Introduction
Posaconazole is an antifungal medication that inhibits the synthesis of ergosterol, a vital component of the fungal cell membrane, ultimately causing cell death. It is effective against a wide range of fungal pathogens, making it a popular treatment for various fungal infections. Accurate and precise quantification of posaconazole in dosage forms is crucial for ensuring proper dosing and therapeutic efficacy, quality control, and pharmacokinetic studies. However, existing methods for measuring posaconazole can be expensive, time-consuming, or unreliable.1–10
High-performance liquid chromatography (HPLC) is a commonly used analytical technique for quantifying drugs in dosage forms. This study aims to develop and validate a new HPLC analytical method that is quick, sensitive, robust, cheap, and reliable for estimating posaconazole in bulk and dosage form.11–23
The HPLC method involves,
▪ Preparing a solution of the active ingredient from the dosage form,
▪ Injecting it into the HPLC system, and
▪ Separating the different components based on their interactions with the stationary phase.
Posaconazole is detected using a UV-Vis detector, which measures the absorbance of posaconazole in the eluent. The peak area or height is then correlated to the concentration of posaconazole in the sample using a standard calibration curve.24–34
The newly developed method was optimized by adjusting chromatographic conditions, including the mobile phase composition, column type, and detection wavelength. Validation was performed in accordance with International Conference on Harmonization (ICH) Q2 R1 guidelines, ensuring the method's accuracy, precision, and specificity. The method was successfully used to estimate posaconazole in its bulk and dosage forms.35–49
Following CONSORT guidelines for reporting randomized trials increased the transparency of our study and improved the quality of our reporting, contributing to the overall usefulness of our findings. Our new analytical method has significant implications for drug delivery, including more accurate and efficient monitoring of posaconazole levels in patients, increasing overall drug efficacy and safety.
Overall, this manuscript presents the development and validation of a new HPLC analytical method for estimating posaconazole in bulk and dosage form, which will provide valuable insights into analytical method development and validation for drug delivery and contribute to the ongoing efforts to improve patient outcomes.50–63
Methods
A HPLC Grade Milli-Q water (Milli Q Direct-Q 3 UV Water Purification System, Merck, United States), Analytical Weighing Balance (BSA224S-CW, Sartorius, Germany), UV Spectrophotometer (UV 1800, Shimadzu, Japan) was used in the study. Posaconazole was received as ex-gratis from Lupin Healthcare limited. Ortho-phosphoric acid (88%) was from Merck Ltd. (Mumbai, India). HPLC grade Acetonitrile (MeCN) and methanol (purity, min 99.8%), was procured from Merck Ltd. (Mumbai, India). A Nylon membrane filter of 0.45 μm was obtained from HiMedia Pvt. Ltd (Mumbai, India). Phenomenex Hyperclone C18 column (5 μm particle size, 100 Å, 250 mm × 4.6 mm id) was procured from Phenomenex (Hyderabad, India), Other reagents and solvents utilized for the method development and validation were of analytical or HPLC grade.
The Shimadzu LC-2010CHT high-performance liquid chromatography model was used in the current research investigation. The device was equipped with a dual-wavelength ultraviolet detector, a column oven, and an autosampler from Shimadzu. The data acquisition of the chromatograms produced was done using LC Solution software version 5.57. A Hyperclone C18 column (250 mm x 4.6 mm in diameter, particle size 5 microns) from Phenomenex, USA. The mobile phase was filtered through a Millipore glass filter using 0.22-micron pore-size nylon membrane filter paper and connected to a glass vacuum filtration unit. The filtered mobile phase was then sonicated in a GT Sonic Professional Ultrasonic Cleaner)GT-2013QTS, GT Sonic, China) for 10 minutes to remove any air bubbles. The pH of the buffer was determined using a pH meter and a glass electrode (μ pH System 361, Systronics, India).
Selection of wavelength
Posaconazole was weighed and mixed with spectroscopy grade methanol to prepare the stock solution (10 mg in 10 mL). A series of concentrations (5 μg/mL, 10 μg/mL, 15 μg/mL, 20 μg/mL, 30 μg/mL) were prepared from the stock solution, and a UV spectrophotometer determined their absorbance maxima. The absorbance maxima were then used for the HPLC method development.13,64–73
Selection of Stationary and Mobile Phase
Choosing the right stationary and mobile phase is critical in HPLC method development. The underlying protocol for the selection of Stationary Phase and Mobile Phase is reposited in the Protocols.io repository which can be accessed by following these references.74,75 To develop the method, the Phenomenex Hyperclone C18 column was selected as the stationary phase, and Acetonitrile and Methanol (as Organic Phase) and 10mM Phosphate buffer (pH 6.8) (Aqueous Phase) were chosen as the mobile phase. The stock solution was prepared in Acetonitrile, and an isocratic mode of elution was employed for the method. The injection volume was set to 20 microliters, and the detection wavelength was selected based on the absorbance maxima determined by UV. The stationary phase was maintained at 25°C, while the flow rate of the mobile phase varied between 0.8 and 1.2 mL per minute. From the stock solution, 1 μg/mL was prepared and used in the analysis.
10 mg of Posaconazole was weighed accurately and transferred to a 10 ml volumetric flask. The volume was made up with Acetonitrile to prepare the stock solution. From the stock solution 1 μg/mL was prepared. Isocratic mode of elution was opted for the method. Injection volume was set to 20 microliters and the detection wavelength was selected based on the results of the determination of absorbance maxima by UV. Stationary phase was maintained at 25oC. Flow rate of the Mobile Phase was varied between 0.8 mL to 1.2 mL per minute.
After the initial method development trials were conducted, an optimized trial was carried forward for analytical method validation according to ICH guidelines.76–94
Validation of the optimized HPLC method
The validation of an analytical method is critical for ensuring that the method is appropriate and reliable for its intended usage. In this study, the optimized analytical technique was subjected to a series of validation experiments recommended by the International Conference on Harmonization (ICH) Q2(R1) guidelines in order to evaluate its performance under various situations.53,88,89,95–125
Specificity
An analytical method's capability to accurately measure the analyte despite the presence of diluents or excipients in the sample is referred to as “specificity.” To assess the specificity of the optimized analytical method, three replicates of a blank solution (diluent), Posaconazole (1 μg/mL), and a commercial formulation (equivalent to 1 μg/mL of Posaconazole) were introduced into the HPLC system. The resulting chromatograms were then examined for any interference from the diluents or excipients at the retention time of the Posaconazole.
Linearity
Ensuring linearity is critical to analytical method development, as it guarantees accurate analyte quantification across a broad range of concentrations. To assess linearity, a range of serial concentrations, spanning from 0.1 to 32 μg/mL, were prepared from a standard Posaconazole drug solution using the mobile phase ratio as a diluent. Quintuplicate injections were made for each concentration, and the resulting peak areas (in mV-min) were plotted against their respective concentrations (in μg/mL) to create a linear regression graph. From this graph, the intercept and slope were calculated via a linear regression equation.
Accuracy
The accuracy of an analytical method is determined by its ability to produce results that are close to the true or accepted value of the measured concentration. To evaluate the accuracy of the developed method, three concentrations from the linearity range (2 μg/mL, 4 μg/mL, and 8 μg/mL) were analyzed in sextuplicate. Intra-day accuracy was assessed by analyzing sextuplicate injections of all three concentrations twice on the same day (at 09:00 and 21:00 hours), while inter-day accuracy was evaluated by analyzing duplicate injections of all three concentrations over three consecutive days. The mean percentage recovery was calculated for each concentration to determine the method's accuracy.
Sensitivity
Sensitivity is a crucial characteristic of an analytical method, as it determines the ability to detect small amounts of the analyte. In this study, the method's sensitivity was evaluated by determining Posaconazole's detection limit (DL) and quantitation limit (QL). The DL is defined as the lowest concentration of Posaconazole that can be detected with reliability and accuracy, while QL is the lowest concentration that can be measured with accuracy and precision. A lower DL and QL indicate a more sensitive method with the ability to detect and quantify low concentrations of the analyte.
The DL and QL are calculated based on the residual standard deviation of the regression line and its slope.
The formula is
Where, σ = residual standard deviation of the regression line
S = slope of the regression line
Precision
Precision is a critical parameter in evaluating the reliability and reproducibility of an analytical method. It assesses the agreement between multiple measurements of the same homogeneous sample under the same experimental conditions. In this study, the precision of the developed analytical method was determined by analyzing four quality control concentrations of Posaconazole, including the quantitation limit, the lower quality control concentration (three times the quantitation limit), higher quality control concentration (70% of the highest concentration from the linearity concentration), and middle-quality control concentration (the mean of the lower and higher quality control concentrations). Sextuplicate injections of all quality control solutions were analyzed twice on the same day at two different times (9:00 and 21:00) to assess intra-day precision. Additionally, duplicate injections of all quality control concentrations were analyzed for inter-day precision for three consecutive days. The peak area for each concentration and the percentage relative standard deviation (%RSD) were calculated to evaluate the method's inter- and intra-day precision.
Robustness
Robustness is a critical factor in assessing the stability and reliability of an analytical method. It ensures that minor variations in operating conditions do not significantly affect the method's performance. In this study, the robustness of the developed analytical method was evaluated by injecting a Posaconazole concentration of 2 μg/mL three times while varying certain conditions such as the acetonitrile ratio in the mobile phase (%), column oven temperature (°C), wavelength (nm), flow rate (mL/min), injection volume (μL), and pH of the aqueous phase. The responses were monitored for any changes, and each condition's peak area and retention time were analyzed to calculate the % RSD.
System suitability
System suitability is an essential aspect of the analytical method validation that assesses the performance of the entire analytical system to ensure that it is suitable for the intended analysis. This includes the evaluation of various parameters, such as peak area and retention time (Rt). To determine system suitability for the developed analytical method, a Posaconazole concentration of 1μg/mL was injected in sextuplicate, and the peak area and Rt for each injection were analyzed. The %RSD of these parameters was calculated to assess the overall performance of the analytical system. This helps ensure that the analytical system performs consistently and reliably, which is crucial for obtaining accurate and precise analytical results.
