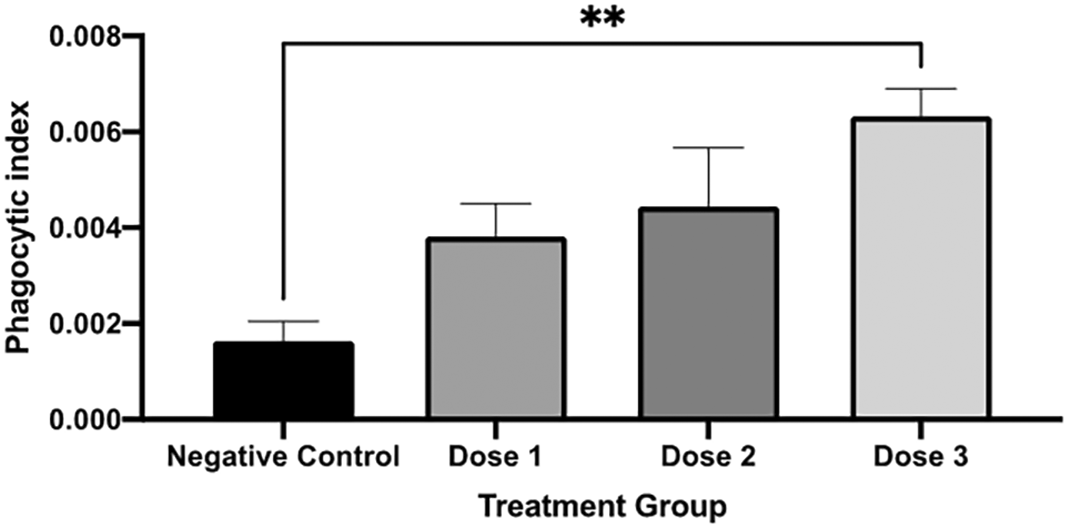
The phagocytic index was significantly higher in dose 3 compared to those in the negative control. A negative control is without secretome treatment. * p < 0.05 vs negative control.
Acute toxicity test
In the preliminary part of the acute toxicity test, the mice received secretome or MEM-α basal medium intramuscularly of the highest possible volume that can be given intramuscularly to mice which is 0.05 mL in each thigh or 0.1 mL per mice. There was no abnormality in the physical examination of the mice in the control and treatment groups. The body weight (Table 2) was also similar between the control and treatment groups. Therefore, the main study was conducted using a 0.1 mL dose per mouse.
The body weight was similar in control and treatment groups.
| Group | Body weight (g) | ||
|---|---|---|---|
| Before treatment | Day 7 | Day 15 (14 days after treatment) | |
| Control | 37.5 ± 2.1 | 38.5 ± 0.7 | 39 ± 1.4 |
| Treatment | 40.8 ± 4.9 | 41.4 ± 4.2 | 42 ± 6.4 |
The relative organ weight was measured by dividing the organ weight by the mouse’s body weight. The relative organ weight was also similar between groups (Table 3).
The relative organ weight was similar in control and treatment groups.
Our study presents the secretome effect on phagocytic activity in mice by carbon clearance assay. Phagocytosis is part of the natural immune system, which acts as a first-line defence against the intrusion of foreign matter. The injected carbon is phagocytes by reticuloendothelial systems (RES) cells which consist of phagocytic cells. This process involves opsonization and complement activation. The faster the clearance, the better the phagocytic activity.9 The phagocytic index in mice receiving the highest dose of secretome was significantly higher than those in control groups. Thus, the phagocytic activity of the mice treated with secretome was better than that of the control group.
