Keywords
organotypic hippocampal slice culture, MAP2, GFAP, intracellular calcium, excitotoxicity, kainic acid
This article is included in the Cell & Molecular Biology gateway.
Neurodegeneration due to neurotoxicity is one of the phenomena in temporal lobe epilepsy. Experimentally, hippocampal excitotoxicity process can occur due to kainic acid exposure, especially in the CA3 area. Neuronal death, astrocyte reactivity and increased calcium also occur in hippocampal excitotoxicity, but few studies have investigated immediate effect after kainic acid exposure. The organotypic hippocampal slice culture (OHSC) is a useful model for studying the neurodegeneration process, but there are still many protocol differences. In this study, minor modifications were made in the OHSC protocol.
OHSC was obtained from three healthy wild type Wistar rats aged P10. Healthy culture slices were obtained and lasted up to 10 days in vitro (DIV 10). Bath application of kainic acid for 48 hours in DIV 10 followed by observation of its initial effects on neurons, astrocytes, and calcium via the expression of MAP2, GFAP, and intracellular calcium imaging, subsequently.
After 48 h of kainic acid administration, there was a significant increase in intracellular calcium intensity (p = 0.006 < α), accompanied by a significant decrease in MAP2 (p = 0.003 < α) and GFAP (p = 0.010 < α) expression.
These findings suggest early neuronal and astrocyte damage at the initial onset of hippocampal injury. This implies that astrocyte damage occurs early before an increase in GFAP that characterizes reactive astrogliosis found in other studies. Damage to neurons and astrocytes may be associated with increased intracellular calcium. It is necessary to develop further research regarding regulation of calcium, MAP2, and GFAP at a spatial time after exposure to kainic acid and strategies to reduce damage caused by excitotoxicity.
organotypic hippocampal slice culture, MAP2, GFAP, intracellular calcium, excitotoxicity, kainic acid
This revised article includes several changes. We have updated the title to highlight the novel aspect of the study, changing it from “Expression of MAP2, GFAP, and Calcium in the CA3 Area of a Modified Organotypic Hippocampal Slice Culture Treated with Kainic Acid” to “Regulation of MAP2, GFAP, and Intracellular Calcium in the CA3 Region Following Kainic Acid Exposure to Organotypic Hippocampal Slice Culture.” The abstract has been adjusted accordingly.
We also corrected the number of animals used in the abstract from two to three. Additionally, we have revised the introduction and discussion sections to clearly differentiate our findings from those of Jeong et al. (2014), de Pablo et al. (2013), and Holopainen et al. (2004). In the methods section, we refined the sentences regarding the number of slices used for each parameter to provide a more accurate and coherent representation of the intended meaning.
The term "calcium expression" has been replaced with "low-resolution calcium imaging," "epileptogenesis" has been changed to "epileptiform activity," and "epilepsy" has been replaced with "seizure," since no in vivo experiments were conducted, as suggested. In all bar diagrams, we have updated and included the sample size (n=x) beneath each bar, and we have updated the Figshare DOI.
The final paragraph of the discussion has been removed as suggested, but we have retained and strengthened the first sentences of the conclusion. The conclusion has been revised to include more details about the possible role of calcium in this research.
See the authors' detailed response to the review by Amin Derouiche
See the authors' detailed response to the review by Abdul Gofir
Neurodegeneration mainly caused by acute and chronic excitotoxicity. The chronic excitotoxicity process can be found in Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), and temporal lobe epilepsy (TLE) (Doble, 1999; Mohd Sairazi et al., 2015). The chemoconvulsant agent kainic acid (KA), which is an AMPA/kainate receptor agonist, is often used experimentally to induce excitotoxicity, as it plays a role in the development of epileptic seizures similar to conditions in epileptic patients (Tanioka et al., 2020; Zhang & Zhu, 2011).
Neuronal death as a result of glutamate excitotoxicity has been extensively studied, both in vivo and in vitro (Holopainen et al., 2004; Liu et al., 2001; Noraberg et al., 1999; Sanon et al., 2005; Sattler & Tymianski, 2001). Glutamate represents the primary excitatory neurotransmitter in the mammalian brain, and it is used by neurons, astrocytes, and other glial cells to process information (Mihály, 2019). The main mechanism behind neuronal death is glutamate excitotoxicity (Cho, 2013; Sanon et al., 2005), which occurs through necrosis, apoptosis, or both (Holopainen et al., 2004; Liu et al., 2001). Other than neuronal death, a significant increase in astrocyte reactivity has been observed in response to central nervous system injury (Jeong et al., 2014; Miller et al., 2017; Yildirim et al., 2019; Zhang et al., 2017; Zhang & Zhu, 2011). Increased astrocyte reactivity (astrogliosis), characterized by increased GFAP expression, is known to exert a protective effect against neuronal death due to nervous system injury (Jeong et al., 2014; Miller et al., 2017; Zhang et al., 2017). Prior studies have found this process to be closely related to calcium level. In the context of epilepsy, the calcium hypothesis suggests an increase in intracellular calcium levels. If this increase is not sufficient to induce excitotoxicity, it can result in temporary or permanent neuroplasticity changes and degeneration. In the long term, these changes can cause abnormal neuronal firing and so lead to epilepsy (Zhang et al., 2019).