Application of the developed analytical method for quantification of posaconazole in the marketed product
The developed HPLC method is considered a valuable tool only after its practical application to quantify Posaconazole in dosage forms. Posaconazole was analyzed in the marketed tablet formulation (Picasa GR 100 mg Tablet, Manufactured by: MSN Laboratories, India, and Marketed by: Intas Pharmaceuticals Limited, India, Expiry Month and Year is April 2024) to test the method's practical feasibility. The marketed formulation's standard stock solution was prepared by accurately weighing 12.6 mg (equivalent to 10 mg of Posaconazole), transferring it to a 10 mL volumetric flask, and filling the volume with the mobile phase. Further dilutions were done with the mobile phase to obtain the desired concentration (1 μg/mL). The validated method was used to analyze the desired concentration, and the Rt and peak area were measured. The amount of Posaconazole in the desired concentration was calculated by comparing the peak areas.33,126–134
Results
Selection of wavelength
The maximum absorbance was observed at 262.20 nm for all Posaconazole concentrations, leading to its selection as the optimal wavelength for subsequent HPLC analysis. This was evident with the Figure 1 which represents the UV spectrum of Posaconazole to determine its Absorption Maxima in different concentrations.
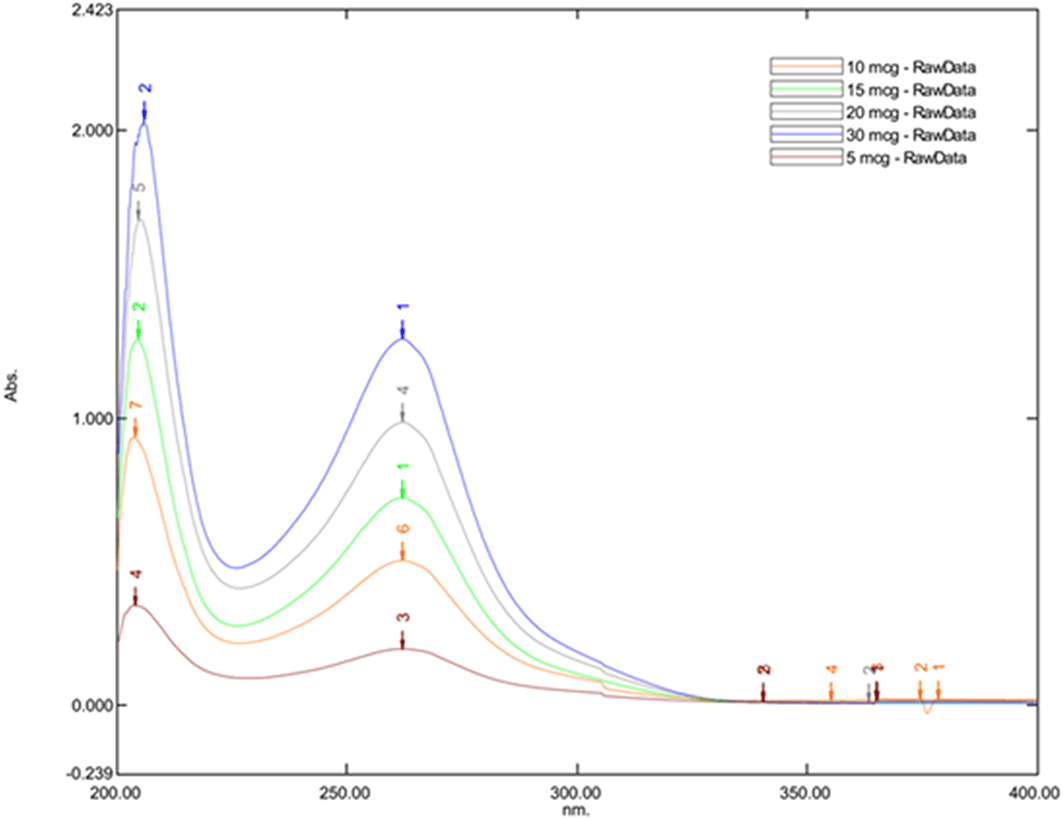
Figure 1. UV spectrum of Posaconazole to determine its absorption maxima in different concentrations.
Initial trials
Thirteen different chromatograms were obtained by altering the ratios of the organic and mobile phases, as well as the flow rate, during the experimental trials. These chromatograms are depicted below.
Trial No. 11 was selected as the optimized method due to its superior performance based on the obtained chromatograms from 13 different trials. The chosen conditions included a specific ratio of the organic phase and mobile phase, as well as a specific flow rate. The optimized method demonstrated excellent peak shape, a high peak area, and a suitable 5-to-8-minutes retention time. This ideal retention time range was considered to be optimal for bioanalytical method development as it allows for the elution of the drug without interference from plasma interferences that typically arise within the first 5 minutes while avoiding excessive consumption of mobile phase beyond 8 minutes, which can increase the cost of the method. Figure 2 shows the different chromatograms of initial trials of method development.
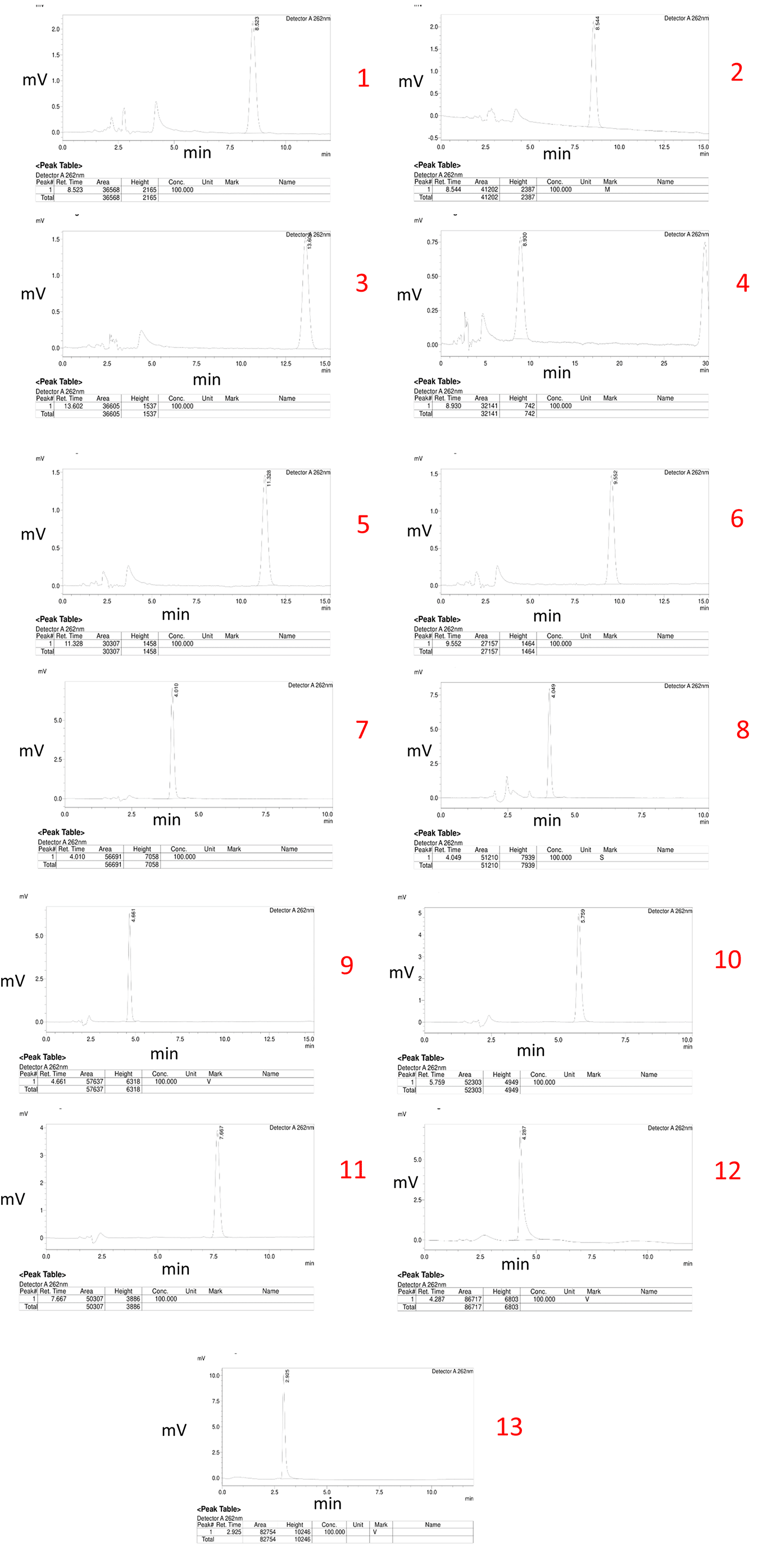
Figure 2. Chromatograms of initial trials of method development.
Optimized Method (Trial 11) Conditions
Stationary Phase: Phenomenex Hyperclone C18 Column
Mobile Phase: Acetonitrile: 10mM Phosphate Buffer pH 6.8
Mobile Phase Ratio: 55:45
Flow Rate: 1 ml/min
Mode: Isocratic elution
Column Temperature: 25oC
Detection Wavelength: 262 nm
Total run time of the instrument: 10 minutes
Validation of the optimized HPLC method
The optimized HPLC method was validated according to the ICH Q2 (R1) guidelines, which involved a range of experiments to evaluate the method's performance under different conditions. Figure 3 shows the optimized chromatogram of the Posaconazole.
