Keywords
Neuroblastoma, oncolytic virotherapy, Zika virus, transcriptomics, proteomics
This article is included in the Oncology gateway.
Paediatric neuroblastoma and brain tumours account for a third of all childhood cancer-related mortality. High-risk neuroblastoma is highly aggressive and survival is poor despite intensive multi-modal therapies with significant toxicity. Novel therapies are desperately needed. The Zika virus (ZIKV) can access the nervous system and there is growing interest in employing ZIKV as a potential therapy against paediatric nervous system tumours, including neuroblastoma.
Here, we perform extensive data mining, integration and re-analysis of ZIKV infection datasets to highlight molecular mechanisms that may govern the oncolytic response in neuroblastoma cells. We collate infection data of multiple neuroblastoma cell lines by different ZIKV strains from a body of published literature to inform the susceptibility of neuroblastoma to the ZIKV oncolytic response. Integrating published transcriptomics, interaction proteomics, dependency factor and compound datasets we propose the involvement of multiple host systems during ZIKV infection.
Through data mining of published literature, we observed most paediatric neuroblastoma cell lines to be highly susceptible to ZIKV infection and propose the PRVABC59 ZIKV strain to be the most promising candidate for neuroblastoma oncolytic virotherapy. ZIKV induces TNF signalling, lipid metabolism, the Unfolded Protein Response (UPR), and downregulates cell cycle and DNA replication processes. ZIKV infection is dependent on sterol regulatory element binding protein (SREBP)-regulated lipid metabolism and three protein complexes; V-ATPase, ER Membrane Protein Complex (EMC) and mammalian translocon. We propose ZIKV non-structural protein 4B (NS4B) as a likely mediator of ZIKVs interaction with IRE1-mediated UPR, lipid metabolism and mammalian translocon.
Our work provides a significant understanding of ZIKV infection in neuroblastoma cells, which will facilitate the progression of ZIKV-based oncolytic virotherapy through pre-clinical research and clinical trials.
Neuroblastoma, oncolytic virotherapy, Zika virus, transcriptomics, proteomics
Higher resolution figures added and now Figures 1- 8
More clarity around the approach used in the paper to synthesize results from multiple studies has been added
Table 1 and 2 have been edited to provide more information about the underlying studies used in the paper
See the authors' detailed response to the review by Griffith D Parks
See the authors' detailed response to the review by Thomas C. N. Leung
See the authors' detailed response to the review by Estanislao Nistal-Villan and Vicent Tur-Planells
The ability to both induce direct oncolysis and provoke an anti-tumoral immune response makes oncolytic virotherapy an attractive candidate to combat aggressive and heterogenous cancers, such as high-risk neuroblastoma. To progress oncolytic virotherapy to clinical trial it is essential to understand the host mechanisms the virus manipulates to kill cancer cells, alongside any pathology as a consequence of infection of normal cells. Through data mining and re-analysing published data we observed that ZIKV efficiently infects and induces oncolysis of paediatric neuroblastoma cells and we propose a potential TNF pathway-driven immune response. ZIKV’s specificity for infection of nervous system cancer cells, while rarely causing nervous system-related pathology in young children, addresses many of its safety concerns. The inclusion of more effective and less toxic novel therapies, such as a potential ZIKV-based therapeutic, in multimodal treatment regimens will pave the way for improving patient long-term health and overall survival.
Neuroblastoma is the most common extracranial solid cancer in children, accounting for 6–10% of all paediatric cancers and disproportionately causing 12–15% of paediatric cancer-related deaths.1 It is an embryonal tumour originating from transformed cells of neural crest lineage and predominately forms tumours in the adrenal medulla and paraspinal sympathetic ganglia. Whilst the majority of patients are diagnosed by the age of 5 years, the median age of patients is 18 months. Prognosis is highly heterogenous and can be predicted by a number of factors, including the presence of metastatic disease, age, chromosomal aberrations and molecular signatures, such as MYC-N amplification.2 Patients are categorised according to internationally agreed risk groups (INRG), and treatment is stratified accordingly.
Outcome for low- and intermediate-risk neuroblastoma is good, with some patients requiring little or no treatment. However, approximately 50% of patients have high-risk disease, for which prognosis is poor, with overall survival of less than 60%.3 Current high-risk neuroblastoma treatment regimens are aggressive. These include multiple rounds of induction chemotherapy, surgical resection, myeloablative chemotherapy, autologous stem cell transplantation and post-consolidation therapy such as immunotherapy.4 The aggressive nature of this regimen carries significant treatment-related mortality and frequently results in long-term toxicities and sequelae impacting the quality of life for surviving patients. Consequently, there is a clear and unmet need for safer and less toxic treatment regimens to combat high-risk neuroblastoma.
