Introduction
Head and neck cancer are the world’s eighth most prevalent tumour in the oral and maxillofacial region, among which the most frequent malignant tumour is oral squamous cell carcinoma (OSCC).1,2 About 880,000 patients suffer with OSCC each year worldwide and approximately half the patients die due to it.2 The lifestyle habits such as tobacco eating, smoking, alcohol drinking, and chewing betel nuts develops the OSCC. Many researchers believe that there are approximately 25 % patients who are positive for OSCC are also positive for HPV so showing high correlation between OSCC and HPV infection.3 Globally there are 3.90 per 100 000 of OSCC cases and the mortality of them is 1.94 per 100,000 globally.4,5
The early staged OSCC cases are treated with surgery only while the rest OSCC cases requires a multi-modal approach that is the chemotherapy and radiotherapy. There is day to day increasing in clinical trials in many researches on improved surgery, radiotherapy and also chemotherapy. It is because there is very high incidence and mortality rate in many countries worldwide. Also there is complex and economic impact for patients which manage to survive this highly disabling disease.6
The contradiction of the OSCC is its simplicity to diagnose it with the routine clinical and histological examination in most of the cases. However very large number of cases are diagnosed at advanced stages. There is impact on prognosis of diseases due to late diagnosis in many cases which hampers the quality of life of patients and financial burden for healthcare systems worldwide.7 In present decades, there have been pivotal advancement in the therapeutic management of OSCC. For efficient strategy to battle with the advanced and metastatic OSCC the immunotherapy is the example.8
Most OSCCs are preceded by oral precursor lesions or conditions (OPMDs). These lesions mainly include “oral leukoplakia, erythroplakia, oral lichen planus, and OSMF”. Histopathological spectrum of these OPMD’s ranges from hyperkeratosis/hyperplasia to different grades of “epithelial dysplasia”, to “carcinoma in situ”, and eventually “invasive squamous cell carcinoma”. The morbidity and mortality of the disease can be decreased by early diagnosis at the premalignant stage. Further histological assessment of OED with various grades helps in the prediction of malignant transformation.9
The immune system, under normal conditions, functions for the protection of the host against various infectious diseases and tumor. Additionally, it helps in clearing the unhealthy and ailing cells. Immune checkpoints, which are the inhibitory regulators of immune system, play a vital role in maintaining self-tolerance. They aid in regulating immune response latency, intensity, and extent, limiting autoimmunity and decreasing collateral tissue damage. Among them is “cytotoxic T lymphocyte-associated protein 4” (CTLA-4). Its expression is significantly higher in regulatory T cells (Tregs) and upregulated in activated conventional T cells. CTLA4 is similar to CD28 which is a T-cell co-stimulatory protein. Both of these molecules compete in binding to CD80 (B7). When triggered by CD80, CTLA4 sends a signal to T cells that is inhibitory. CD28 transmits a stimulatory signal. Cell surface proteins known as “programmed cell death 1” (PD-1) and “programmed cell death ligand 1” (PD-L1) are essential for the proper functioning of immunological checkpoints.10 The expression of “PD-L1” positive cells has a direct correlation with poorer prognostic outcome and its correlation is inversed with CD8+T cells infiltrating tumor.11
Development of tumor, “immune privilege” regions, viral persistence, and immune evasion are some important roles of “PD-L1”. “PD-L1” ligation on tumor cell surface protects the tumor from cell death. Therefore, dysfunctional or exhausted T cells are reactivated by blockade of the “PD-1/PD-L1” axis by restoring tumor-specific immunity that eliminates the cancer cells. Nivolumab is a commercially available human monoclonal anti-programmed death-1 antibody. It is clinically approved in several countries after undergoing a successful phase III clinical trial and it is reportedly active against recurrent/metastatic head and neck cancers.12
Positive tumor “PD-L1” expression is determined by immunohistochemistry (IHC), which predicts the clinical response. Nivolumab and pembrolizumab are the PD-1/PD-L1 checkpoint inhibitors which have shown clinical effectiveness in advanced treatment of head and neck cancer.13
The presence of expression “PD-L1” is seen in the cell membrane and cytoplasm of tumor cells and infiltrating lymphocytes. Physiologically, it is seen in the placenta, pancreatic islet cells and mesenchymal stem cells. Higher expression of “PD-L1” is corresponds with the poorly differentiated tumor, advanced cancer staging, nodal as well as distant metastasis.14
In HNSCC, the degree of inter-observer variability in “PD-L1” assessment represents an obstacle even after the preliminary analysis. This is owing to the variety of scoring systems available for “PD-L1”, like the “tumor-proportion score” (TPS), the immune-cell (IC) score, and the “combined-positive score” (CPS), which is recommended in the HNSCC.15
In 2016, new medications that target the immune checkpoint “PD-1/PD-L1” were being evaluated for the treatment of instances of advanced HNSCC. A new immunotherapy treatment approach was launched, and early studies on select patients revealed encouraging outcomes. Due to the fact that it is expressed in various malignancies, including HNSCC, PD-L1 has become a frequently investigated biomarker for immune checkpoint therapy.16
Expression of “PD-1/PD-L1” differs in numerous cancers and OPMDs. The function of expression of “PD-1 and PD-L1” has explored in OPMDs. The over- expression of “PD-1/PD-L1” was seen higher in cases of actinic cheilitis as compared to healthy individuals but lower in cases of OSCC.17 This investigation was performed with the aim of identifying the immunological checkpoint molecules that are the target before OPMDs proceed to OSCC.18
This study focuses on correlating “PD-L1” immunoexpression to clinicopathological characteristics and its prognostic importance in OPMD and OSCC, a new role for “PDL1” has been discovered.
Methods
This study was conducted in “Department of Oral and Maxillofacial Pathology and Microbiology”, “Sharad Pawar Dental College and Hospital”, Datta Meghe Institute of Higher Education and Research (DMIHER), Sawangi (M), Wardha, Maharashtra, India. Prior to the study, ethical approval was obtained from the Institutional Ethical Committee of Datta Meghe Institute of Medical Sciences. (DMIMS (DU)/IEC/2020-21/9425). The informed written consent was obtained from the participants before the study.
The study included surgical tissue samples from patients by taking consent who underwent surgery for OSCC from 2003 to 2019 retrospectively at the same facility. The tissue specimens were retrieved from the department’s archives. 62 cases altogether, including 31 OPMD cases and 31 OSCC cases were chosen. The histopathological grading of OSCC followed the Broder’s grading system as well differentiated, moderately differentiated and poorly differentiated squamous cell carcinoma.
Study design
In this cross-sectional study, a total of 62 samples divided into three groups: The groups are as follows:
Group I: 31 samples with Oral Potentially Premalignant Disorders
Group II: 31 samples with Oral Squamous Cell Carcinoma
For OPMD group, 31 cases of hyperkeratosis and oral sub mucous fibrosis (OSMF) were selected. The study’s inclusion criteria took into account instances with OSCC that had been clinically and histopathologically diagnosed and were largely operated surgically.
The cases that were excluded from the study were those with head neck cancer history in the past, recurrent or distant disease and also patients who had undergone pre-operative treatments except biopsy.