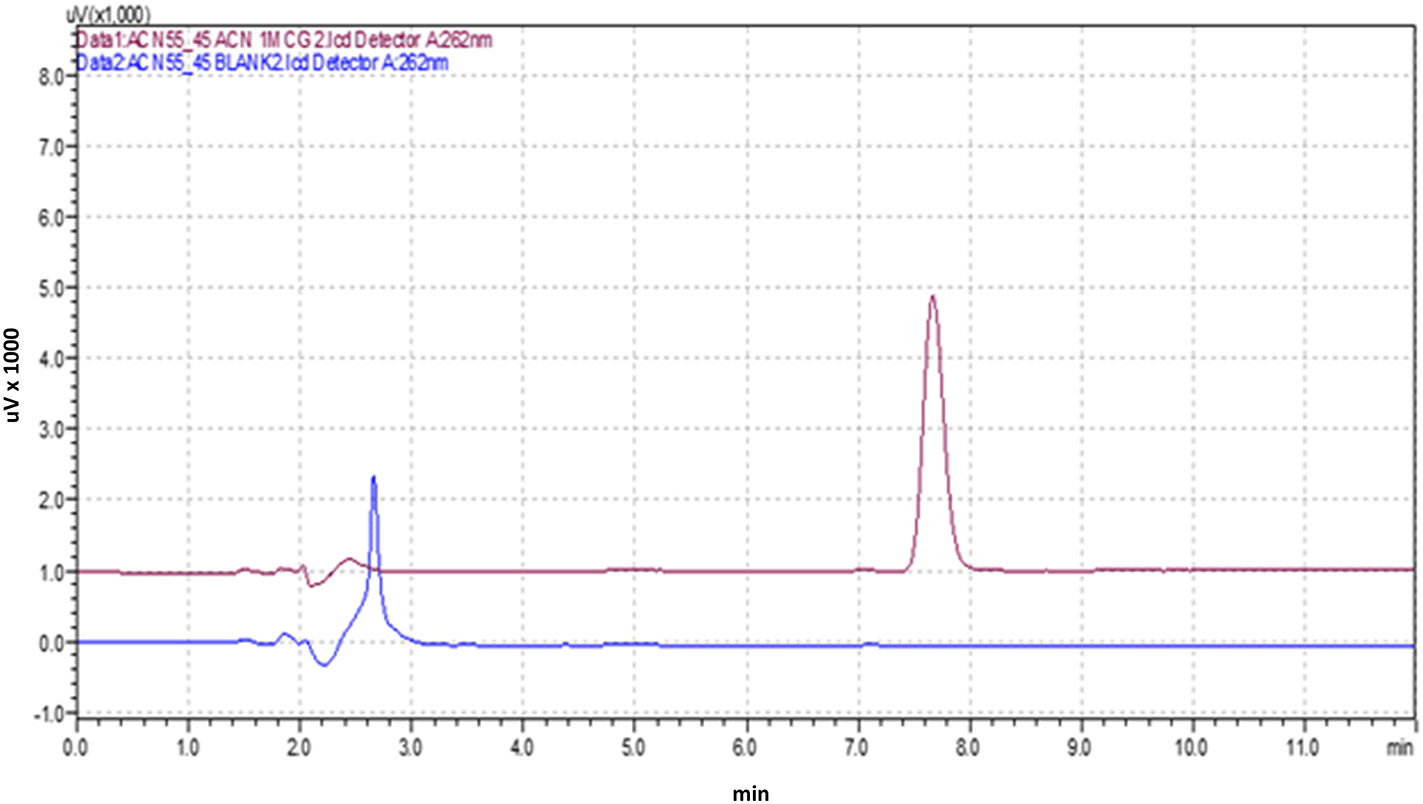
Figure 3. Chromatogram of the Posaconazole with Blank.
Specificity
The developed HPLC method exhibited high specificity in determining Posaconazole, as indicated by the absence of interferences at the retention time of 7.634 minutes for pure Posaconazole and 7.691 minutes for the Posaconazole present in the marketed formulation. This suggests that the method is capable of accurately identifying Posaconazole in the presence of other components.
Linearity
Posaconazole was quantified in quintuplicate for a concentration range of 0.1 to 32 μg/mL. Individual linear plots were generated for each trial, and the mean value was computed. The plotted linear regression graph showed a linear regression equation of y = 58203x-3671 for the mean value, with a coefficient of determination (R2) of 1. Notably, none of the consecutive R2 values from the five linear regression plots was less than 0.999. This indicated that the optimized method was highly linear within the Posaconazole concentration range, and further experimentation was warranted. Figure 4 represents the linearity of the method via a linear graph.
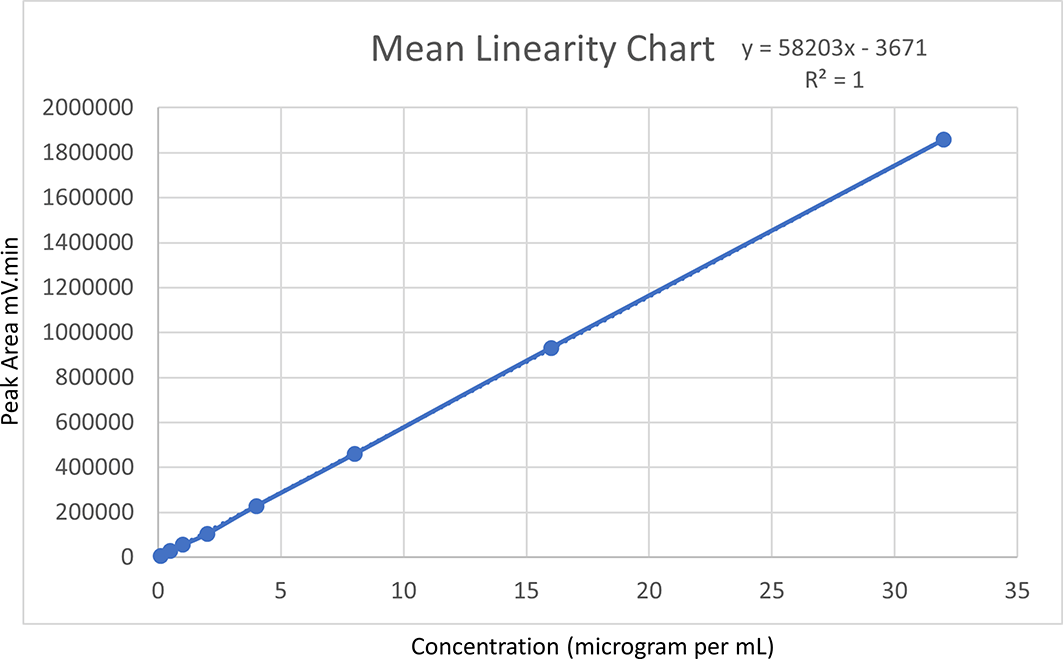
Figure 4. Mean linearity chart.
Accuracy
Intraday and interday analyses were conducted to assess the accuracy of the optimized HPLC method for quantifying Posaconazole. In the intraday analysis, sextuplicate injections were made at three different Posaconazole concentrations ranging from 2 to 8 μg/mL, while in the inter-day analysis, duplicate injections were made at the same three concentrations. The mean percentage recovery for each concentration was calculated and found to be 91.86%, 89.22%, and 87.46% for 2 μg/mL, 4 μg/mL, and 8 μg/mL, respectively, in the intraday analysis. Similarly, the mean percentage recovery for the inter-day analysis was 94.71%, 89.15%, and 88.09% for the same concentrations. The results showed that the mean recovery percentage for both intraday and inter-day analyses of Posaconazole was within the range of 85%-115%, indicating the optimized method's accuracy and suitability for the precise quantification of Posaconazole. Data represented in the Table 1 evident the accuracy of the method.
Table 1. Accuracy of the method.
| Time in hours | Concentration in mcg | Retention time | Area | Linear equation | % Recovery |
|---|
| 0 | 2 | 7.632 | 102744 | Y=58203x-3671 | 91.42 |
| 0 | 4 | 7.638 | 201361 | 88.07 |
| 0 | 8 | 7.633 | 401422 | 87.00 |
| 13 | 2 | 7.641 | 103262 | 91.86 |
| 13 | 4 | 7.639 | 204050 | 89.22 |
| 13 | 8 | 7.639 | 403561 | 87.46 |
| 26 | 2 | 7.644 | 106575 | 94.71 |
| 26 | 4 | 7.642 | 203875 | 89.15 |
| 26 | 8 | 7.646 | 406513 | 88.09 |
Sensitivity
The detection limit is an important parameter in the analytical method validation process as it determines the lowest concentration of analyte that can be detected with acceptable accuracy and precision. In this study, the detection limit of Posaconazole was calculated based on the residual standard deviation of the regression line and its slope. The limit of detection and limit of quantitation were determined to be 0.24 and 0.74 μg/mL, respectively. This suggests that the developed HPLC method is highly sensitive and can detect Posaconazole even at very low concentrations.
Precision
Four quality control concentrations of Posaconazole were analyzed in duplicate during intraday and interday experiments to assess the precision of the developed analytical method. The mean peak area of the four concentrations (1.5 μg/mL, 4.5 μg/mL, 34.26 μg/mL, and 64 μg/mL) was calculated for both experiments. The results showed that the percentage relative standard deviation of the peak area for all concentrations was less than 2%, indicating the high precision of the method. This precision study confirms the suitability of the optimized method for the accurate quantification of Posaconazole. Therefore, the developed technique shows excellent potential for the precise quantification of Posaconazole, offering a reliable method for pharmaceutical research and analysis. Data represented in the Table 2 evident the precision of the method.