Secretomes, as well as MSCs, have been known to have an immunomodulatory effect. MSCs contain various soluble proteins such as cytokines, chemokines, growth factors, and extracellular vesicles (EV).10 The secretome protein interacts with host cells, including endogenous stem cells and progenitor cells. It influences the function of those cells, such as regulating immune response, mediating chemokines activation, and influencing the inflammatory response.11 A study on human bone marrow stem cells (hBMSC) showed that its secretome, but not the hBMSC-self, can increase the phagocytic activity of macrophages. The increase in macrophage phagocytic activity induced by the secretome was related to the increase in macrophage CD206 expression. CD206 is a marker of M2 macrophage, an anti-inflammatory macrophage that recognizes the foreign matter as phagocyted.12 The M2 macrophage, an anti-inflammatory macrophage, has been reported to have higher phagocytic affinity than the M1 macrophage under low and high density of cell target.13
The phagocytosis ability of macrophages is diverse depending on the stimulation. Our study also examined the expression of IL-10 in the secretome. We found that the secretome contained IL-10 in the range of 0.019-1.951 pg/mL. Macrophage stimulation by IL-10 resulted in higher phagocytosis activity than unstimulated macrophages.14 Meanwhile, stimulation by IL-4 resulted in higher macrophage activity than stimulation by IFN-gamma.15 The study also showed that IL-10 and IL-4 induced human macrophage phagocytes mycobacteria faster than those on M1 macrophages.16 However, another study showed that IL-10 could increase IL-4-induced M2 polarization and increase the migration of eosinophils.17 The primary function of Il-10 is to inhibit the excessive response of the immune system.
Consequently, it can impair the immune response to infection. However, IL-10 is also necessary for tackling viral and parasitic infections, depending on the course of infection.18,19 Therefore, using IL-10 for immunomodulation should consider the pathogenesis of the infection and the administration timing.
The miRNA profiling showed that some miRNA are involved in hypo signalling pathways which is gaining attention for their roles in the immune system function and homeostasis.20 However, further studies are needed to gather more evidence about the MSC secretome miRNA roles in the immune system.
We also studied the safety of secretomes in mice by conducting an acute toxicity test. After a single intramuscular injection of the secretome, there was no toxicity sign or symptom in the injected mice. The therapeutic activity of stem cells has been known as the result of its secreted product in their medium, secretomes. The secretome, the cell-free medium of stem cells, has been gaining more interest from researchers since it is more tolerable due to its cell-free nature, easier to administrate, and easier to store.21–23 Our study has shown that the secretome derived from human UCMSCs is safe when injected intramuscularly in mice. Further studies are needed to determine its safety in long-term administration and other routes of administration.
The secretome derived from human UCMSCs shows immunomodulatory activity by modulating phagocytosis. The study also shows the safety of single administration of secretome. One of the limitations of our study is that the carbon clearance assay was only conducted on male mice to avoid the physiological variation related to the estrus cycle in female mice.
figshare: accute toxicity data. https://doi.org/10.6084/m9.figshare.22338229
figshare: Secretome carbon clearance assay dataset. https://doi.org/10.6084/m9.figshare.22217824
figshare: miRNA profiling of MSC secretome. https://doi.org/10.6084/m9.figshare.22224742
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
ARRIVE checklist for ‘In vivo immunomodulatory effect and safety of MSC-derived secretome’. https://doi.org/10.6084/m9.figshare.22566628.v1
The author acknowledges the involvement of:
1. Kepolisian Republik Indonesia for funding this research.
2. PT Tristem Medika Indonesia has provided the MSC secretome.
3. Mrs. Sumartiningsih from Laboratorium Riset Terpadu, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, for assistance in working on the ELISA technique.
4. Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Gadjah Mada, has given permission to access the TEM facilities.
5. Ms. Rahmi Ayu and Mr. Suroso from Laboratorium Farmakologi dan Toksikologi, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, for assistance in handling experimental animals and conducting in vivo tests.
6. Laboratorium Penelitian dan Pengujian Terpadu, Universitas Gadjah Mada that, provides whole blood check sevices for supplementary data on in vivo tests.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: In vitro and in vivo investigations of human umbilical cord mesenchymal stem cell secretome.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: neuroscience, cell based therapies
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 07 Oct 24 |
||
|
Version 1 19 Apr 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)