Immunohistochemical method for detection of PD-L1
The IHC for PD-L1 monoclonal antibody, which is a nuclear proliferative marker was carried out in the “Department of Oral and Maxillofacial Pathology & Microbiology”, “Sharad Pawar Dental College”, DMIHER, Sawangi (Meghe), Wardha. Sections were hydrated with increasing grades of alcohol and brought to distilled water and treated with hydrogen peroxide (H2O2) to eliminate endogenous peroxidase activity. The tissues were then incubated sequentially with:
• Primary antibody, which binds to specific tissue antigens.
• Secondary antibody, which binds to the primary antibody; it is a polyvalent antibody that will bind to primary antibodies derived from rabbit, mouse, rat and guinea pig.
A coloured precipitate is formed by adding peroxidase substrate (hydrogen peroxidase) to chromogen at the tissue antigen sites. Counter staining with hematoxylin aided in visualization.
Procedure
1. Fixation of tissues was done by using 10% formalin (neutral buffered) for 24 hours which were later routinely processed and embedded in paraffin wax.
2. Silane coated slides provided proper adherence of tissue sections to the glass slides. Sections were floated on silane-coated slides in a 45°C water bath. Air drying of sections was done overnight at 37°C which were heated at 56°C for one hour.
3. Sections were dewaxed thoroughly in xylene and hydrated through descending grades of alcohol to water as seen in Table 1.
Table 1. Dewaxing in Xylene and hydration in graded alcohol.
| Xylene | 3 changes | 10 minutes each |
|---|
| 1:1 Xylene/Alcohol | 1 change | 10 minutes |
| 100% Alcohol | 2 changes | 5 minutes each |
| 90% Alcohol | 2 changes | 5 minutes each |
| 80% Alcohol | 2 changes | 5 minutes each |
| 70% Alcohol | 2 changes | 5 minutes each |
Sections were kept in running tap water for 5 minutes and were later rinsed in distilled water.
Antigen retrieval
M sodium citrate buffer (pH 6.0) was used to heat the slides in the microwave oven for 12 mins for antigen retrieval which was then bench cooled for 20 mins. The same cycle was repeated again. Then sections were washed thrice by shaking gently in TBS for 5 mins each.
Blocking of endogenous peroxidise
Individual sections were outlined by NCL-Pen. Sections were incubated in Peroxidase block (0.3% H2O2 in methanol) for 20 mins. This is to inactivate the “endogenous peroxidise” activity to avoid false positive reactions when using the same or similar enzyme as label. These sections were then washed with TBS 3 times each for 5 mins.
Application of primary antibody
Blocking serum was poured off and wiped gently around each section. Covering of sections was done using prediluted polyclonal antibody and incubation was done in a humidifying chamber for 1 hour at room temperature. Wash buffer was later used for washing the sections 3 times for 10 mins each.
Application of secondary antibody (Biotinylated rabbit anti mouse IgG)
Wash buffer was wiped gently from around each section. Addition and incubation of biotinylated secondary antibody was done at room temperature for 60 minutes in a humidifying chamber. Then these slides were washed with TBS 3 times each for 10 minutes.
Application of 3,3’ Diaminobenzidine substrate solution (DAB)
Slides were cleaned (wiped) as before. Application of substrate chromogen solution was done which should be enough for covering the section. Incubation was done at room temperature for twenty minutes. Preparation of DAB chromogen working solution was done by mixing 5ul of concentrated DAB in 50ml of substrate buffer.
Counterstaining
Sections were washed for 2 mins in water and were later counterstained with Harris’s hematoxylin for 30 sec. Dehydration of sections was done Sections were dehydrated through ascending grades of alcohol, cleared in xylene and mounted in DPX.
Assessment of immunohistochemically stained sections: IHC analysis
Examination of sections stained with “PD-L1” antibody was done under Leica DMLB2 (Leica microscope) at 40×.
At the site of the target antigen (DAB chromogen brown end product), examination of the sections was done for the presence of a coloured end product. This indicated a positive staining result and proper performance of kit reagents.
The cells which showed the presence of brown colour (membranous staining) were considered positive for the “PD-L1.”The positive score of the stained cells was regardless of the intensity of staining. Cells that did not show a clear staining were excluded. Each section had a minimum of 1000 cells. Evaluation of tissue sections positive for “PD-L1” was done by locating the epithelial linings and was labelled by scanning the sections at 10X. The cells were counted at 40× under Leica DMLB2 (Leica microscope) in 5 different fields. “PD-L1” labelled cells counting was done.
Five randomly chosen fields from the representative areas were evaluated at a 40× magnification. Each field’s membranous/cytoplasmic cell positive was recorded, regardless of its level. Cells that were positive for “PD-1” and “PD-L1” in the sub-epithelial region were counted up to sixty micrometres from the basement membrane. This was carried out in accordance with the oral mucosa’s superficial lamina propria. In each example, the basal and spinous layer were evaluated for “PD-L1” expression. The proportion of positively labelled cells to all cells was calculated.
The evaluation and tracing was done with the help of computer aided analysis system Leica Q-Win ProV3.5.0, Leica Microsystems (Switzerland) Ltd.
Tonsils showing expression of “PD-L1” were used as positive control. For negative control, one section was taken from each positive control and primary antibody were excluded. It was then incubated with serum. The rinsing was done 3 times for 10 mins in PBS after primary reaction of antigen and antibody. Incubation of the HRP-labelled anti-mouse polymer was done for 30 mins at room temperature in a humidifying chamber. There were antigen-antibody reactions in the chromogen buffer solution of “3,3′ diaminobenzidine”. Final counterstaining was done using “Mayer’s Hematoxylin”.
Then the assessment of “PD-L1” immunoexpression was done manually by 3 different observers in blinded manner.
The evaluation was done into 4 categories according to percentages stained for malignant cells (0 = negative; 1 = 1-33%; 2 = 34-66%; 3 = 67-100%). More than 10% “PD-L1” expression show poor survival outcomes. So even 1% PD-L1 expressed cancer cells were considered as positive.
Sample size calculation
Considering the prevalence of Oral Potentially Malignant Disorder as 2% in outpatient department of Oral Medicine and Radiology, using Single Proportion Formula, sample size was calculated by using the formula:
Where,
And Zα/2 is the critical value of the Normal distribution at a/2 (e.g. for a confidence level of 95%, a is 0.05 and the critical value is 1.96), MOE is the margin of error, p is the sample proportion, and N is the population size.
The sample size was calculated to be 31. There are total 2 groups, hence, total 62 samples were required.
Statistical analysis
The Chi-square test will be used to analyse differences in categorical variables. Survival analysis will be done by Kaplan-Meier method, and the log-rank test will be used to test for significant differences by SPSS 17.0 version 8, SPSS Inc., Chicago, IL.
Results
Figure 1 representing the hematoxylin and eosinophilic stained tissue section showing well differentiated squamous cell carcinoma and thereby Figure 2 showing transmembranous PD-L1 immunostaining in it. Figure 3 represents haematoxylin and eosin stained tissue section at 400× magnification and Figure 4 represents transmembranous PD-L1 immunostaining at 400× in oral squamous cell carcinoma.