Table 2. Precision of the method.
| Concentration in mcg | 0 Hours | 13 hours | 26 hours | %RSD |
|---|
| Retention time | Area | Retention time | Area | Retention time | Area | Retention time | Area |
|---|
| 1.5 | 7.632 | 77441 | 7.641 | 78642 | 7.644 | 79113 | 0.08 | 1.01 |
| 4.5 | 7.638 | 194388 | 7.639 | 196151 | 7.642 | 197540 | 0.03 | 0.81 |
| 34.26 | 7.633 | 1695217 | 7.639 | 1718915 | 7.646 | 1732927 | 0.09 | 1.11 |
| 64 | 7.635 | 3213814 | 7.634 | 3253934 | 7.643 | 3233874 | 0.07 | 0.62 |
Robustness
To assess the robustness of the developed HPLC analytical method, triplicate injections of Posaconazole at a concentration of 2 μg/mL were performed while varying the operating conditions such as the pH of the buffer, the acetonitrile ratio in the mobile phase, the column oven temperature, the wavelength, the flow rate, and the injection volume. The results showed that the mean percentage relative standard deviation (%RSD) of peak area and retention time were less than 2% for all the varied conditions. This indicates that the optimized methodology has a high degree of robustness, ensuring precise and accurate quantification of Posaconazole even in the presence of small variations in the operating conditions. Data represented in the Table 3 evident the robustness of the method.
Table 3. Robustness of the method.
| Condition | Retention time | Area | Standard retention time | Standard area | %RSD of retention time | %RSD of area |
|---|
| +2 nm Wavelength | 7.641 | 102225 | 7.636 | 104890 | 0.04 | 1.82 |
| -2 nm wavelength | 7.628 | 102709 | 0.07 | 1.49 |
| +0.2 ml Flow Rate | 7.443 | 107548 | 1.81 | 1.77 |
| -0.2 ml Flow Rate | 7.851 | 102052 | 1.96 | 1.94 |
| +0.2 pH | 7.611 | 105729 | 0.23 | 0.56 |
| -0.2 pH | 7.698 | 105444 | 0.57 | 0.37 |
| +2 mcl Injection Volume | 7.637 | 107884 | 0.01 | 1.99 |
| -2 mcl Injection Volume | 7.635 | 102026 | 0.01 | 1.95 |
| +2oC Column Temperature | 7.633 | 103537 | 0.02 | 0.92 |
| -2oC Column Temperature | 7.634 | 103159 | 0.02 | 1.18 |
| +2% Mobile Phase | 7.594 | 103500 | 0.39 | 0.94 |
| -2% Mobile Phase | 7.688 | 104414 | 0.48 | 0.32 |
System suitability
The suitability of the analytical system for the intended application of Posaconazole quantification in its bulk and dosage forms was evaluated by injecting Posaconazole at a concentration of 1 μg/mL in sextuplicate and analyzing the retention time and mean peak area. The consistent results obtained from the sextuplicate trials indicated the system's suitability for precise and accurate quantification of Posaconazole in its various forms. Data represented in the Table 4 evident the system suitability of the method.
Table 4. System suitability of the method.
| Flow rate | Rt | Area | NTP | HETP | TF 5% | TF 10% | K factor |
|---|
| 1 | 7.634 | 55012 | 5910 | 25.382 | 1.132 | 1.107 | 1.62 |
| 1 | 7.637 | 55825 | 5797 | 25.874 | 1.133 | 1.105 | 1.62 |
| 1 | 7.635 | 55936 | 5848 | 25.65 | 1.135 | 1.105 | 1.62 |
| 1 | 7.635 | 55970 | 5858 | 25.605 | 1.132 | 1.103 | 1.62 |
| 1 | 7.636 | 56191 | 5766 | 26.014 | 1.135 | 1.106 | 1.62 |
| 1 | 7.637 | 55842 | 5828 | 25.736 | 1.131 | 1.102 | 1.62 |
| %RSD | 0.015861 | 0.727357 | 0.859556 | 0.856041 | 0.147689 | 0.168548 | 0.03 |
Application of the developed analytical method for quantification of Posaconazole in novel nanoformulations and marketed product
The analytical method developed for quantifying Posaconazole was successfully applied to the practical analysis of Posaconazole in a marketed tablet formulation (Picasa GR 100, INTAS, India). This practical application demonstrated the feasibility and accuracy of the developed HPLC method in quantifying Posaconazole in real-world samples, indicating its potential as a reliable and efficient tool for quality control and assessment of Posaconazole-containing pharmaceutical products. The results highlight the effectiveness of the developed method in analyzing Posaconazole in dosage forms, affirming its potential as a valuable analytical tool for pharmaceutical industries.
Quantification of Posaconazole in the marketed formulation
The validated HPLC method was employed to determine the concentration of Posaconazole in the marketed tablet formulation (Picasa GR 100, INTAS, India). A standard stock solution of 10 μg/mL of Posaconazole was prepared and introduced into the HPLC system for analysis. The retention time and mean peak area were carefully recorded and analyzed to determine the concentration of Posaconazole. The analysis results indicated that the mean peak area and retention time of POSA were consistent with the anticipated values, which is a strong indication of the reliability of the analytical method. Moreover, the recovery percentage obtained from the analysis was 99%, signifying high precision and accuracy. These observations demonstrate the effectiveness of the HPLC method in determining the concentration of Posaconazole in the marketed formulation with high levels of reliability, precision, and accuracy.
Discussion
In this study, we successfully developed and validated a reverse-phase high-performance liquid chromatography (HPLC) method to accurately quantify Posaconazole in bulk and dosage forms. Accurate measurement of Posaconazole is crucial for ensuring the quality, safety, and effectiveness of pharmaceutical formulations. Our method addresses this need, offering a reliable and efficient approach for quality control and assessment of products containing this widely used antifungal drug.
To ensure the reliability and robustness of our developed HPLC method, we applied specific acceptance criteria for the validation parameters. These acceptance criteria were as follows:
1. Specificity: The absence of interference in the retention time of posaconazole served as the acceptance criterion for specificity. This ensured that the developed method accurately identified and quantified posaconazole without any interference from other components.
2. Linearity: We assessed linearity using the coefficient of determination (R2) value, with an acceptance criterion of R2 greater than 0.999. This demonstrated a strong linear relationship between the analyte concentration and the corresponding peak area, ensuring accurate quantification across a range of concentrations.
3. Accuracy: Our acceptance criterion for accuracy was set at 85%-115%. This range ensured that the measured concentrations of posaconazole using our method fell within an acceptable percentage deviation from the true or expected values, indicating the method’s ability to provide accurate results.
4. Precision: For precision, we set the acceptance criterion as a percentage relative standard deviation (%RSD) of the peak area not exceeding 2%. This criterion evaluated the method’s repeatability and reproducibility by assessing the consistency of results obtained from multiple injections of the same sample.
5. Robustness: In terms of robustness, we used %RSD of area and retention time as acceptance criteria, with a maximum limit of 2%. This criterion evaluated the method’s ability to withstand deliberate variations in experimental conditions, while maintaining consistent and reliable results.
Our method exhibited excellent linearity within a concentration range of 2-20 μg/mL, meeting the acceptance criterion for linearity (R2 > 0.999). The accuracy of our method fell within the predefined acceptance range of 85%-115%, demonstrating its ability to provide accurate quantification. The precision results, with a %RSD of the peak area below 2%, confirmed the method’s repeatability and reproducibility. Additionally, the method showed robustness by maintaining consistent results within the accepted %RSD limits for area and retention time.
One of the key advantages of our HPLC method lies in its simplicity and efficiency. With a straightforward sample preparation procedure and a relatively short analysis time, our method is practical for high-throughput analysis in diverse laboratory settings. Utilizing a commercially available HPLC column and a well-optimized mobile phase composition ensures optimal elution of Posaconazole.
Moreover, our method offers high sensitivity and accuracy. It demonstrates excellent linearity across a wide concentration range, facilitating precise quantification of Posaconazole in different sample matrices. The method’s accuracy is supported by the close agreement between the measured concentrations and known reference standards.
While our method shows robustness and reproducibility, we acknowledge certain limitations. Specifically, we did not investigate the potential impurities or contaminants that could interfere with the analysis. Exploring impurity profiling and assessing potential interferences would provide a more comprehensive understanding and enhance the method’s applicability.
Future studies could focus on employing different extraction and purification techniques. These efforts would further enhance the reliability of our method and broaden its potential applications.
The repeated analyses we conducted yielded highly consistent results, underscoring the reproducibility of our method. With its precision and accuracy confirmed through comparison with known reference standards, our method proves reliable for accurately quantifying Posaconazole. The comprehensive validation and repetition steps we undertook instill confidence in the method’s robustness and reliability across different experimental settings.
While our method demonstrates robustness and reproducibility within the tested conditions, it is important to evaluate its performance when applied to different instruments or laboratories. Method transferability and inter-laboratory comparisons should be considered to ensure broader application and standardization.
By providing a reliable and efficient means of analyzing Posaconazole, our developed method significantly contributes to the quality assurance and assessment of pharmaceutical products containing this drug. This is particularly valuable for ensuring the therapeutic effectiveness and safety of these formulations, ultimately benefiting patient care and treatment outcomes.
In summary, our study successfully developed and validated an HPLC method for accurate Posaconazole quantification. The method’s reliability, simplicity, and high sensitivity make it suitable for widespread use in various pharmaceutical formulations and matrices. By adhering to specific acceptance criteria, we have established a robust and reliable method that meets stringent standards for specificity, linearity, accuracy, precision, and robustness.
Conclusion
In conclusion, this study successfully developed and validated a reverse-phase HPLC analytical method for quantifying Posaconazole in bulk and dosage forms. The method showed excellent linearity, precision, accuracy, and specificity. The study adhered to the CONSORT guidelines, ensuring the validity and reproducibility of the results. This analytical method can be used for quality control and assessment of Posaconazole-containing pharmaceutical products, thereby contributing to the development of safe and effective drugs for patients.
Acknowledgements
We thank all the colleagues of Department of Pharmaceutics, MCOPS, MAHE for their support.
Bibliography
- 1.