Kainic acid is commonly used for glutamate excitotoxicity model. Damage due to kainic acid mainly occurs in the hippocampus. Such damage may be caused by the high density of kainic receptors in that area (Mihály, 2019; Zhang & Zhu, 2011). The CA3 circuit plays a role in the epileptiform activity within the hippocampus. Song et al. found hyperexcitability due to hypoxic-hypoglycemic injury to be more visible in the CA3 area than in other hippocampal areas (Song et al., 2018). The maturation of synapses, receptors, and intrinsic fiber pathways in the OHSC also occurs in in-vivo conditions, which renders it a useful model for studying age-related conditions in a maintained environment (Holopainen et al., 2004). To date, various methods have been used to make OHSCs. The preparation of acute hippocampal slices at room temperature gives rise to better results, especially with regard to electrophysiological observations (Eguchi et al., 2020). In contrast, OHSC processing requires cold temperatures during slicing in order to reduce the damage that may occur throughout the surgical process, although there are still many variations when it comes to the use of cold temperatures (Lein et al., 2011; Opitz-Araya & Barria, 2010; Simoni & Yu, 2006; Varela et al., 2012).
Only a few prior studies have investigated the immediate impact of hippocampal insult to neuron and astrocyte (Jeong et al., 2014; Zhao et al., 2003). This study aimed to observe the immediate effect of kainic acid on neurons, astrocytes, and intracellular calcium in the CA3 area of modified OHSC.
This study used three healthy wild type Wistar rats aged postnatal day 10 (P10), weight range 10-15 grams, regardless of sex. The sample size was decided from Federer’s formula (Ihwah et al., 2018). The number of treatments are two, and the result from the formula is 8, so we used 9 slices for each subgroup. All of the Wistar rats were purchased from Animal Lab of Biosains Institute Universitas Brawijaya. All the procedures carried out in this study were approved by the Health Research Ethics Commission, Faculty of Medicine, Brawijaya University Malang (No.50/EC/KEPK-S3/02/2019).
We obtained approximately 18-20 hippocampal slices per rat and cultured them to make the appropriate organotypic hippocampal slice culture (OHSC; made at the Biosciences Institute of Universitas Brawijaya). The OHSC was divided into two groups (allocated randomly), namely the untreated group and the kainate group. The kainate group was treated with kainic acid (Abcam, AB120100) at a dose of 8 μM by bath application for 48 h and put in an incubator at 37 oC. After that, both groups (kainate group and untreated group) were stained for calcium, MAP2, and GFAP (each subgroup consist of 9 slices) and observed with confocal laser scanning microscopy (CLSM). Sliced culture photographs were taken from a stereomicroscope observation at 40× magnification. The photos were processed with Adobe Photoshop ver. 2020. We examined the healthiness of the slices macroscopically, using stereomicroscope. The healthy slices will look thinner and more transparent, because the dead cells and tissue debris have disappeared. Another marker is we can see the different region of hippocampus clearly (Simoni & Yu, 2006). On day in vitro 13 (DIV 13), some slices were not very healthy macroscopically, so we exclude some slices for statistical analysis. The excluded slices conditions are: not getting thinner and transparent, also the color become whitish opaque or more darker. The remaining slices deemed to be of sufficient quality were utilized for calcium imaging (five slices), MAP2 staining (three slices), and GFAP staining (three slices). Parameter observations with CLSM were done in the Central Laboratory of Biological Sciences Universitas Brawijaya.
The slicing medium and the culture medium were prepared before surgery. More specifically, the slicing medium was prepared using 71.49 g of HEPES (Sigma, H3375, Lot SLBB55267) in 300 ml of Earle’s Balanced Salt Solution (EBSS) 10X (Sigma, Lot RNBG8086). The culture medium comprised 50 ml of Minimum Eagle Medium (MEM, Sigma, M0769-10x1L, Lot SLBS6945), 18 ml of EBSS, 5 ml of EBSS + D-glucose (Merck, CAS-No: 50-99), 1 ml of penicillin-streptomycin (Gibco, Ref 15070-063, Lot 2145472), 25 ml of horse serum (Gibco, Ref 16050-122, Lot 2208948), and 0.06 ml of nystatin (Abcam, ab141118, Lot GR305852-5).