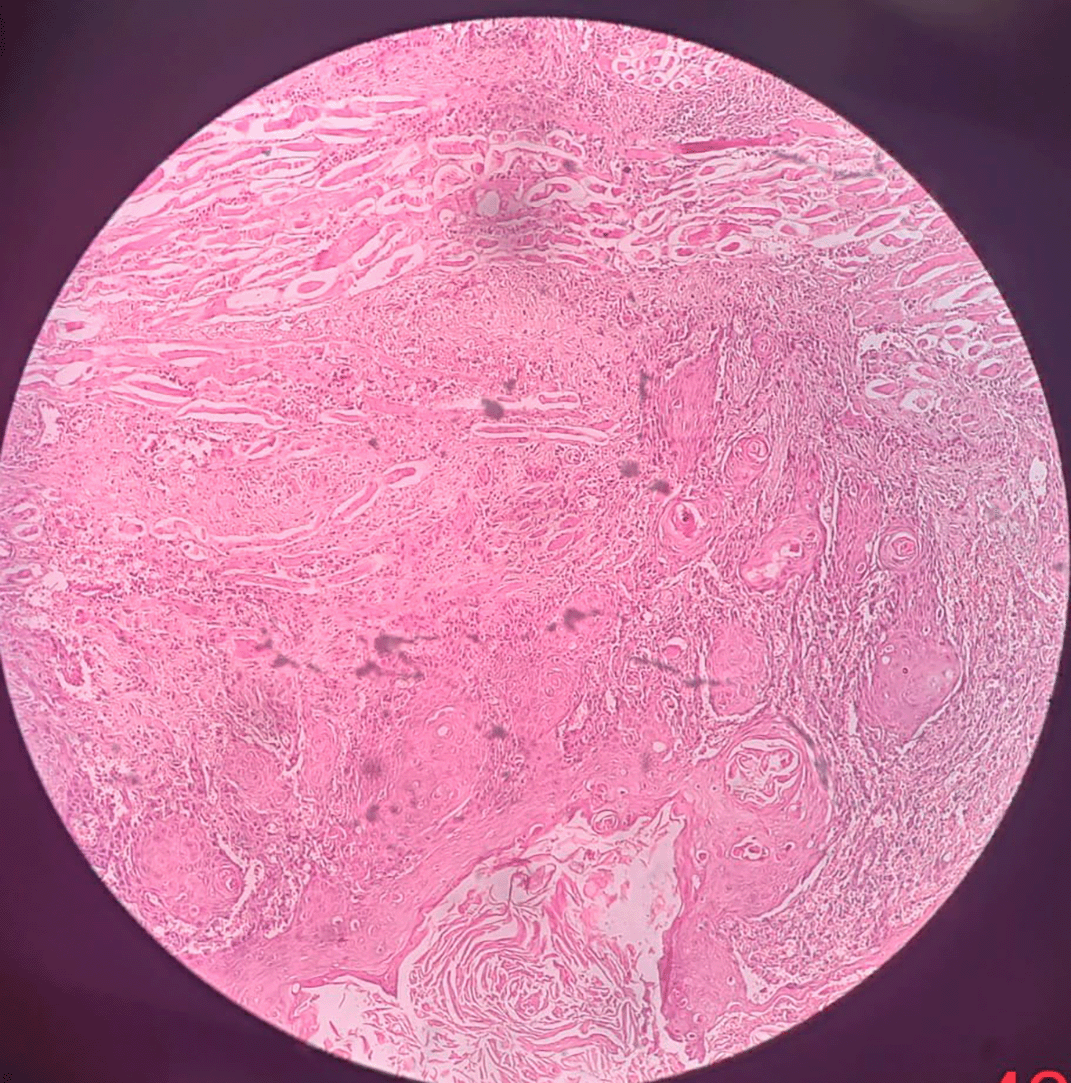
Figure 1. The Hematoxylin and Eosinophilic stained tissue section showing oral squamous cell carcinoma at 100× magnification.
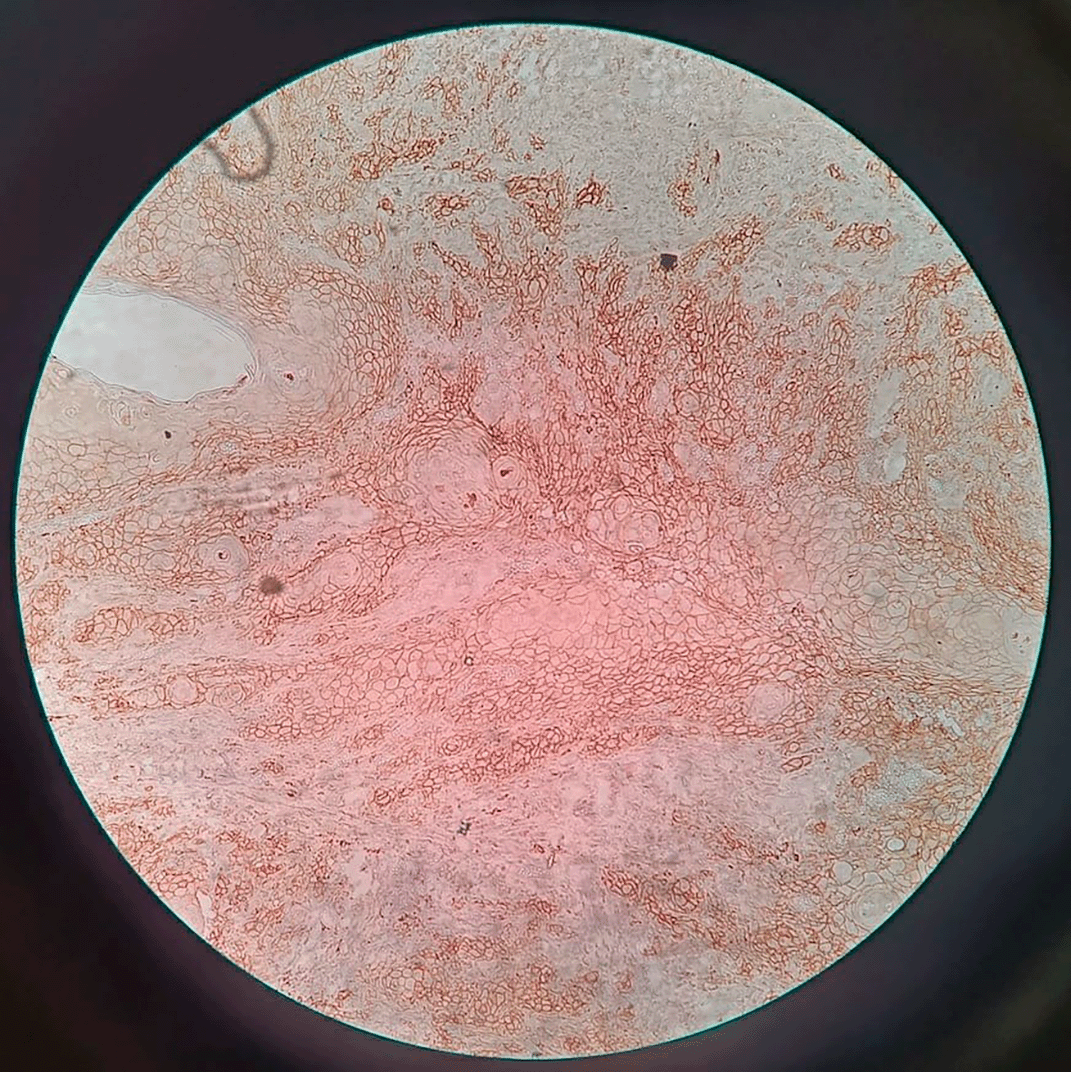
Figure 2. Transmembranous PD-L1 immunostaining in oral squamous cell carcinoma at 100× magnification.