Abou El-Alamin MM, Sultan MA, Atia MA, et al.:
Novel Application of Pentabromobenzyl Column for Simultaneous Determination of Eight Antifungal Drugs Using High-performance Liquid Chromatography.
Comb. Chem. High Throughput Screen.
2020; 23(10): 991–1001. PubMed Abstract
| Publisher Full Text
- 2.
Alffenaar JWC, Wessels AMA, van Hateren K , et al.:
Method for therapeutic drug monitoring of azole antifungal drugs in human serum using LC/MS/MS.
J. Chromatogr. B Analyt. Technol. Biomed. Life Sci.
2009; 878(1): 39–44. PubMed Abstract
| Publisher Full Text
- 3.
Gomez-Lopez A, Alcazar-Fuoli L, Bernal-Martínez L:
Simultaneous quantification of systemic azoles and their major metabolites in human serum by HPLC/PDA: role of azole metabolic rate.
Diagn. Microbiol. Infect. Dis.
2018; 92(1): 78–83. PubMed Abstract
| Publisher Full Text
- 4.
Allegra S, Fatiguso G, De Francia S, et al.:
Evaluation of posaconazole pharmacokinetics in adult patients with invasive fungal infection.
Biomedicines.
2017; 5(4). PubMed Abstract
| Publisher Full Text
| Free Full Text
- 5.
de Araújo JIR , de Moura FL , Soares MFLR, et al.:
Stability study and oxidative degradation kinetics of posaconazole.
Microchem. J.
2019; 151: 104181. Publisher Full Text
- 6.
Assress HA, Nyoni H, Mamba BB, et al.:
Target quantification of azole antifungals and retrospective screening of other emerging pollutants in wastewater effluent using UHPLC –QTOF-MS.
Environ. Pollut.
2019; 253: 655–666. PubMed Abstract
| Publisher Full Text
- 7.
Baietto L, D’Avolio A, Ventimiglia G, et al.:
Development, validation, and routine application of a high-performance liquid chromatography method coupled with a single mass detector for quantification of itraconazole, voriconazole, and posaconazole in human plasma.
Antimicrob. Agents Chemother.
2010; 54(8): 3408–3413. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 8.
Baietto L, D’avolio A, Marra C, et al.:
Development and validation of a new method to simultaneously quantify triazoles in plasma spotted on dry sample spot devices and analysed by HPLC-MS.
J. Antimicrob. Chemother.
2012; 67(11): 2645–2649. PubMed Abstract
| Publisher Full Text
- 9.
Basu SS, Petrides A, Mason DS, et al.:
A rapid UPLC-MS/MS assay for the simultaneous measurement of fluconazole, voriconazole, posaconazole, itraconazole, and hydroxyitraconazole concentrations in serum.
Clin. Chem. Lab. Med.
2017; 55(6): 836–844. PubMed Abstract
| Publisher Full Text
- 10.
Beste KY, Burkhardt O, Kaever V:
Rapid HPLC-MS/MS method for simultaneous quantitation of four routinely administered triazole antifungals in human plasma.
Clin. Chim. Acta.
2012; 413(1-2): 240–245. PubMed Abstract
| Publisher Full Text
- 11.
Garcia CV, Bitencourt AS, Oliveira SS, et al.:
Analytical Study of the Antifungal Posaconazole in Raw Material: Quantitative Bioassay, Decomposition Chemical Kinetics, and Degradation Impurities by LC-QTOF-MS.
J. AOAC Int.
2021; 104(4): 1055–1064. PubMed Abstract
| Publisher Full Text
- 12.
Garcia CV, Bitencourt AS, Oliveira SS, et al.:
Analytical Study of the Antifungal Posaconazole in Raw Material: Quantitative Bioassay, Decomposition Chemical Kinetics, and Degradation Impurities by LC-QTOF-MS.
J. AOAC Int.
2021; 104(4): 1055–1064. PubMed Abstract
| Publisher Full Text
- 13.
Virginia Garcia C, da Bitencourt AS , Oliveira SS, et al.:
UV Spectrophotometric method for determination of posaconazole: comparison to HPLC.
Journal of Basic and Applied Pharmaceutical Sciences Rev Ciênc Farm Básica Apl.
2015; 36(4): 491–495.
- 14.
Bressán IG, Mendez ML, Gimenez MI:
Validation of a Reversed-Phase Ultra-High-Performance Liquid Chromatographic Method with Photodiode Array Detection for the Determination of Voriconazole in Human Serum and Its Application to Therapeutic Drug Monitoring.
Ther. Drug Monit.
2018; 40(2): 276–283. PubMed Abstract
| Publisher Full Text
- 15.
Buckner SL, Ceesay MM, Pagliuca A, et al.:
Measurement of Posaconazole, Itraconazole, and Hydroxyitraconazole in Plasma/Serum by High-Performance Liquid Chromatography With Fluorescence Detection.
- 16.
Couchman L, Buckner SL, Morgan PE, et al.:
An automated method for the simultaneous measurement of azole antifungal drugs in human plasma or serum using turbulent flow liquid chromatography-tandem mass spectrometry.
Anal. Bioanal. Chem.
2012; 404: 513–523. PubMed Abstract
| Publisher Full Text
- 17.
Cáceres DH, Zapata JD, Granada SD, et al.:
Estandarización y validación en Colombia de una metodología basada en HPLC para la determinación de la concentración sérica de posaconazol.
Rev. Iberoam. Micol.
2016; 33(4): 230–236. PubMed Abstract
| Publisher Full Text
- 18.
Campoli P, Al Abdallah Q, Robitaille R, et al.:
Concentration of antifungal agents within host cell membranes: A new paradigm governing the efficacy of prophylaxis.
Antimicrob. Agents Chemother.
2011; 55(12): 5732–5739. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 19.
Chae H, Cho SY, Yu H, et al.:
Determination of posaconazole concentration with LC-MS/MS in adult patients with hematologic malignancy.
Clin. Chim. Acta.
2015; 450: 220–226. PubMed Abstract
| Publisher Full Text
- 20.
Chahbouni A, Wilhelm AJ, Den Burger JCG, et al.:
Validated Liquid Chromatography-Tandem Mass Spectroscopy Method for the Simultaneous Quantification of Four Antimycotic Agents in Human Serum.
- 21.
Chhun S, Rey E, Tran A, et al.:
Simultaneous quantification of voriconazole and posaconazole in human plasma by high-performance liquid chromatography with ultra-violet detection.
J. Chromatogr. B Analyt. Technol. Biomed. Life Sci.
2007; 852(1-2): 223–228. PubMed Abstract
| Publisher Full Text
- 22.
Crombag MRBS, Huisman C, Kemper EM, et al.:
Posaconazole Treatment in Hematology Patients: A Pilot Study of Therapeutic Drug Monitoring.
- 23.
Cunliffe JM, Noren CF, Hayes RN, et al.:
A high-throughput LC-MS/MS method for the quantitation of posaconazole in human plasma: Implementing fused core silica liquid chromatography.
J. Pharm. Biomed. Anal.
2009; 50(1): 46–52. PubMed Abstract
| Publisher Full Text
- 24.
Decosterd LA, Rochat B, Pesse B, et al.:
Multiplex ultra-performance liquid chromatography-tandem mass spectrometry method for simultaneous quantification in human plasma of fluconazole, itraconazole, hydroxyitraconazole, posaconazole, voriconazole, voriconazole-N-oxide, anidulafungin, and caspofungin.
Antimicrob. Agents Chemother.
2010; 54(12): 5303–5315. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 25.
Docci L, Klammers F, Ekiciler A, et al.:
In Vitro to in vivo Extrapolation of Metabolic Clearance for UGT Substrates Using Short-Term Suspension and Long-Term Co-cultured Human Hepatocytes.
AAPS Journal.
2020; 22(6): 131. PubMed Abstract
| Publisher Full Text
- 26.
Prasad VD, Reddy VR, Aparna P:
Validated Gradient Stability Indicating UPLC Method for the Determination of Related Substances of Posaconazole in Bulk Drug.
Am. J. Anal. Chem.
2015; 06(12): 965–976. Publisher Full Text
- 27.
Durgun ME, Kahraman E, Güngör S, et al.:
Optimization and Characterization of Aqueous Micellar Formulations for Ocular Delivery of an Antifungal Drug, Posaconazole.
Curr. Pharm. Des.
2020; 26(14): 1543–1555. PubMed Abstract
| Publisher Full Text
- 28.
Elkhabaz A, Sarkar S, Simpson GJ, et al.:
Characterization of Phase Transformations for Amorphous Solid Dispersions of a Weakly Basic Drug upon Dissolution in Biorelevant Media.
Pharm. Res.
2019; 36(12): 174. PubMed Abstract
| Publisher Full Text
- 29.
Elkhabaz A, Moseson DE, Brouwers J, et al.:
Interplay of Supersaturation and Solubilization: Lack of Correlation between Concentration-Based Supersaturation Measurements and Membrane Transport Rates in Simulated and Aspirated Human Fluids.
Mol. Pharm.
2019; 16: 5042–5053. PubMed Abstract
| Publisher Full Text
- 30.
Elkhabaz A, Moseson DE, Sarkar S, et al.:
Crystallization Kinetics in Fasted-State Simulated and Aspirated Human Intestinal Fluids.
Cryst. Growth Des.
2021; 21(5): 2807–2820. Publisher Full Text
- 31.
Farowski F, Cornely OA, Vehreschild JJ, et al.:
Quantitation of azoles and echinocandins in compartments of peripheral blood by liquid chromatography-tandem mass spectrometry.
Antimicrob. Agents Chemother.
2010; 54(5): 1815–1819. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 32.
Fatiguso G, Favata F, Zedda I, et al.:
A simple high performance liquid chromatography–mass spectrometry method for Therapeutic Drug Monitoring of isavuconazole and four other antifungal drugs in human plasma samples.