Age recommendation to make OHSC for rat is P0-15 and P0-5 for mice (Muller et al., 2001). We used P10 Wistar rat, because technically it was easier for us than younger rats. The brain size was not too small so it was easier to slice the hippocampus. The pups remained with the mother, mostly in a dark environment at a temperature of 26oC with 70% relative humidity. On P10 we took the pups for euthanasia. The OHSC was made according to the method proposed by de Simoni (Simoni & Yu, 2006), albeit with slight modifications (Opitz-Araya & Barria, 2010). Briefly, cervical dislocation followed by decapitation were performed aseptically and without any prior anesthesia (Simoni & Yu, 2006). Cervical dislocation is appropriate and acceptable method of euthanasia for small rats weighing <200 grams. Cervical dislocation causes rapid loss of consciousness, and we performed decapitation immediately after cervical dislocation. Additionally the procedure was performed by a skilled technician and we require uncontaminated brain tissue since anesthetic drug can interfere brain properties (Hollands, 1986; Karmarkar et al., 2010; Leary et al., 2020). The brain was removed from the skull and then soaked for 1 minute in a beaker placed on ice. The beaker contained a slicing medium that had been perfused with 5% CO2/95% O2 for 10 – 20 minutes. Next, the brain was transferred to a petri dish containing the slicing medium (2.4 g HEPES and 10 ml EBSS) and the hippocampus isolation was performed. Following the isolation, the next step involved making slices with a thickness of 350 μm using a tissue slicer (Stoelting Tissue Slicer 51425, RRID:SCR_022902). The hippocampal slices were then placed on an omnipore membrane (Merck, Ref: JHWP01300, Lot: R0MB18744) within a cell culture insert (Merck, Ref: PICM03050, Lot: R0DB59407) and a six-well plate filled with 1.3 ml of the culture medium (EBSS, glucose, penicillin, horse serum, Na bicarbonate, amphotericin, L-glutamine, EBSS, and MEM). All slices that were ready for culturing were put into an incubator at 37 oC for 10 days. The culture medium was changed every two days.
The full protocol for this procedure can be found here: http://dx.doi.org/10.17504/protocols.io.81wgby6pnvpk/v1.
The staining of microtubule-associated protein-2 (MAP2 ) (GeneTex Cat# GTX50810, RRID: AB 11170769) and glial fibrillary acidic protein (GFAP) (Santa Cruz Biotechnology Cat# sc-33673, RRID:AB_627673) were done at DIV 10 via the following procedure. Tissue slices were fixed using 4% paraformaldehyde (PFA) for 15 min, then washed with phosphate buffered saline (PBS) for three times (5 minutes each). After the PBS was removed, 0.1% Triton X-100 was added for 30 min, and the slice culture was washed with PBS for three times (5 minutes each). After washing, 10% bovine serum albumin (BSA) was added for 30 min at room temperature. The primary antibody incubation MAP2 (1: 500) and GFAP (1: 500) was performed overnight at 4°C, then washed with PBS for three times (5 minutes each). An incubation using a secondary antibody, Goat anti-Rabbit IgG-F (1: 500) for MAP2 (Santa Cruz Biotechnology Cat# sc-53805, RRID:AB_783978) and anti-mouse Rhodamine (1: 500) for GFAP (Rockland Cat# 610-1002, RRID:AB_219636) for 1 h at room temperature. It was subsequently washed with PBS for three times (5 minutes each), and the sample was ready to be observed using CLSM with a magnification of 100× and a wavelength of 488 nm (MAP2) and 543 nm (GFAP).
The full protocol for this procedure can be found here: http://dx.doi.org/10.17504/protocols.io.q26g7y4b1gwz/v1
Fluo-4 was used for low resolution calcium imaging (Abcam Fluo-4 assay unit (calcium), AB228555). Nine mL of HHBS + 1 mL 10X F127 were mixed into a 15 mL conical tube, 20 μL of Fluo-4 was added. It was mixed in a dark room until homogeneous. Next, 100 μL of Fluo-4 AM dye-loading solution was added in a petri cell dish, followed by incubation in a cell incubator for 1 h. The observation of calcium fluorescence used CLSM at 100× magnification with the fluorescence intensity monitor at maximum Ex/Em = 490/525 nm.
The full protocol for this procedure can be found here: http://dx.doi.org/10.17504/protocols.io.kxygx9zewg8j/v1.
The data normality test was performed using the Shapiro-Wilk test. Normally distributed data in both groups were compared using the independent sample t-test. Significant differences in the data were indicated by a p < 0.05. Statistical analysis was performed with SPSS for Windows 25.0 (SCR_016479).
Examples of the organotypic hippocampal slice culture results using a modified method from de Simoni (Simoni & Yu, 2006) and Opitz-Araya (Opitz-Araya & Barria, 2010) can be seen in Figure 1. Images of several slices of culture were taken at DIV 0, 3, and 7. On DIV 0, slices were seen dark-colored, and became more transparent and thinner on the 3rd and 7th day; the hippocampal architecture still visible. The slice thickness at DIV 0 was 350 μm, and the thickness at DIV 13 was about 150-200 μm, estimated by CLSM.