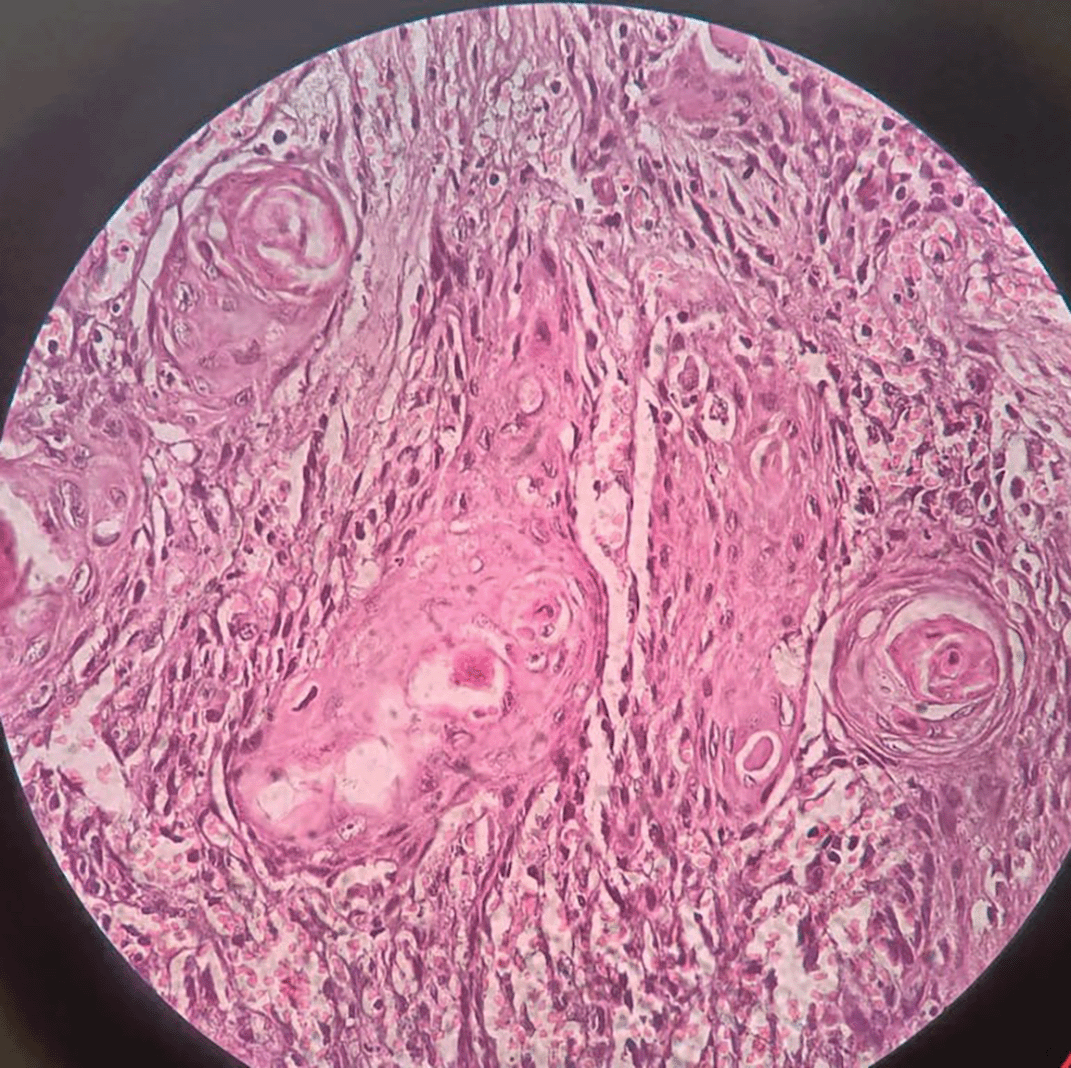
Figure 3. The Hematoxylin and Eosinophilic stained tissue section showing oral squamous cell carcinoma at 400× magnification.
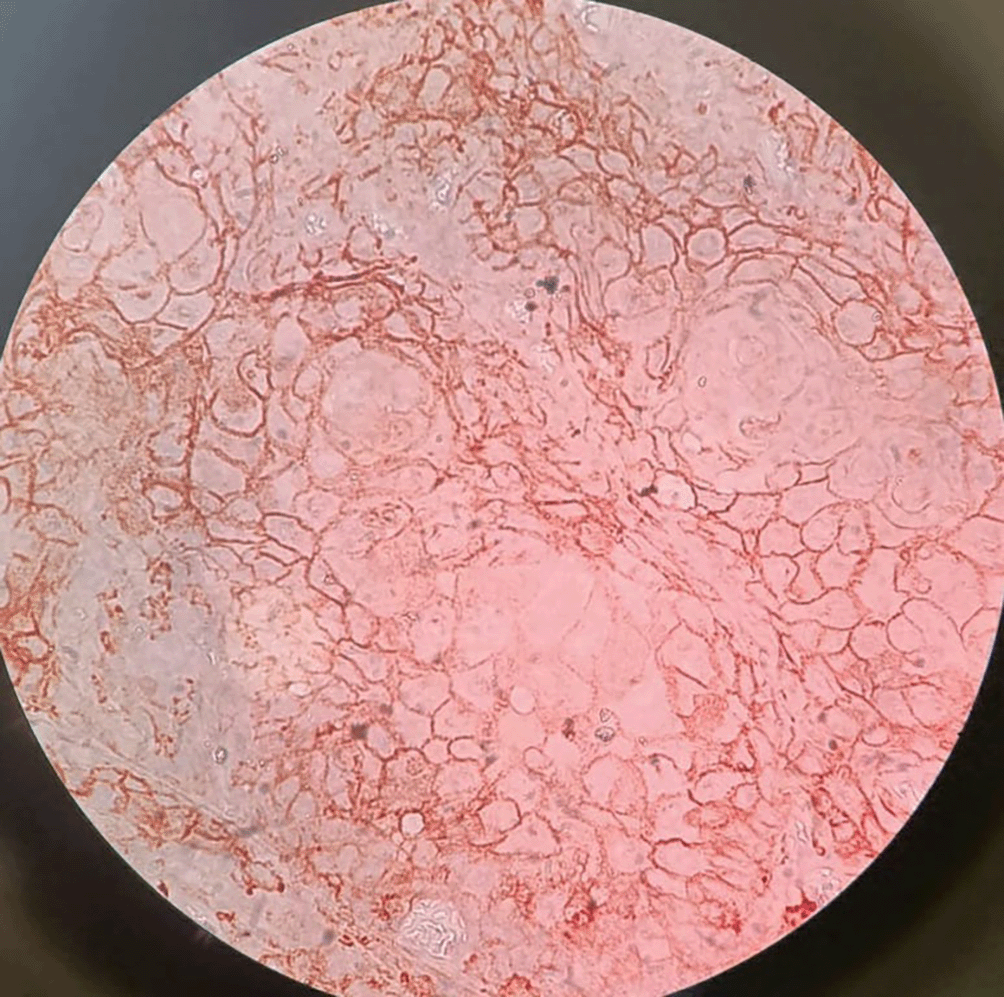
Figure 4. Transmembranous PD-L1 immunostaining in oral squamous cell carcinoma at 400× magnification.