J. Pharm. Biomed. Anal.
2017; 145: 718–724. PubMed Abstract
| Publisher Full Text
- 33.
Xu F, Xu Y, Liu G, et al.:
Separation of twelve posaconazole related stereoisomers by multiple heart-cutting chiral–chiral two-dimensional liquid chromatography.
J. Chromatogr. A.
2020; 1618: 460845. PubMed Abstract
| Publisher Full Text
- 34.
Feng W, Liu H, Chen G, et al.:
Structural Characterization of the Oxidative Degradation Products of an Antifungal Agent SCH 56592 by LC-NMR and LC-MS.2001; 25.
Reference Source
- 35.
Franceschi L, D’Aronco S, Furlanut M:
Development and validation of a liquid chromatography-tandem mass spectrometry method for the determination of voriconazole and posaconazole in serum samples from patients with invasive mycoses.
J. Bioanal. Biomed.
2011; 03(5): 92–97. Publisher Full Text
- 36.
Fule R, Amin P:
Hot Melt Extruded Amorphous Solid Dispersion of Posaconazole with Improved Bioavailability: Investigating Drug-Polymer Miscibility with Advanced Characterisation.
Biomed. Res. Int.
2014; 2014: 1–16. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 37.
Garcia CV, Costa GR, Mendez ASL:
Stability-indicating HPLC method for posaconazole bulk assay.
Sci. Pharm.
2012; 80(2): 317–327. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 38.
Garg EU:
Clinical Applications of Mass Spectrometry in Drug Analysis Methods and Protocols Methods in Molecular Biology 1383.Reference Source
- 39.
Gharibi S, Kimble B, Vogelnest L, et al.:
Pharmacokinetics of posaconazole in koalas (Phascolarctos cinereus) after intravenous and oral administration.
J. Vet. Pharmacol. Ther.
2017; 40(6): 675–681. PubMed Abstract
| Publisher Full Text
- 40.
Ghosal A, Hapangama N, Yuan Y, et al.:
IDENTIFICATION OF HUMAN UDP-GLUCURONOSYLTRANSFERASE ENZYME(S) RESPONSIBLE FOR THE GLUCURONIDATION OF POSACONAZOLE (NOXAFIL).2004.
- 41.
Gordien JB, Pigneux A, Vigouroux S, et al.:
Simultaneous determination of five systemic azoles in plasma by high-performance liquid chromatography with ultraviolet detection.
J. Pharm. Biomed. Anal.
2009; 50(5): 932–938. PubMed Abstract
| Publisher Full Text
- 42.
Greenblatt DJ, Harmatz JS, Ryan MJ, et al.:
Sustained Impairment of Lurasidone Clearance after Discontinuation of Posaconazole: Impact of Obesity, and Implications for Patient Safety.
J. Clin. Psychopharmacol.
2018; 38(4): 289–295. PubMed Abstract
| Publisher Full Text
- 43.
Hamdy DA, Belal TS:
A comparative study of newly developed HPLC-DAD and UHPLC-UV assays for the determination of posaconazole in bulk powder and suspension dosage form.
J. Anal. Methods Chem.
2014; 2014: 1–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 44.
Choudhary H, Singh S, Singh R, et al.:
Rapid and Simple Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC) Method for Simultaneous Quantifications of Triazole Antifungals in Human Serum.
Mycopathologia.
2021; 186(1): 27–39. PubMed Abstract
| Publisher Full Text
- 45.
Henry H, Sobhi HR, Scheibner O, et al.:
Comparison between a high-resolution single-stage Orbitrap and a triple quadrupole mass spectrometer for quantitative analyses of drugs.
Rapid Commun. Mass Spectrom.
2012; 26(5): 499–509. PubMed Abstract
| Publisher Full Text
- 46.
Jenkins N, Black M, Schneider HG:
Simultaneous determination of voriconazole, posaconazole, itraconazole and hydroxy-itraconazole in human plasma using LCMS/MS.
Clin. Biochem.
2018; 53: 110–115. PubMed Abstract
| Publisher Full Text
- 47.
Kahle K, Langmann P, Schirmer D, et al.:
Simultaneous determination of voriconazole and posaconazole concentrations in human plasma by high-performance liquid chromatography.
Antimicrob. Agents Chemother.
2009; 53(7): 3140–3142. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 48.
Kai M, Tanaka R, Suzuki Y, et al.:
Simultaneous quantification of plasma levels of 12 antimicrobial agents including carbapenem, anti-methicillin-resistant Staphylococcus aureus agent, quinolone and azole used in intensive care unit using UHPLC-MS/MS method.
Clin. Biochem.
2021; 90: 40–49. PubMed Abstract
| Publisher Full Text
- 49.
Kathirvel S, Raju R, Seethadevi B, et al.:
Stability Indicating RP-HPLC Method for the Determination of Process Related Impurities in Posaconazole API.2014; 4.
Reference Source
- 50.
Pathak SM, Kumar AR, Musmade P, et al.:
A simple and rapid high performance liquid chromatographic method with fluorescence detection for the estimation of fexofenadine in rat plasma-Application to preclinical pharmacokinetics.
Talanta.
2008; 76(2): 338–346. PubMed Abstract
| Publisher Full Text
- 51.
Pathak SM, Kumar AR, Subramanian G, et al.:
Development and validation of a reversed-phase liquid chromatographic method with fluorescence detection for the study of Saquinavir pharmacokinetics in rat plasma.
Anal. Chim. Acta.
2007; 594(2): 248–256. PubMed Abstract
| Publisher Full Text
- 52.
Pathak SM, Musmade PB, Bhat KM, et al.:
Validated HPLC method for quantitative determination of talinolol in rat plasma and application to a preclinical pharmacokinetic study.
Bioanalysis.
2010; 2(1): 95–104. Publisher Full Text
- 53.
Attari Z, Kumar L, Mallikarjuna Rao C, et al.:
Reversed-Phase HPLC Method for Determination of Temozolomide in Rat Plasma and Brain: Simple, Sensitive and Robust Method.
Pharm. Chem. J.
2018; 52(3): 266–270. Publisher Full Text
- 54.
Kumar L, Sreenivasa Reddy M, Managuli RS, et al.:
Full factorial design for optimization, development and validation of HPLC method to determine valsartan in nanoparticles.
Saudi Pharm. J.
2015; 23(5): 549–555. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 55.
Bhaskaran NA, Kumar L, Reddy MS, et al.:
An analytical “quality by design” approach in RP-HPLC method development and validation for reliable and rapid estimation of irinotecan in an injectable formulation.
Acta Pharma.
2021; 71(1): 57–79. Publisher Full Text
- 56.
Mutalik SP, Mullick P, Pandey A, et al.:
Box–Behnken design aided optimization and validation of developed reverse phase HPLC analytical method for simultaneous quantification of dolutegravir sodium and lamivudine co-loaded in nano-liposomes.
J. Sep. Sci.
2021; 44(15): 2917–2931. Publisher Full Text
- 57.
Hegde AR, Padya BS, Soman S, et al.:
A simple, precise, and sensitive HPLC method for quantification of letrozole in rat plasma: development, validation, and preclinical pharmacokinetics.
J. Anal. Sci. Technol.
2021; 12(1). Publisher Full Text
- 58.
Padya BS, Hegde AR, Mutalik SP, et al.:
Analytical and bioanalytical HPLC method for simultaneous estimation of 5-fluorouracil and sonidegib.
Bioanalysis.
2022; 14(1): 29–45. Publisher Full Text
- 59.
Managuli RS, Gourishetti K, Shenoy RR, et al.:
Preclinical pharmacokinetics and biodistribution studies of asenapine maleate using novel and sensitive RP-HPLC method.
Bioanalysis.
2017; 9(14): 1037–1047. Publisher Full Text
- 60.
Sree N, Dengale SJ, Mutalik S, et al.:
Dronedarone HCl-Quercetin Co-amorphous System: Characterization and RP-HPLC Method Development for Simultaneous Estimation.2020. Publisher Full Text
- 61.
Mullick P, Mutalik SP, Hegde AR, et al.:
Simultaneous Estimation of Apremilast and Betamethasone Dipropionate in Microsponge-Based Topical Formulation using a Stability Indicating RP-HPLC Method: A Quality-by-Design Approach.
J. Chromatogr. Sci.
2021; 59(10): 928–940. Publisher Full Text
- 62.
Managuli RS, Kumar L, Chonkar AD, et al.:
Development and Validation of a Stability-Indicating RP-HPLC Method by a Statistical Optimization Process for the Quantification of Asenapine Maleate in Lipidic Nanoformulations.
J. Chromatogr. Sci.
2016; 54(8): 1290–1300. PubMed Abstract
| Publisher Full Text
- 63.
Marghade S, Musmade PB, Moorkoth S:
High-performance liquid chromatographic assay for ziprasidone in plasma samples: Application to pharmacokinetic studies in rats.
J. Chromatogr. Sci.
2012; 50(10): 902–908. PubMed Abstract
| Publisher Full Text
- 64.
Kim H, Kumari P, Lin CC, et al.:
Simultaneous High-Performance Liquid Chromatographic Determination of SCH 59884 (Phosphate Ester Prodrug of SCH 56592), SCH 207962 and SCH 56592 in Dog Plasma.2002; 27.
Reference Source
- 65.
Kim H, Likhari P, Kumari P, et al.:
High-Performance Liquid Chromatographic Analysis of the Anti-Fungal Agent SCH 56592 in Dog Serum.2000; 738.
Reference Source
- 66.
Kim H, Lin CC, Laughlin M, et al.:
Chiral High-Performance Liquid Chromatographic Analysis of Antifungal SCH 56592 and Evaluation of Its Chiral Inversion in Animals and Humans.2000; 12.
- 67.
Kim H, Kumari P, Laughlin M, et al.:
U Se of High-Performance Liquid Chromatographic and Microbiological Analyses for Evaluating the Presence or Absence of Active Metabolites of the Antifungal Posaconazole in Human Plasma.2003; 987.