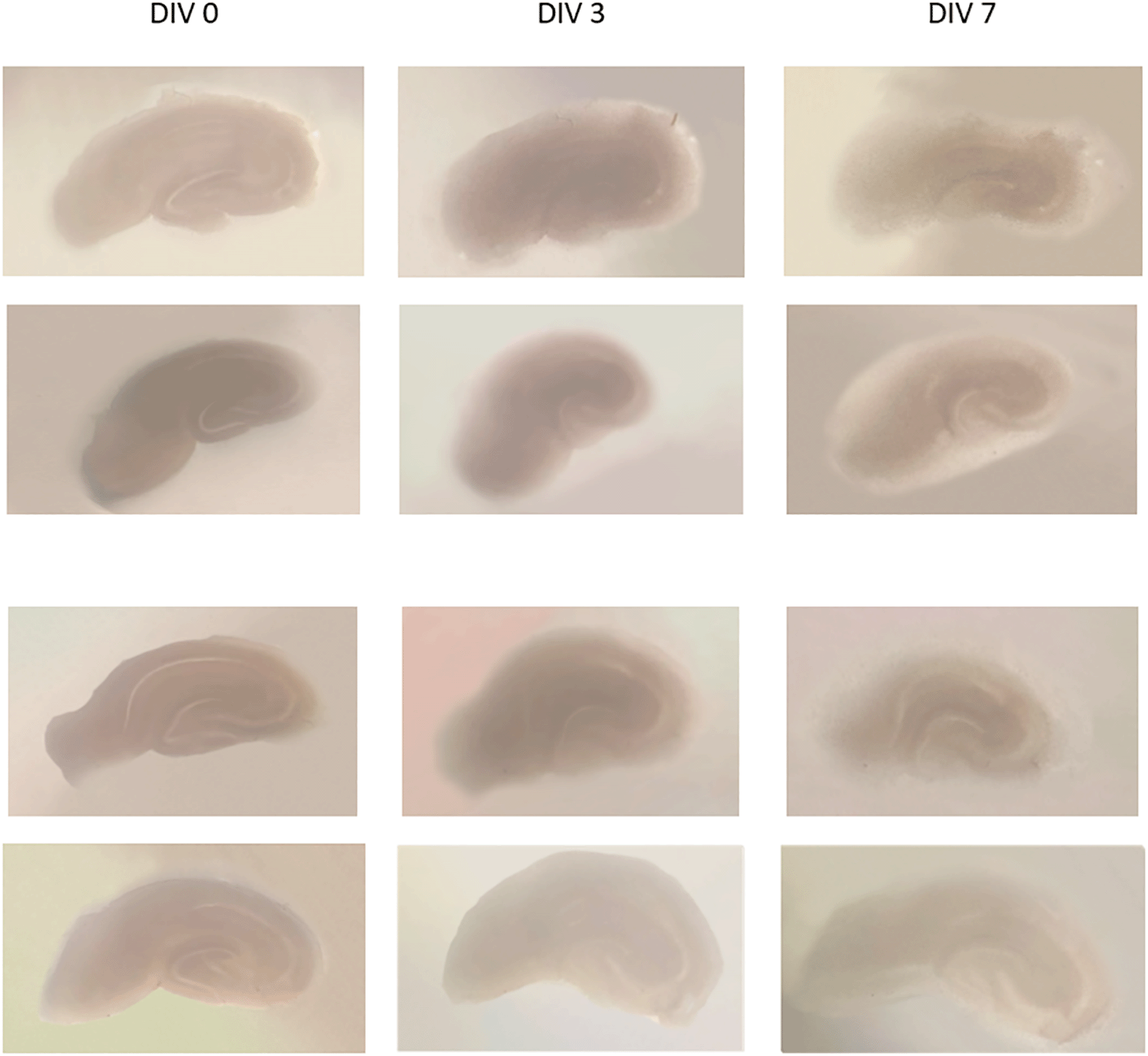
It appears that the slices are thinner and become transparent, with parts of the hippocampus still visible.
DIV = day in vitro.
CLSM image of MAP2 expression in untreated and treated group shown in Figure 2(a-b), GFAP expression in Figure 3 (a-b), and intracellular calcium expression in Figure 4(a-b). The mean intensity of MAP2, GFAP, and intracellular calcium expression in the untreated and kainate groups can be seen in Figures 2-4(c). MAP2 ( Figure 2c) and GFAP ( Figure 3c) expression decreased significantly in the kainate group compared to the untreated group. There was a significant increase of intracellular calcium expression in treated versus untreated group ( Figure 4c).

Comparison of MAP2 expression in the untreated group (a) and kainate group (b). c. There was a significant decrease of MAP2 expression in kainate group (p = 0.003 < ).
MAP2 = microtubule-associated protein 2. The denomination of expression intensity uses the arbitrary unit (AU).

Comparison of GFAP expression in the untreated group (a) and kainate group (b).
c. There was a significant decrease of GFAP expression in kainate group (p = 0.010 < ).
GFAP = glial fibrillary acidic protein. The denomination of expression intensity uses the arbitrary unit (AU).