Evaluation of 31 OPMD and 31 OSCC cases were done and results were obtained as follows.
OPMD and OSCC was more prevalent in 4th and 5th decade of life. In OPMD, the age range of patient was 27-60yrs with the mean age of 44.83 ± 10.83. Whereas, In OSCC the age ranging from 27-75 years with the mean age of 48.80 ± 12.1. There was no significant Correlation between PD-L1 score with age of OPMD cases (χ2-value 3.68; P = 0.45) (Table 2) and OSCC cases (χ2-value = 0.009 and P = 0.99) (Table 4).
Table 2. Correlation between PD-L1 score with age in years of OPMD patients.
| PD-L1 Score | 25-40 yrs | 41-55 yrs | >55 yrs | Total | χ2-value |
|---|
| 0% | 3(42.86%) | 4(57.14%) | 0(0%) | 7(22.58%) | 3.68
P = 0.45, NS |
| 1-33% | 5(29.41%) | 7(41.18%) | 5(29.41%) | 17(54.84%) |
| 34-66% | 2(28.57%) | 2(28.57%) | 3(42.86%) | 7(22.58%) |
| 67-100% | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
| Total | 10(32.26%) | 13(41.94%) | 8(25.81%) | 31(100%) |
Table 3. Correlation between PD-L1 score with gender of OPMD patients.
| PD-L1 Score | Male | Female | Total | χ2-value |
|---|
| 0% | 7(100%) | 0(0%) | 7(22.58%) | 3.88
P = 0.14, NS |
| 1-33% | 11(64.71%) | 6(35.29%) | 17(54.84%) |
| 34-66% | 6(85.71%) | 1(14.29%) | 7(22.58%) |
| 67-100% | 0(0%) | 0(0%) | 0(0%) |
| Total | 24(77.42%) | 7(22.58%) | 31(100%) |
Table 4. Correlation between PD-L1 score with age in years of OSCC patients.
| PD-L1 Score | 25-40 yrs | 41-55 yrs | >55 yrs | Total | χ2-value |
|---|
| 0% | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0.009
P = 0.99, NS |
| 1-33% | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
| 34-66% | 4(28.57%) | 6(42.86%) | 4(28.57%) | 14(45.16%) |
| 67-100% | 5(29.41%) | 7(41.18%) | 5(29.41%) | 17(54.84%) |
| Total | 9(29.03%) | 13(41.94%) | 9(29.03%) | 31(100%) |
Males were more frequently affected than female with OPMD and OSCC. In OPMD cases, there were 24(77.4%) males and 7(22.6%) females. In OSCC cases, there were 25(80.6%) males and 6(19.4%) females. However, we found no significant correlation between PD-L1 score and gender of OPMD cases (χ2-value = 3.88 and P value = 0.14) (Table 3) and OSCC cases (χ2-value = 0.07 and P = 0.79) (Table 5).
Table 5. Correlation between PD-L1 score with gender of OSCC patients.
| PD-L1 Score | Male | Female | Total | χ2-value |
|---|
| 0% | 0(0%) | 0(0%) | 0(0%) | 0.07
P = 0.79, NS |
| 1-33% | 0(0%) | 0(0%) | 0(0%) |
| 34-66% | 11(78.57%) | 3(21.43%) | 14(45.16%) |
| 67-100% | 14(82.35%) | 3(17.65%) | 17(54.84%) |
| Total | 25(80.65%) | 6(19.35%) | 31(100%) |
We further evaluated the clinical diagnosis of OPMD and OSCC cases. Among 31 OPMD, 23(74.2%) cases were Leukoplakia and 8(25.8%) were OSMF. A significant correlation was seen between PD-L1 score with clinical Diagnosis of OPMD patients with the χ2-value = 26.08 and P = 0.0001 (Table 6). We have classified the tumor into four categories according to AJCC classification for TNM: stage I, stage II, stage III, stage IV and among 31 cases we found 2(6.5%), 5(16.1%), 7(22.6%), 17(54.8%) patients respectively. Among 31 OSCC patients, in stage I, 2(14.29%) patient showed score 2. In stage II, 3(21.43%) showed score 2 and 2(11.76%) showed score 3. In stage III, 6(42.86%) patients showed score 2 and 1(5.88%) patients showed score 3. In stage IV, 3(21.43%) patients showed score 2 and 14(82.35%) showed score 3. There was significantly positive correlation between PD-L1 score with TNM Staging of OSCC patients with χ2-value = 12.74 and P = 0.005 (Table 7).
Table 6. Correlation between PD-L1 score with clinical diagnosis of OPMD patients.
| PD-L1 Score | Leukoplakia | OSMF | Total | χ2-value |
|---|
| 0% (0) | 0(0%) | 7(22.58%) | 7(22.58%) | 26.08
P = 0.0001, S |
| 1-33% (1) | 16(94.12%) | 1(5.88%) | 17(54.84%) |
| 34-66% (2) | 7(22.58%) | 0(0%) | 7(22.58%) |
| 67-100% (3) | 0(0%) | 0(0%) | 0(0%) |
| Total | 23(74.19%) | 8(25.81%) | 31(100%) |
Table 7. Correlation between PD-L1 score with TNM staging of OSCC patients.
| PD-L1 Score | Stage I | Stage II | Stage III | Stage IV | Total | χ2-value |
|---|
| 0% | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 12.74
P = 0.005, S |
| 1-33% | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
| 34-66% | 2(14.29%) | 3(21.43%) | 6(42.86%) | 3(21.43%) | 14(45.16%) |
| 67-100% | 0(0%) | 2(11.76%) | 1(5.88%) | 14(82.35%) | 17(54.84%) |
| Total | 2(6.45%) | 5(16.13%) | 7(22.58%) | 17(54.84%) | 31(100%) |
In OPMD, we histologically divided the patients into 5 categories i.e. hyperkeratosis, no dysplasia, mild dysplasia, moderate dysplasia and severe dysplasia having 14(45.16%), 8(25.81%), 2(6.45%), 3(9.68%), 4(12.9%) patients in each category respectively. In leukoplakia, 16(94.12%) patients showed score 1 and 7(22.58%) patients showed score 2. In OSMF, 7(22.58%) patients showed Score 0 and 1(5.88%) patients showed score 1. We corelate PD-L1 score with histopathological diagnosis of OPMD and observed, there were 14(82.35%) patients in hyperkeratosis showing score 1. 7(100%) patients showed no dysplasia with score 0 and 1(5.88%) were with score 1. Patients showing mild dysplasia were 2(11.76%) with score 1. In moderate dysplasia there were 3(9.68%) patients in score 2. In severe dysplasia, 4(57.14%) patients showed score 2. A remarkable correlation was observed between PD-L1 score with Histopathological diagnosis of OPMD patients with χ2-value of 56.52 and P-value = 0.0001 (Table 8).