Reference Source
- 68.
Kourentas A, Vertzoni M, Symillides M, et al.:
In vitro evaluation of the impact of gastrointestinal transfer on luminal performance of commercially available products of posaconazole and itraconazole using BioGIT.
Int. J. Pharm.
2016; 515(1-2): 352–358. PubMed Abstract
| Publisher Full Text
- 69.
Kourentas A, Vertzoni M, Barmpatsalou V, et al.:
The BioGIT System: a Valuable in vitro Tool to Assess the Impact of Dose and Formulation on Early Exposure to Low Solubility Drugs After Oral Administration.
AAPS Journal.
2018; 20(4): 71. PubMed Abstract
| Publisher Full Text
- 70.
Krieter P, Flannery B, Musick T, et al.:
Disposition of posaconazole following single-dose oral administration in healthy subjects.
Antimicrob. Agents Chemother.
2004; 48(9): 3543–3551. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 71.
Krüger R, Vogeser M, Burghardt S, et al.:
Impact of glucuronide interferences on therapeutic drug monitoring of posaconazole by tandem mass spectrometry.
Clin. Chem. Lab. Med.
2010; 48(12): 1723–1731. PubMed Abstract
| Publisher Full Text
- 72.
Lignell A, Löwdin E, Cars O, et al.:
Posaconazole in human serum: A greater pharmacodynamic effect than predicted by the non-protein-bound serum concentration.
Antimicrob. Agents Chemother.
2011; 55(7): 3099–3104. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 73.
Locatelli M, Kabir A, Innosa D, et al.:
A fabric phase sorptive extraction-High performance liquid chromatography-Photo diode array detection method for the determination of twelve azole antimicrobial drug residues in human plasma and urine.
J. Chromatogr. B Analyt. Technol. Biomed. Life Sci.
2017; 1040: 192–198. PubMed Abstract
| Publisher Full Text
- 74.
annamalai.rama, annamalai.rama:
Selection of Stationary Phase of HPLC for Posaconazole Estimation.
protocols.io.
March 2023.
- 75.
annamalai.rama, annamalai.rama:
Selection of buffer for the HPLC estimation of Posaconazole.
protocols.io.
March 2023.
- 76.
Mahaparale SP, Yadav AM, Mahaparale S:
BIOANALYTICAL METHOD DEVELOPMENT AND VALIDATION OF POSACANAZOLE BY RP-HPLC METHOD.
- 77.
McShane AJ, Wang S:
Development and validation of a liquid chromatography-tandem mass spectrometry assay for the simultaneous quantitation of 5 azole antifungals and 1 active metabolite.
Clin. Chim. Acta.
2017; 474: 8–13. PubMed Abstract
| Publisher Full Text
- 78.
Mistretta V, Dubois N, Denooz R, et al.:
Simultaneous determination of seven azole antifungal drugs in serum by ultra-high pressure liquid chromatography and diode array detection.
Acta Clin. Belg.
2014; 69(1): 53–61. Publisher Full Text
- 79.
Molinelli AR, Rose CH:
Quantification of the triazole antifungal compounds voriconazole and posaconazole in human serum or plasma using liquid chromatography electrospray tandem mass spectrometry (HPLC-ESI-MS/MS).
Methods in Molecular Biology.
Vol. 1383. .
Humana Press Inc.;
2016; pp. 39–47. Publisher Full Text
- 80.
Moseson DE, Jordan MA, Shah DD, et al.:
Application and limitations of thermogravimetric analysis to delineate the hot melt extrusion chemical stability processing window.
Int. J. Pharm.
2020; 590: 119916. Publisher Full Text
- 81.
Mueller SC, Karasch I, Lakner J, et al.:
Validation of an Isavuconazole High-Performance Liquid Chromatography Assay in Plasma for Routine Therapeutic Drug Monitoring Applications.2018.
Reference Source
- 82.
Müller C, Gehlen D, Blaich C, et al.:
Reliable and Easy-To-Use Liquid Chromatography-Tandem Mass Spectrometry Method for Simultaneous Analysis of Fluconazole, Isavuconazole, Itraconazole, Hydroxy-Itraconazole, Posaconazole, and Voriconazole in Human Plasma and Serum.
Ther. Drug Monit.
2017; 39(5): 505–513. PubMed Abstract
| Publisher Full Text
- 83.
Störzinger D, Swoboda S, Lichtenstern C, et al.:
Development and validation of a high-performance liquid chromatography assay for posaconazole utilizing solid-phase extraction.
Clin. Chem. Lab. Med.
2008; 46(12): 1747–1751. PubMed Abstract
| Publisher Full Text
- 84.
Nasser ST, Abdulrassol AA:
Formulation and characterization of anti-fungal (Posaconazole) o/w nanoemulsion.
Int. J. Drug Deliv..
2021; 11(2): 322–328. Publisher Full Text
- 85.
Neubauer W, König A, Bolek R, et al.:
Determination of the antifungal agent posaconazole in human serum by HPLC with parallel column-switching technique.
J. Chromatogr. B Analyt. Technol. Biomed. Life Sci.
2009; 877(24): 2493–2498. PubMed Abstract
| Publisher Full Text
- 86.
Nomeir AA, Kumari P, Hilbert MJ, et al.:
Pharmacokinetics of SCH 56592, a New Azole Broad-Spectrum Antifungal Agent, in Mice, Rats, Rabbits, Dogs, and Cynomolgus Monkeys.2000; 44.
Reference Source
- 87.
Patel A, Patel K, Patel K, et al.:
Therapeutic drug monitoring of posaconazole delayed release tablet while managing COVID-19–associated mucormycosis in a real-life setting.
Mycoses.
2022; 65(3): 312–316. PubMed Abstract
| Publisher Full Text
- 88.
Patel K, Verriboina SK, Vasantharaju SG:
Stability indicating assay method development and validation of simultaneous estimation of chlorzoxazone, diclofenac sodium and paracetamol in bulk drug and tablet by RP-HPLC.
Res. J. Pharm. Technol.
2021; 14(9): 5024–5028. Publisher Full Text
- 89.
Krishna V, Reddy MS:
Isocratic High Performance Liquid Chromatographic (HPLC) Determination of Rifampicin in Presence of Isoniazid.
Res. J. Pharm. Tech.
2014; 7(3).
Reference Source
- 90.
Rochat B, Pascual A, Pesse B, et al.:
Ultra-performance liquid chromatography mass spectrometry and sensitive bioassay methods for quantification of posaconazole plasma concentrations after oral dosing.
Antimicrob. Agents Chemother.
2010; 54(12): 5074–5081. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 91.
Ruan LH, Fan LL, Wang K, et al.:
The Effect of Posaconazole and Isavuconazole on the Pharmacokinetics of Erdafitinib in Beagle Dogs by UPLC-MS/MS.
Front. Pharmacol.
2021; 12: 12. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 92.
Santana ACSGV, Danda LJDA, Nunes LCC, et al.:
Simultaneous Quantification of Benznidazole and Posaconazole by HPLC-DAD Using QbD Approach.
J. Chromatogr. Sci.
2019; 57(2): 156–162. PubMed Abstract
| Publisher Full Text
- 93.
Santana ACSGV, Nadvorny D, da Rocha Passos TD , et al.:
Influence of cyclodextrin on posaconazole stability, release and activity: Improve the utility of the drug.
J. Drug Deliv. Sci. Technol.
2019; 53: 101153. Publisher Full Text
- 94.
Schuster C, Paal M, Lindner J, et al.:
Isotope dilution LC-orbitrap-HRMS with automated sample preparation for the simultaneous quantification of 11 antimycotics in human serum.
J. Pharm. Biomed. Anal.
2019; 166: 398–405. PubMed Abstract
| Publisher Full Text
- 95.
Shen JX, Krishna G, Hayes RN:
A sensitive liquid chromatography and mass spectrometry method for the determination of posaconazole in human plasma.
J. Pharm. Biomed. Anal.
2007; 43(1): 228–236. PubMed Abstract
| Publisher Full Text
- 96.
Shen JX, Tama CI, Hayes RN:
Evaluation of automated micro solid phase extraction tips (μ-SPE) for the validation of a LC-MS/MS bioanalytical method.
J. Chromatogr. B Analyt. Technol. Biomed. Life Sci.
2006; 843(2): 275–282. Publisher Full Text
- 97.
Skaggs CL, Ren GJ, Elgierari ETM, et al.:
Simultaneous quantitation of five triazole anti-fungal agents by paper spray-mass spectrometry.
Clin. Chem. Lab. Med.
2020; 58(5): 836–846. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 98.
Škríba A, Patil RH, Hubáček P, et al.:
Rhizoferrin glycosylation in rhizopus microsporus.
J. Fungi.
2020; 6(2): 1–10. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 99.
Smith A, Dowis J, French D:
Quantification of Serum Voriconazole, Isavuconazole, and Posaconazole by Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS).
Curr. Protoc. Toxicol.
2018; 76(1): e47. PubMed Abstract
| Publisher Full Text
- 100.
Tang PH:
Determination of posaconazole in plasma/serum by high-performance liquid chromatography with fluorescence detection.
Separations.
2017; 4(2). Publisher Full Text
- 101.
Tang S, Chen J, Cannon J, et al.:
Dendrimer-based posaconazole nanoplatform for antifungal therapy Dendrimer-based posaconazole nanoplatform for antifungal therapy.
Drug Deliv.
2021; 28(1): 2150–2159. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 102.
Tang S, Chen J, Cannon J, et al.:
Dendrimer-based posaconazole nanoplatform for antifungal therapy.
Drug Deliv.
2021; 28(1): 2150–2159. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 103.
Toussaint B, Lanternier F, Woloch C, et al.:
An ultra performance liquid chromatography-tandem mass spectrometry method for the therapeutic drug monitoring of isavuconazole and seven other antifungal compounds in plasma samples.