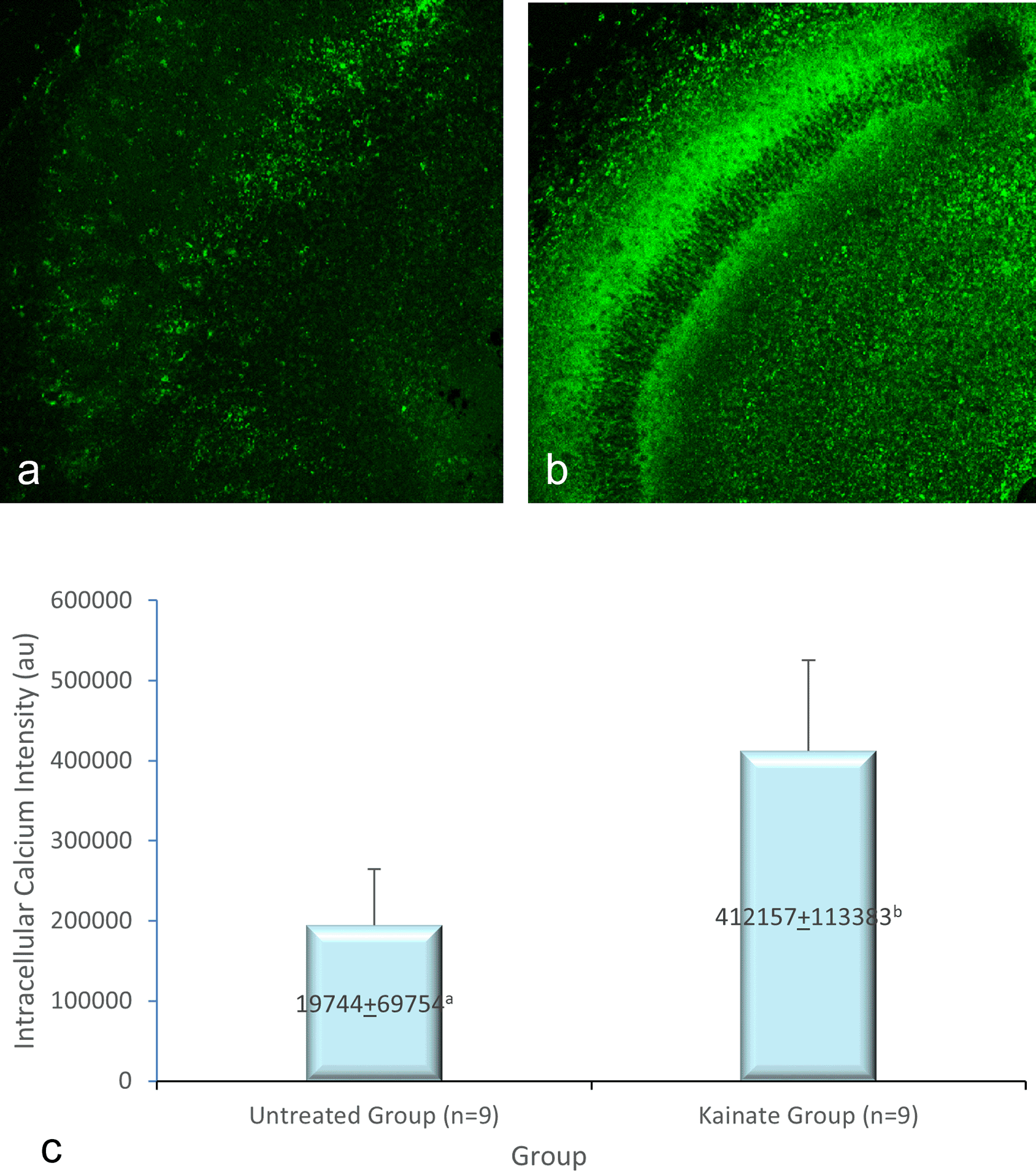
Comparison of calcium imaging intensity in the untreated group (a) and kainate group (b). c. There was a significant increment of intracellular calcium imaging intensity in kainate group (p = 0.006 < α). The denomination of intensity uses the arbitrary unit (AU).
Based on the results of the Shapiro-Wilk test, it was found that the data on calcium, MAP2, and GFAP intensities in the two observation groups had a p-value more significant than the significance level α = 0.05, which means that all the observation groups proved to be normally distributed.
The comparison test between the untreated and the kainate group using the independent sample t-test is shown in the Table 1.
Mean of MAP2 intensity in the kainate group was significantly lower than the untreated group (379579 ± 27334 AU vs 163941 ± 51296 AU; p = 0.003 < α). Likewise, we found significant difference in GFAP expression (p = 0.010 < α) between untreated group (366242 ± 19028 AU) and kainate group (177850 ± 67950 AU).
There is a significant difference (p = 0.006 < α) of intracellular calcium imaging intensity in kainate group (412.157 ± 113.383 AU) compared to untreated group (194.744 ± 69.754 AU). This means that the treated culture exposed to 8 μM of kainic acid for 48 h shows an increased intracellular calcium.