Table 8. Correlation between PD-L1 score with histopathological diagnosis of OPMD patients.
| PD-L1 Score | Hyperkeratosis | No Dysplasia | Mild Dysplasia | Moderate Dysplasia | Severe Dysplasia | Total |
|---|
| 0% | 0(0%) | 7(100%) | 0(0%) | 0(0%) | 0(0%) | 7(22.58%) |
| 1-33% | 14(82.35%) | 1(5.88%) | 2(11.76%) | 0(0%) | 0(0%) | 17(54.84%) |
| 34-66% | 0(0%) | 0(0%) | 0(0%) | 3(42.86%) | 4(57.14%) | 7(22.58%) |
| 67-100% | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
| Total | 14(45.16%) | 8(25.81%) | 2(6.45%) | 3(9.98%) | 4(12.90%) | 31(100%) |
| χ2-value | 56.52, P-value = 0.0001, Significant |
Among 31 OSCC patients, in WDSCC, 5(35.71%) patients showed score 2 and only 1(5.88%) patient showed score 3. In MDSCC, 9(64.29%) patients showed score 2 and majority of patients i.e. 12(70.59%) showed score 3. In PDSCC, all the patients i.e. 4(23.53%) showed score 3. So, we found significantly positive Correlation between PD-L1 score with histopathological diagnosis of OSCC patients with the χ2-value = 6.96 and P = 0.032) (Table 9).
Table 9. Correlation between PD-L1 score with histopathological diagnosis of OSCC patients.
| PD-L1 Score | WDSCC | MDSCC | PDSCC | Total | χ2-value |
|---|
| 0% | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 6.96
P = 0.032, S |
| 1-33% | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
| 34-66% | 5(35.71%) | 9(64.29%) | 0(0%) | 14(45.16%) |
| 67-100% | 1(5.88%) | 12(70.59%) | 4(23.53%) | 17(54.84%) |
| Total | 6(19.35%) | 21(67.74%) | 4(12.90%) | 31(100%) |
According to percentages of stained tumor cells we evaluate PD-L1 score into four categories. (0 = negative; 1 = 1-33%; 2 = 34-66%; 3 = 67-100%). In OPMD cases, we observed 7(22.6%), 17(54.8%), 7(22.6%) showing score 0,1 and 2 respectively. There were 0 cases showing score 3. However, in OSCC cases, we observed 14(45.2%) and 17(54.8%) showing score 2 and 3 respectively. Whereas there were no cases showing score 0 and 1. The χ2 value was 43.33 and both the groups showing significantly positive correlation (P = 0.00001) (Table 10).
Table 10. Distribution of patients in two groups according to their PD-L1 Score.
| PD-L1 Score | OPMD | OSCC | χ2-value |
|---|
| 0% | 7(22.6%) | 0(0%) | 43.33
P = 0.00001, S |
| 1-33% | 17(54.8%) | 0(0%) |
| 34-66% | 7(22.6%) | 14(45.2%) |
| 67-100% | 0(0%) | 17(54.8%) |
| Total | 31(100%) | 31(100%) |
Discussion
Carcinomas represent the commonest form of human cancer. Worldwide, oral carcinomas rank from 6th to 8th as the most prevalent cancers.19 Despite advanced treatment modalities, the overall five-year survival rate of OSCC patients remains unchanged. The prognosis of a tumor does not depend upon a single factor, rather it is multifactorial. The contribution of various factors to the tumor progression decides the outcome of the tumor. Therefore, it is of prime importance to evaluate multiple factors (tumor markers) for correct assessment of the tumor prognosis. A thorough literature review showed a rarity of such a study on OSCC. Hence, this study was designed keeping in mind this need.20
OPMDs are proportionately common with a global prevalence rate of 1–5% showing similar age, site and gender predilections as OSCC. It is observed that oral leukoplakia (OL) is often associated with OSCC. However, the exact rate of malignant transformation of OMPDs is unknown. In the present study, a similar pattern was seen in 77% cases. Surveillance directed to OPMDs could have been an important factor in early detection and diagnosis of OSCC in the population of this study. These results show the importance of considering the chances of development of OSCC in cases of leukoplakia and OSMF. This adds to the need of obtaining biopsy specimens from all lesions from this group.20
In OPMD, the age range of patient was 27-60 yrs with the mean age of 44.83 ± 10.83. According to Graham R. Ogden21 the age range of occurrence of OPMD in population was 11 to ≥50 years and 40% of OPMD cases were seen in 21–30 years of age group. Whereas, In OSCC the age ranging from 27-75 years with the mean age of 48.80 ± 12.12 (χ2-value 0.11; P = 0.94 NS) was there. Our results are similar with that of Hong zhi Quan et al.22 who stated that, OSCC patients were in age range of 33 to 78 years with the mean age of 56 years. The OPMD cases at an earlier age occurrence are more as compared to OSCC and it may convert into OSCC.23 In context to the gender predilection in patients with OPMD and OSCC, the male predominance was observed. These results were in accordance with previous study by Preeti Sharma et al.24 and Shenoi et al.25 Age was not related to “PD-L1” positive (P > 0.05). This outcome was consistent with research by Hui Tang et al.,26 who found no connection between PD-L1 expression and age. On correlation of gender of patents with the “PD-L1” score we found no significant correlation between “PD-L1” score and gender of OPMD cases (χ2-value = 3.88, P = 0.14). “PD-L1” expression was independent of gender of OPMD and OSCC group. Same as our results, Hui Tang et al.27 also found no significant correlation between “PD-L1” expression with age and gender in HNSCC (OR = 1.09, 95% CI: 0.51-2.34; OR = 0.87, 95% CI: 0.56-1.36; OR = 1.23, 95% CI: 0.96–1.56).
We evaluated the Clinical presentation and site of the lesion of OPMD and OSCC patients. In OPMD, 23 (74.2%) cases were Leukoplakia and 8(25.8%) were OSMF, predominantly involving buccal mucosa. In OSCC, the most common involved site was the gingivobuccal sulcus 15(48.38%) followed by buccal mucosa 11(35.48%), alveolar mucosa 4(12.90%) and labial mucosa 1(3.22%). Juana Maria García-Pedrero et al.20 noted that most common involved site was the tongue. In regions like America and Europe, the most common site of OSCC is considered to be the border of tongue.28 In southern Asian countries, the habit of areca nut and tobacco chewing makes the buccal mucosa as an occasional site for OSCC. In a study done by Jainkittivong et al.,29 it was reported that around 50% of their total cases of OSCC were of gingival and alveolar ridge. This could be justified by the association of different etiologic factors and OSCC development in their specific population. In their research done in Nigeria, observed that the most commonly affected sites of OSCC are upper and lower gingiva, followed by the tongue. The most commonly affected OSCC sites were border of tongue and floor of mouth, as reported by majority of the studies which focused on Brazilian and other countries of the west populations.20 The clinical staging of OSCC is determined by the tumor’s size, the involvement of nearby lymph nodes, and the presence or absence of metastases, which is referred to as TNM tumour staging. We have further divided the tumor into 4 main categories according to AJCC classification for TNM: stage I-IV and observed that 2(6.5%) cases were in stage I, 5(16.1%) in stage II, 7(22.6%) in stage III, 17(54.8%) in stage IV. There was significantly positive correlation between “PD-L1” score with TNM Staging of OSCC patients (χ2-value = 12.74; P = 0.005, S). This result is in accordance with Jing He et al.,30 a significant positive correlation was found between expression of “PD-L1” and the TNM staging.