J. Chromatogr. B Analyt. Technol. Biomed. Life Sci.
2017; 1046: 26–33. PubMed Abstract
| Publisher Full Text
- 104.
Verdier MC, Bentué-Ferrer D, Tribut O, et al.:
Liquid chromatography-tandem mass spectrometry method for simultaneous quantification of four triazole antifungal agents in human plasma.
Clin. Chem. Lab. Med.
2010; 48(10): 1515–1522. PubMed Abstract
| Publisher Full Text
- 105.
Vogeser M, Rieger C, Ostermann H, et al.:
A routine method for the quantification of the novel antimycotic drug posaconazole in plasma using liquid chromatography-tandem mass spectrometry.
Clin. Chem. Lab. Med.
2009; 47(5): 579–584. PubMed Abstract
| Publisher Full Text
- 106.
Wadsworth JM, Milan AM, Anson J, et al.:
Development of a liquid chromatography tandem mass spectrometry method for the simultaneous measurement of voriconazole, posaconazole and itraconazole.
Ann. Clin. Biochem.
2017; 54(6): 686–695. PubMed Abstract
| Publisher Full Text
- 107.
Dabir J, Mathew EM, Moorkoth S:
Analytical method development and validation of RP-HPLC method for simultaneous estimation of N-acetyl cysteine and cefexime from its fixed dose combination.
Res. J. Pharm. Technol.
2016; 9(7): 835–842. Publisher Full Text
- 108.
Mathew EM, Moorkoth S, Rane PD, et al.:
Cost-Effective HPLC-UV Method for Quantification of Vitamin D 2 and D 3 in Dried Blood Spot: A Potential Adjunct to Newborn Screening for Prophylaxis of Intractable Paediatric Seizures.2019; 88.
- 109.
Tejasvini CS, Sekhar S, Verma R, et al.:
DEVELOPMENT AND VALIDATION OF STABILITY-INDICATING ASSAY RP-HPLC METHOD FOR THE ESTIMATION OF ANIDULAFUNGIN AND RELATED COMPOUNDS IN PARENTERAL DOSAGE FORM.
Rasayan J. Chem.
2022; 15(1): 280–287. Publisher Full Text
- 110.
Gadag S, Narayan R, Nayak Y, et al.:
Bioanalytical RP-HPLC method validation for resveratrol and its application to pharmacokinetic and drug distribution studies.
J. Appl. Pharm. Sci.
2022; 12(2): 158–164. Publisher Full Text
- 111.
Patil PH, Desai M, Rao RR, et al.:
Assessment of pH-shift drug interactions of palbociclib by in vitro micro-dissolution in bio relevant media: An analytical QbD-driven RP-HPLC method optimization.
J. Appl. Pharm. Sci.
2022; 12(5): 78–87. Publisher Full Text
- 112.
Goudar N, Tejas B, Sathyanarayana MB, et al.:
QUANTITATIVE DETERMINATION AND VALIDATION OF ETORICOXIB AND PARACETAMOL COMBINED TABLET DOSAGE FORM BY REVERSE PHASE-HPLC.
Rasayan J. Chem.
2022; 15(3): 1702–1708. Publisher Full Text
- 113.
Gopalan D, Patil PH, Jagadish PC, et al.:
QbD-driven HPLC method for the quantification of rivastigmine in rat plasma and brain for pharmacokinetics study.
J. Appl. Pharm. Sci.
2022; 12(6): 56–067. Publisher Full Text
- 114.
Peraman R, Chiranjeevi P, Reddy YP, et al.:
Beta-Alanine and Tris-(hydroxyl methyl) Aminomethane as Peak Modifiers in the Development of RP-HPLC Methods Using Aceclofenac and Haloperidol Hydrochloride as Exemplar Drugs.
J. Chromatogr. Sci.
2021; 59(10): 899–908. PubMed Abstract
| Publisher Full Text
- 115.
Arumugam K, Chamallamudi MR, Gilibili RR, et al.:
Development and validation of a HPLC method for quantification of rivastigmine in rat urine and identification of a novel metabolite in urine by LC-MS/MS.
Biomed. Chromatogr.
2011; 25(3): 353–361. PubMed Abstract
| Publisher Full Text
- 116.
Jitta SR, Salwa KL, Gangurde PK, et al.:
Development and Validation of High-Performance Liquid Chromatography Method for the Quantification of Remdesivir in Intravenous Dosage Form.
Assay Drug Dev. Technol.
2021; 19(8): 475–483. PubMed Abstract
| Publisher Full Text
- 117.
Kumar G, Mullick P, Nandakumar K, et al.:
Box–Behnken Design-Based Development and Validation of a Reverse-Phase HPLC Analytical Method for the Estimation of Paclitaxel in Cationic Liposomes.
Chromatographia.
2022; 85(7): 629–642. Publisher Full Text
- 118.
Naik S, Mullick P, Mutalik SP, et al.:
Full Factorial Design for Development and Validation of a Stability-Indicating RP-HPLC Method for the Estimation of Timolol Maleate in Surfactant-Based Elastic Nano-Vesicular Systems.
J. Chromatogr. Sci.
2022; 60(6): 584–594. PubMed Abstract
| Publisher Full Text
- 119.
Kolate NS, Mishra H, Kini SG, et al.:
A Validated RP-HPLC Method for Quantification of Steviol Glycoside: Rebaudioside A in Extracts of Stevia Rebaudiana Leaf.
Chromatographia.
2021; 84(1): 21–26. Publisher Full Text
- 120.
Sravani AB, Mathew EM, Ghate V, et al.:
A Sensitive Spectrofluorimetric Method for Curcumin Analysis.
J. Fluoresc.
2022; 32(4): 1517–1527. Publisher Full Text
- 121.
Navya Sree KS, Girish Pai K, Verma R, et al.:
Validation of HPLC method for quantitative determination of gefitinib in polymeric nanoformulation.
Pharm. Chem. J.
2017; 51(2): 159–163. Publisher Full Text
- 122.
Rathod RU, Navyasree KS, Bhat K:
Quantification of Sofosbuvir in Human Plasma: RP-HPLC Method Development and Validation.
Pharm. Chem. J.
2018; 52(7): 663–673. Publisher Full Text
- 123.
Phani Sekhar Reddy G, Navyasree KS, Jagadish PC, et al.:
Analytical Method Development and Validation for HPLC-ECD Determination of Moxifloxacin in Marketed Formulations.
Pharm. Chem. J.
2018; 52(7): 674–679. Publisher Full Text
- 124.
Sushmitha GS, Pai G, Krishna M, et al.:
Development of multiple time point stability indicating assay method and validation of nabumetone by RP-HPLC.
Res J Pharm Technol.
2018; 11(11): 4813–4820. Publisher Full Text
- 125.
Avadhani KS, Amirthalingam M, Reddy MS, et al.:
Development and validation of RP-HPLC method for estimation of epigallocatechin -3-gallate (EGCG) in lipid based nanoformulations.
Res J Pharm Technol.
2016; 9(6): 725–730. Publisher Full Text
- 126.
Wang S, Liu C, Chen Y, et al.:
Aggregation of Hydroxypropyl Methylcellulose Acetate Succinate under Its Dissolving pH and the Impact on Drug Supersaturation.
Mol. Pharm.
2018; 15(10): 4643–4653. PubMed Abstract
| Publisher Full Text
- 127.
Wang M, Jiang J, Cai Y, et al.:
In vitro and in vivo evaluation of a posaconazole-sulfobutyl ether-β-cyclodextrin inclusion complex.
Biomed. Chromatogr.
2018; 32(12): e4364. PubMed Abstract
| Publisher Full Text
- 128.
Verweij-van Wissen CPWGM, Burger DM, Verweij PE, et al.:
Simultaneous determination of the azoles voriconazole, posaconazole, isavuconazole, itraconazole and its metabolite hydroxy-itraconazole in human plasma by reversed phase ultra-performance liquid chromatography with ultraviolet detection.
J. Chromatogr. B Analyt. Technol. Biomed. Life Sci.
2012; 887-888: 79–84. PubMed Abstract
| Publisher Full Text
- 129.
Xiao Y, Xu YK, Pattengale P, et al.:
A Rapid High-Performance LC-MS/MS Method for Therapeutic Drug Monitoring of Voriconazole, Posaconazole, Fluconazole, and Itraconazole in Human Serum.
J. Appl. Lab. Med.
2017; 1(6): 626–636. PubMed Abstract
| Publisher Full Text
- 130.
Yang M, Dong Z, Zhang Y, et al.:
Preparation and evaluation of posaconazole-loaded enteric microparticles in rats.
Drug Dev. Ind. Pharm.
2017; 43(4): 618–627. PubMed Abstract
| Publisher Full Text
- 131.
Yang Y, Zhu X, Zhang F, et al.:
Stability-indicating HPLC method development and structural elucidation of novel degradation products in posaconazole injection by LC-TOF/MS, LC-MS/MS and NMR.
J. Pharm. Biomed. Anal.
2016; 125: 165–177. PubMed Abstract
| Publisher Full Text
- 132.
Yoon SJ, Lee K, Oh J, et al.:
Experience with therapeutic drug monitoring of three antifungal agents using an LC-MS/MS method in routine clinical practice.
Clin. Biochem.
2019; 70: 14–17. PubMed Abstract
| Publisher Full Text
- 133.
Zhang M, Moore GA, Barclay ML, et al.:
A simple high-performance liquid chromatography method for simultaneous determination of three triazole antifungals in human plasma.
Antimicrob. Agents Chemother.
2013; 57(1): 484–489. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 134.
Zhong W, Yang X, Tong W, et al.:
Structural characterization of a novel degradant of the antifungal agent posaconazole.
J. Pharm. Biomed. Anal.
2012; 66: 40–49. PubMed Abstract
| Publisher Full Text
Comments on this article Comments (0)