The OHSC protocol according to de Simoni, has been widely used (Abutbul et al., 2012; Kang et al., 2010; Stephen et al., 2015), as well as the method of Opitz-Araya (McQuate & Barria, 2020; Park et al., 2020; Yang et al., 2020). The two methods are mainly similar, however, Opitz-Araya immersed the brain in the slicing medium with perfusion before performing hippocampus isolation (Opitz-Araya & Barria, 2010). Low temperature is required to decrease metabolic activity (Eguchi et al., 2020), glutamate release, excitotoxicity, and purify the blood to aid in the visualization of the cell in slices. Perfusion of the slicing medium with a mixture of 95% O2 gas and 5% CO2 aimed to reduce the hypoxic-ischemic damage to the brain that can occur between decapitation time and the brain's placement in the medium (Varela et al., 2012). Combining the two methods produced the desired results, and the culture was viable until the 10th day. The culture slices were thinner and more transparent compared to day 0. This is an indicator of culture health macroscopically (Opitz-Araya & Barria, 2010; Simoni & Yu, 2006). We could not use the unhealthy slices, so we exclude them for statistical analysis. We used only the healthy ones. The minimum requirement for comparation analysis is triplicate (we used 5 for calcium, 3 for MAP2, and 3 for GFAP analysis). The statistical analysis still can be done, and the result was significant, in accordance with the visual image obtained.
The three main findings of this study were significant decrease of MAP2 and GFAP expression, which are indicators of neuronal and astrocyte injury in the CA3 area, accompanied by a significant increase in intracellular calcium expression at the same time, that is, after 48 h of exposure to kainic acid. MAP2 is a cytoskeletal protein located in the perikaryal and dendrites that specific for neurons, functions for microtubule stabilization, and participates in the dendrite branching processes during the development of hippocampal neurons. This molecule is thought to play a role in synaptic plasticity mechanisms (Kim et al., 2020; Yildirim et al., 2019), which also occurs in epilepsy (Pitkänen & Lukasiuk, 2011). Expression of the MAP2 protein is primarily regulated during brain development and maturation (Blümcke et al., 2001). These proteins are frequently used as markers of dendrites and neuron maturity and integrity (Melani et al., 2005; Pitkänen & Lukasiuk, 2011). The decrease in expression of MAP2 in this study is appropriate with prior studies on OHSC (Hoskison et al., 2007; Noraberg et al., 1999). One study reported neuronal death in CA3 areas, especially after 12, 24, and 48 hours of 5mM KA exposure. The highest neuron mortality was found on exposure to KA for 48 hours, with necrosis as the underlying death process (Holopainen et al., 2004). Other researchers showed that neurons could still be observed within 3 hours after KA injection into the mice's cortex even though they did not appear healthy morphologically. On day 7, neurons disappeared in the entire hemisphere, indicating delayed neuronal death, supporting the apoptotic process (Jeong et al., 2014). Hoskinson et al. demonstrated that loss of MAP2 dendrite in CA1 areas is an indicator of excitotoxic damage after neuronal injury, both in vivo and in vitro, this phenomenon is dependent on Ca2+ permanently (Hoskison et al., 2007). Lopim et al. found that the number of hippocampal neurons in epileptic mice decreased compared to normal mice and that the total number of neurons was negatively correlated with the frequency of epileptic seizures. The number of cell deaths occurred in the initial period (30 d) after the seizure onset (Lopim et al., 2016).
Astrocytes secrete several inhibitory factors in conditions of injury, ischemia, blood-brain barrier damage, inflammatory reactions, abnormal metabolism, and oxidative stress (Zhang et al., 2017). The active inflammatory status in astrocytes is characterized by the upregulation of GFAP (Siracusa et al., 2019). These external factors influence axon regeneration and recovery of CNS neurological function. GFAP is one of the best markers for astrocyte activation due to injury or stress to the CNS (Zhang et al., 2017). During development, astrocytes also play a role in synaptic transmission, differentiation and migration of neurons, and axon growth. GFAP is an intermediate protein filament expressed in mature astrocytes. This protein is vital for adaptation to neuronal changes and activity during brain development. GFAP also plays a role in neural-glial interactions. Changes in GFAP levels can cause damage to the relationship between neurons and between neurons and glial cells (Yildirim et al., 2019). Jeong et al. found that astrocytes swelled within 3 hours of KA injection into the mice cortex. After that, GFAP+ astrocytes began to disappear from day one and increased until day 7. The location of the loss of astrocytes is the same as delayed neuronal death, thus supporting the suggestion that astrocytes may play a role in the onset of delayed neuronal death (Jeong et al., 2014). The decreased expression of MAP2 after exposure to kainic acid in this study indicated damage to neurons due to administration of kainic acid.
This study found that kainic acid caused early damage to neurons and astrocytes, particularly at the onset of exposure. Astrocyte activation, characterized by increased GFAP expression, inhibits the inflammatory response after injury, limiting cell damage (Zhang et al., 2017). Decreased astrocyte activation in GFAP-/-Vim-/- mice with an ischemic stroke resulted in larger infarct size. This implies that astrocyte activation is essential for protecting brain tissue in stroke and that GFAP plays a role in this process, especially in the cell resistance to oxidative stress (De Pablo et al., 2013). In this study, the decreased expression of GFAP after exposure to kainic acid indicated damage to astrocytes due to administration of kainic acid.