We found significantly positive correlation between “PD-L1” score with histopathological diagnosis of OSCC patients. (χ2-value = 6.96; P = 0.032, S). Similarly, Chen et al.31 found that “PD-L1” was significantly associated with the OSCC pathological grade.
The management of OPMD is related to the histopathological diagnosis which may be range from hyperkeratosis and further with various grades of dysplasia. In OPMD, we observed that 14(45.16%) showed hyperkeratosis, 8(25.81%) showed no dysplasia, 2(6.45%) showed mild dysplasia, 3(9.68%) showed moderate dysplasia and 4(12.9%) showed severe dysplasia. Erythroplakias having histopathologic features of severe dysplasia or carcinoma in situ (Ca in situ), should be excised with a clear margin. The malignant transforming rate of oral leukoplakia (OL) ranges between 1 and 17.5% per year. It is generally managed either by patient follow up or excision of lesion depending on the histopathological evaluation and severity of dysplasia. It is still unclear whether the excision of leukoplakia or erythroplakia prevents the development of OSCC. However, a study done on leukoplakia has documented that 20% cases regress due to the elimination of risk factors. A retrospective study was done comprising of 94 cases of surgically resected leukoplakia and erythroplakia with a mean 6.8 years follow up, along with 175 cases which did not undergo surgical resection of the lesion with mean 5.5 years follow up. The study revealed that 12% cases with surgical resection and 4% cases without resection developed OSCC. This led to a conclusion that surgery does not provide protection against malignant transformation of leukoplakia. In a meta-analysis and study on surgical treatment of leukoplakia (with dysplasia), it was revealed that the risk of developing OSCC reduces by surgical intervention by does not eliminate it completely. Efforts should be made to modify habits leading to OSCC and patient counselling should be done at earliest to reduce the risk of transformation of leukoplakia into malignancy. Cases with risk of malignant transformation should be kept on a regular follow-up. The follow up intervals are however entirely based on clinicians’ subjective assessment of clinical appearance of the disease and reported dysplasia and not evidence based. The first 2 years carry the greatest risk of malignant transformation and around 1% may transform on annual basis. Patients should remain on regular follow-up and re-biopsy of suspected lesions should be done by experienced clinicians if clinically indicated.32,33
In the present study, the histopathologic features of epithelial dysplasia were seen in 30% cases of OPMDs. Mello et al.34 has reported a high proportion of epithelial dysplasia (73%) in OPMDs. Prevention of malignant transformation of the lesion is the main goal for identifying oral pre-cancerous lesions. Regular surveillance of the lesion, change of lifestyle like use of tobacco and alcohol, retinoid or antioxidant treatment and surgery are some of the treatment options for oral precancerous lesions. However, none of these options have shown significance in reducing the malignant transformation in the long-term follow-up studies.
In present study of OSCC patients most of the cases were of moderately differentiated tumors, and around 50% cases were in late cancer stages (stage III/IV). In all patients, 6(19.4%) were WDSCC, 21(67.7%) were MDSCC and 4 (12.9%) were PDSCC.
This study concluded that the host immune system by “PD-L1” expression may be escaped by OPMD lesions on dysplastic cells of epithelium as well as the recruited subepithelial cells. This results in the development of invasive SCC. So to prevent their malignant transformation and to inhibit the “PD-L1’ pathways there are immunological approaches which gives new treatment modalities.35
So according to our results, there is a crucial role of “PD-L1’ in the progression of tumor, the only limitation of the study is the sample size.
We interpreted that there is propensity of OPMD lesions showing more prevalence of “PD-L1” positive expression when we compared it to the normal mucosa. But in contrast it shows lower PD-L1 positivity than OSCC. This shows a condition where there are biological adaptations has been confirmed already which directed towards malignant transformation. Though there is still control of the immune the system. So, we can note that the premalignant lesions show the intermediate “PD-L1” expression which lie between the normal or hyperplastic lesions to the OSCC. As expected, researches which very clearly differentiate between the hyperplastic lesion, low grade dysplasia and high grade dysplastic lesions had not evaluated any significant difference amongst all these kind of lesions.30,36
Acknowledgements
I acknowledge the support of Research House Team, Dr. S.Z. Quazi, Director Research and Development, DMIHER.
References
- 1.
Ghapanchi J, Ranjbar Z, Mokhtari MJ, et al.:
The LncRNA H19 rs217727 Polymorphism Is Associated with Oral Squamous Cell Carcinoma Susceptibility in Iranian Population.
Biomed. Res. Int.
2020 Apr 2; 2020: 1–6. Publisher Full Text
- 2.
Sung H, Ferlay J, Siegel RL, et al.:
Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries.
CA Cancer J. Clin.
2021 May; 71(3): 209–249. PubMed Abstract
| Publisher Full Text
- 3.
Chai AWY, Lim KP, Cheong SC:
Translational genomics and recent advances in oral squamous cell carcinoma.
Semin. Cancer Biol.
2020 Apr; 61: 71–83. PubMed Abstract
| Publisher Full Text
- 4.
Global Burden of Disease Cancer CollaborationFitzmaurice C, Abate D, et al.:
Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study.
JAMA Oncol.
2019 Dec 1; 5(12): 1749–1768. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 5.
Ling Z, Cheng B, Tao X:
Epithelial-to-mesenchymal transition in oral squamous cell carcinoma: Challenges and opportunities.
Int. J. Cancer.
2021 Apr 1; 148(7): 1548–1561. PubMed Abstract
| Publisher Full Text
- 6.
Caruntu A, Caruntu C:
Recent Advances in Oral Squamous Cell Carcinoma.
J. Clin. Med.
2022 Oct 29; 11(21): 6406. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 7.
Grafton-Clarke C, Chen KW, Wilcock J:
Diagnosis and referral delays in primary care for oral squamous cell cancer: a systematic review.
Br. J. Gen. Pract.
2019 Feb; 69(679): e112–e126. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 8.
Caruntu A, Scheau C, Tampa M, et al.:
Complex Interaction Among Immune, Inflammatory, and Carcinogenic Mechanisms in the Head and Neck Squamous Cell Carcinoma.
Adv. Exp. Med. Biol.
2021; 1335: 11–35. PubMed Abstract
| Publisher Full Text
- 9.
Aittiwarapoj A, Juengsomjit R, Kitkumthorn N, et al.:
Oral Potentially Malignant Disorders and Squamous Cell Carcinoma at the Tongue: Clinicopathological Analysis in a Thai Population.
Eur. J. Dent.
2019 Jul; 13(3): 376–382. PubMed Abstract
| Publisher Full Text
- 10.
Akhtar M, Rashid S, Al-Bozom IA:
PD− L1 immunostaining: what pathologists need to know.
Diagn. Pathol.
2021 Dec; 16(1): 1–2. Publisher Full Text
- 11.
Tekade SA, Chaudhary MS, Tekade SS, et al.:
Early Stage Oral Submucous Fibrosis is Characterized by Increased Vascularity as Opposed to Advanced Stages.
J. Clin. Diagn. Res. JCDR.
2017 May; 11(5): ZC92–ZC96. PubMed Abstract
| Publisher Full Text
- 12.
Planes-Laine G, Rochigneux P, Bertucci F, et al.:
PD-1/PD-L1 Targeting in Breast Cancer: The First Clinical Evidences are Emerging—A Literature Review.
Cancers.
2019 Jul 22; 11(7): 1033. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 13.
de Vicente JC , Rodríguez-Santamarta T, Rodrigo JP, et al.:
PD-L1 Expression in Tumor Cells Is an Independent Unfavorable Prognostic Factor in Oral Squamous Cell Carcinoma.
Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol.
2019 Mar; 28(3): 546–554. PubMed Abstract
| Publisher Full Text
- 14.
Deliverska E, Forghani P, Parusheva S:
Evaluation of programmed death 1 (PD-1) and programmed death ligand-1 (PD-L1) as tumor biomarkers an in patients with head and neck squamous cell carcinoma.
J. Med. Dent. Pract.
2020 Jun 4; 7(2): 1198–1202. Publisher Full Text
- 15.
Crosta S, Boldorini R, Bono F, et al.:
PD-L1 testing and squamous cell carcinoma of the head and neck: a multicenter study on the diagnostic reproducibility of different protocols.
Cancers.
2021 Jan 14; 13(2): 292. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 16.
Lenouvel D, González-Moles MÁ, Talbaoui A, et al.:
An update of knowledge on PD-L1 in head and neck cancers: Physiologic, prognostic and therapeutic perspectives.
Oral Dis.
2020 Apr; 26(3): 511–526. PubMed Abstract
| Publisher Full Text
- 17.
Meng X, Huang Z, Teng F, et al.:
Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy.
Cancer Treat. Rev.
2015 Dec; 41(10): 868–876. Publisher Full Text
- 18.
Kujan O, van Schaijik B , Farah CS:
Immune Checkpoint Inhibitors in Oral Cavity Squamous Cell Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review.
Cancers.
2020 Jul 17; 12(7): 1937. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 19.
Marocchio LS, Lima J, Sperandio FF, et al.:
Oral squamous cell carcinoma: an analysis of 1,564 cases showing advances in early detection.
J. Oral Sci.
2010 Jun; 52(2): 267–273. PubMed Abstract
| Publisher Full Text
- 20.
García-Pedrero JM, Martínez-Camblor P, Diaz-Coto S, et al.:
Tumor programmed cell death ligand 1 expression correlates with nodal metastasis in patients with cutaneous squamous cell carcinoma of the head and neck.
J. Am. Acad. Dermatol.
2017 Sep; 77(3): 527–533. PubMed Abstract
| Publisher Full Text
- 21.
Ogden GR:
Alcohol and oral cancer.
Alcohol Fayettev N.
2005 Apr; 35(3): 169–173. Publisher Full Text
- 22.
Quan H, Liu S, Shan Z, et al.:
Differential expression of programmed death-1 and its ligand, programmed death ligand-1 in oral squamous cell carcinoma with and without oral submucous fibrosis.
Arch. Oral Biol.
2020 Nov; 119: 104916. PubMed Abstract
| Publisher Full Text
- 23.
Kadashetti V, Shivakumar KM, Chaudhary M, et al.:
Influence of risk factors on patients suffering from potentially malignant disorders and oral cancer: A case-control study.
J. Oral Maxillofac. Pathol. JOMFP.
2017; 21(3): 455–456. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 24.
Sharma P, Saxena S, Aggarwal P:
Trends in the epidemiology of oral squamous cell carcinoma in Western UP: an institutional study.
Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res.
2010; 21(3): 316–319. Publisher Full Text
- 25.
Shenoi R, Devrukhkar V, Chaudhuri, et al.:
Demographic and clinical profile of oral squamous cell carcinoma patients: a retrospective study.
Indian J. Cancer.
2012; 49(1): 21–26. Publisher Full Text
- 26.
Tang H, Zhou X, Ye Y, et al.:
The different role of PD-L1 in head and neck squamous cell carcinomas: A meta-analysis.
Pathol. Res. Pract.
2020 Jan; 216(1): 152768. PubMed Abstract
| Publisher Full Text
- 27.
Lin YM, Sung WW, Hsieh MJ, et al.:
High PD-L1 Expression Correlates with Metastasis and Poor Prognosis in Oral Squamous Cell Carcinoma.
PLoS One.
2015; 10(11): e0142656. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 28.
Ohaegbulam KC, Assal A, Lazar-Molnar E, et al.:
Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway.
Trends Mol. Med.
2015 Jan 1; 21(1): 24–33. Publisher Full Text
- 29.
Jainkittivong A, Swasdison S, Thangpisityotin M, et al.:
Oral Squamous Cell Carcinoma: A Clinicopathological Study of 342 Thai Cases.
J. Contemp. Dent. Pract.
2009 Sep 1; 10: 33–41. Publisher Full Text
- 30.
He J, Chen XF, Xu MG, et al.:
Relationship of programmed death ligand-1 expression with clinicopathological features and prognosis in patients with oral squamous cell carcinoma: A meta-analysis.
Arch. Oral Biol.
2020 Jun; 114: 104717. PubMed Abstract
| Publisher Full Text
- 31.
Chen XJ, Tan YQ, Zhang N, et al.:
Expression of programmed cell death-ligand 1 in oral squamous cell carcinoma and oral leukoplakia is associated with disease progress and CD8+ tumor-infiltrating lymphocytes.
Pathol. Res. Pract.
2019 Jun; 215(6): 152418. Publisher Full Text
- 32.
Sathiyasekar AC, Chandrasekar P, Pakash A, et al.:
Overview of immunology of oral squamous cell carcinoma.
J. Pharm. Bioallied Sci.
2016 Oct; 8(Suppl 1): 8–12. Publisher Full Text
- 33.
Gatti RA, Good RA:
Aging, immunity, and malignancy.
Geriatrics.
1970 Sep; 25(9): 158–168. PubMed Abstract
- 34.
Mello FW, Miguel AFP, Dutra KL, et al.:
Prevalence of oral potentially malignant disorders: A systematic review and meta-analysis.
J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol.
2018 Aug; 47(7): 633–640. Publisher Full Text
- 35.
Deshmukh V, Shekar K:
Oral Squamous Cell Carcinoma: Diagnosis and Treatment Planning.
Oral Maxillofac. Surg. Clin.
2021; 1853–1867. Publisher Full Text
- 36.
Yoshida S, Nagatsuka H, Nakano K, et al.:
Significance of PD-L1 Expression in Tongue Cancer Development.
Int. J. Med. Sci.
2018 Nov 22; 15(14): 1723–1730. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 37.
Durge S:
Association of PD-L1 immunoexpression with the clinicopathological characteristics and its prognostic significance in OPMD and OSCC. [Data set].
Zenodo.
2023. Publisher Full Text




Comments on this article Comments (0)