As previously mentioned, kainic acid causes excessive activation of glutamate receptors, leading to excitotoxicity (Cho, 2013; Mohd Sairazi et al., 2015; Sanon et al., 2005). Vargas et al. reported that ionotropic glutamate receptors expression was common in young mice's slice cultures (mean age P10). In the adult hippocampus, astrocytes expressed KAR (kainate receptor) after the status epilepticus induced by kainic acid, especially GluK1. This increase is thought to be related to astrocytes' function as glutamate sensors after status epilepticus, as an attempt to save neurons from injury. Besides, KAR expression is also thought to increase calcium signal and hyperexcitability, which causes epileptic seizures (Vargas et al., 2013). A study reported a loss of GFAP immunoreactivity within 24 hours of intracortical injection of kainic acid in mice. This loss of GFAP precedes neuronal death (Jeong et al., 2014). Loss of GFAP immunoreactivity at the onset of traumatic brain injury has also been reported (Zhao et al., 2003).
The role of astrocytes in changes in neuronal activity is found in epilepsy. Astrocytes can trigger the induction and development of inflammatory status via Ca2+ signaling and are closely related to disease grading. Ca2+ variations that affect neuronal activity in releasing gliotransmitters are also one of the astrocytes' roles. The glutamate transporter is found in several neuron cell types, and astrocytes primarily play a role in glutamate uptake. GLT-1 is a glutamate transporter found in astrocytes, which plays a role in removing extracellular glutamate and increasing epileptogenic foci levels. Glutamine synthetase was reduced in the hippocampus of TLE patients compared to healthy patients. This downregulation causes glutamate-glutamine circulation and increases the accumulation of transmitters in the extracellular space and astrocytes. This indicates a condition of hyperexcitability that depends on astrocytes. AMPA receptors, especially the subtypes formed by the GLuR1 to GluR4 sub-units, are highly expressed in astrocytes. Epilepsy patients show increased expression of the GluR1 variant, which causes prolonged channel opening, thereby increasing the influx of Na+ and Ca2+ ions, blocking astroglia Kir channels, increasing depolarization, and reducing the capacity of astrocytes in the K+ ion buffer (Siracusa et al., 2019).
The third finding in this study was an increase in intracellular calcium expression after exposure to kainic acid. OHSC is an in vitro preparation used to study intact neural circuits in chronic conditions, including epileptogenesis. After tissue incision makes deafferentation, the neural tissue rearranges itself and exhibits epileptiform-like activity, which can be visualized by a calcium indicator. This calcium activity is present in both astrocytes and neurons (Gee et al., 2015). The increase in intracellular calcium influx and oxidative stress due to excessive activation of glutamate receptors by KA causes cell damage and increases in protease and endonuclease enzymes that damage membranes, cytoskeletal proteins, and DNA fragmentation. This oxidative stress plays a role in the death of neurons and glial cells (Mohd Sairazi et al., 2015). The significant increase in intracellular calcium expression in the kainate group implies that calcium plays a significant role in neuron and astrocyte damage due to kainic acid administration.
The strength of this study is the demonstration of early damage of neuron and astrocyte by kainic acid, accompanied with high intracellular calcium expression. Only few prior studies have investigated it together (Yang et al., 2020). This early damage could lead to epileptiform activity. However, we only measure the effect of excitotoxicity at one time only. Therefore, this study’s results can still be developed to further determine the course of neurons, astrocytes, as well as calcium in the epileptiform activity and to find strategies to minimize excitotoxicitiy.
The reduction of MAP2 and GFAP expression in this study indicates the loss of neurons and also astrocytes at the initial onset of exposure to kainic acid, which can be analogous to the deterioration of neuron and astrocyte function at the onset of insult leading to seizure. The immediate rise in calcium levels likely contributes to this phenomenon. Understanding this calcium-mediated mechanism provides valuable insight into potential therapeutic strategies to mitigate excitotoxic damage and prevent the progression to epileptiform activity.
Figshare: Expression of MAP2, GFAP, and Calcium in CA3 Area of a Modified Organotypic Hippocampal Slice Culture Treated with Kainic Acid, https://doi.org/10.6084/m9.figshare.28045007.v1 (Husna et al., 2024).
This project contains the following underlying data:
1. UNT CA SLICE B (.png) = Calcium Intensity Untreated Group
2. UNT CA SLICE C (.png) = Calcium Intensity Untreated Group
3. UNT GFAP SLICE B (.png) = GFAP Expression Untreated Group
4. UNT MAP 2 SLICE B (.png) = MAP 2 Expression Untreated Group
5. AK8 CA SLICE B (.png) = Calcium Intensity Kainate Group
6. AK8 CA SLICE C (.png) = Calcium Intensity Kainate Group
7. AK8 GFAP SLICE B (.png) = GFAP Expression Kainate Group
8. AK8 GFAP SLICE C (.png) = GFAP Expression Kainate Group
9. AK8 MAP 2 SLICE B (.png) = MAP 2 Expression Kainate Group
10. AK8 MAP 2 SLICE C (.png) = MAP 2 Expression Kainate Group
11. MAP 2Untreated (.tif ) = MAP 2 Expression Untreated Group
12. MAP 2Kainate (.tif ) = MAP 2 Expression Kainate Group
13. GFAPKainate (.tif ) = GFAP Expression Kainate Group
14. GFAPUntreated (.tif ) = GFAP Expression Untreated Group
15. CalciumKainate (.tif ) = Calcium Intensity Kainate Group
16. CalciumUntreated (.tif ) = Calcium Intensity Untreated Group
17. MAP 2Kainate300 (.tif ) = MAP 2 Expression Kainate Group (300 dpi)
18. MAP 2Untreated300 (.tif ) = MAP 2 Expression Untreated Group (300 dpi)
19. GFAPKainate300 (.tif ) = GFAP Expression Kainate Group (300 dpi)
20. GFAPUntreated300 (.tif ) = GFAP Expression Untreated Group (300 dpi)
21. CalciumKainate300 (.tif ) = Calcium Intensity Kainate Group (300 dpi)
22. CalciumUntreated300 (.tif ) = Calcium Intensity Untreated Group (300 dpi)
23. DIV0-1 (.tif ) = Hippocampal Slice Culture Day in vitro 0
24. DIV0-2 (.tif ) = Hippocampal Slice Culture Day in vitro 0
25. DIV0-3 (.tif ) = Hippocampal Slice Culture Day in vitro 0
26. DIV0-4 (.tif ) = Hippocampal Slice Culture Day in vitro 0
27. DIV0-1300 (.tif ) = Hippocampal Slice Culture Day in vitro 0 (300 dpi)
28. DIV0-2300 (.tif ) = Hippocampal Slice Culture Day in vitro 0 (300 dpi)
29. DIV0-3300 (.tif ) = Hippocampal Slice Culture Day in vitro 0 (300 dpi)
30. DIV0-4300 (.tif ) = Hippocampal Slice Culture Day in vitro 0 (300 dpi)
31. DIV3-1 (.tif ) = Hippocampal Slice Culture Day in vitro 3
32. DIV3-2 (.tif ) = Hippocampal Slice Culture Day in vitro 3
33. DIV3-3 (.tif ) = Hippocampal Slice Culture Day in vitro 3
34. DIV3-4 (.tif ) = Hippocampal Slice Culture Day in vitro 3
35. DIV3-1300 (.tif ) = Hippocampal Slice Culture Day in vitro 3 (300 dpi)
36. DIV3-2300 (.tif ) = Hippocampal Slice Culture Day in vitro 3 (300 dpi)
37. DIV3-3300 (.tif ) = Hippocampal Slice Culture Day in vitro 3 (300 dpi)
38. DIV3-4300 (.tif ) = Hippocampal Slice Culture Day in vitro 3 (300 dpi)
39. DIV7-1 (.tif ) = Hippocampal Slice Culture Day in vitro 7
40. DIV7-2 (.tif ) = Hippocampal Slice Culture Day in vitro 7
41. DIV7-3 (.tif ) = Hippocampal Slice Culture Day in vitro 7
42. DIV7-4 (.tif ) = Hippocampal Slice Culture Day in vitro 7
43. DIV7-1300 (.tif ) = Hippocampal Slice Culture Day in vitro 7 (300 dpi)
44. DIV7-2300 (.tif ) = Hippocampal Slice Culture Day in vitro 7 (300 dpi)
45. DIV7-3300 (.tif ) = Hippocampal Slice Culture Day in vitro 7 (300 dpi)
46. DIV7-4300 (.tif ) = Hippocampal Slice Culture Day in vitro 7 (300 dpi)
47. Calcium (.tif ) = Calcium Intensity Graphic
48. GFAP (.tif ) = GFAP Expression Graphic
49. MAP 2 (.tif ) = MAP 2 Expression Graphic
50. Calcium600 (.tif ) = Calcium Intensity Graphic (600 dpi)
51. GFAP600 (.tif ) = GFAP Expression Graphic (600 dpi)
52. MAP 2600 (.tif ) = MAP 2 Expression Graphic (600 dpi)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Special thanks to Shahdevi Nandar Kurniawan, Wahyu Nur Laili Fajri, Pupimadita Tizar Afdora, and Choirunil Chotimah for their assistance during this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: morphology, immunocytochemistry, astrocyte, hippocampus
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: morphology, immunocytochemistry, astrocyte, hippocampus
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neurodegeneration and neurovascular
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neurodegeneration and neurovascular
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 03 Jan 25 |
read | |
|
Version 2 (revision) 25 May 23 |
read | read |
|
Version 1 12 Jan 23 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)