Oncolytic virotherapy exploits viruses that preferentially infect and destroy cancer cells via two distinct routes of therapeutic action. Following infection, intense viral replication induces oncolysis, releasing virions into the tumour microenvironment to infect neighbouring tumour cells. Induction of a tumour-specific immune response is a crucial secondary mechanism employed by oncolytic virotherapy that can address highly heterogeneous tumours such as high-risk neuroblastoma and central nervous system (CNS) tumours. There is significant interest in combining immuno-modulating cancer therapies with oncolytic virotherapy to augment the anti-tumoral immune response. Oncolytic virotherapy clinical studies have in general reported low toxicity and minimal adverse effects in patients, mainly low-grade constitutional symptoms.5
Zika virus (ZIKV) is a mosquito-borne flavivirus consisting of historical African and epidemic-associated Asian lineages. The latter can access the central nervous system and may cause microcephaly in the developing foetus through infection of neural stem and progenitor cells, causing cell death and growth reduction.6,7 By contrast, ZIKV rarely causes adverse effects in children and adults, with the majority of cases (50–80%) being asymptomatic.8 In symptomatic children, ZIKV may cause short-term side effects, namely rash, fever and gastrointestinal symptoms, and in rare instances in adults can cause more severe conditions, such as Guillain-Barré Syndrome, meningitis and encephalitis.8,9
Since 2017, the concept of employing the ZIKV as oncolytic virotherapy against brain tumours has gained momentum. ZIKV induces an oncolytic event in infected paediatric brain tumour cells in vitro and in vivo assays and induces an immune response against spontaneous canine brain tumours.10–12 An initial study assessing ZIKV infection in multiple neuroblastoma cell lines demonstrated ZIKV’s potential as a novel neuroblastoma oncolytic virotherapy.13 Here, we survey over 35 studies that have used neuroblastoma cell lines to model ZIKV infection. These studies focused on understanding ZIKV pathology and assessing anti-viral compounds. Through re-analysis and integration of the transcriptomics, proteomics and dependency factor screens from these studies, we propose multiple molecular mechanisms to be implicated in ZIKV infection of neuroblastoma which help to determine its potential as oncolytic virotherapy.
RNA-Seq data files (.fastq.gz paired-end) were acquired from the European Nucleotide Archive (ENA) (accession: PRJNA630088). Bonenfant et al., generated this data by infecting SH-SY5Y cells in monolayer culture with ZIKV PRVABC59 at MOI 5 for 1 hour and collected RNA at 24hpi vial TRIzol extraction.14 RNA-seq libraries were prepared and sequenced using an Illumina NextSeq500. Our RNA-Seq processing pipeline consisted of FastQC (V0.11.9-0) (RRID:SCR_014583),15,16 Trim Galore (V0.6.6-0) (RRID:SCR_011847), HISAT2 (V2.2.0) (RRID:SCR_015530),17 SAMTOOLS (V1.11) (RRID:SCR_002105)18 and Subread (V2.0.1) (RRID:SCR_009803).19 Reads were aligned against the Homo sapiens GRCh38 genome.
Differential gene expression analysis was performed using DESeq2 (RRID:SCR_015687)20 to compare the ZIKV-infected SH-SY5Y cells versus the non-infected control cells at 24hpi (n = 3). Differentially expressed genes (DEGs) were plotted on bar charts, volcano and scatter plots using GraphPad Prism (9.2.0) (RRID:SCR_002798). DEGs (padj < 0.05, fold change > 1.5) were submitted to Database for Annotation, Visualization and Integrated Discovery (DAVID) (DAVID 2021 (Dec. 2021)) (RRID:SCR_001881)21,22 as official gene symbols for Gene Ontology (GO) (RRID:SCR_002811) (Biological Process Direct),23,24 Kyoto Encyclopedia of Genes and Genomes (KEGG) (RRID:SCR_012773)25–27 and Reactome (RRID:SCR_003485) pathway analysis. ZIKV-induced DEGs were mapped onto Pathview (RRID:SCR_002732).28 Significance values of DEG and pathway analysis were corrected for multiple testing using the Benjamini and Hochberg method (padj < 0.05).
We sourced 130 and five high-confidence interactions of ZIKV NS4B and NS2B-3, respectively, in SK-N-BE2 paediatric neuroblastoma cells from IMEx - The International Molecular Exchange Consortium (RRID:SCR_002805) (IM-26452).29 Scaturro et al., produced this interactome through stable expression of HA-tagged ZIKV proteins in SK-N-BE2 cells, isolation of ZIKV-host protein complexes through HA-affinity purifications, then sample preparation and run for LC–MS/MS (n = 4).30 Raw data was processed using MaxQuant with Andromeda search engine. High-confidence interactions were determined by Bayesian statistical modelling, with log2(fold change) ≥ 2.5; unadjusted one-sided P ≤ 0.05.
The viral-host interactome was submitted to STRING (RRID:SCR_005223)31 for high confidence (0.7) evidence-based physical subnetwork analysis to identify host-host interactions. We integrated and mapped the viral-host and STRING derived host-host interactions in Cytoscape (3.9.1) (RRID:SCR_003032)32 to identify the interaction of ZIKV NS4B with host protein complexes. ZIKV NS4B host interaction partners were also submitted to DAVID to identify the interaction between NS4B and host pathways. ZIKV dependency factors were integrated into Cytoscape map to highlight known protein interaction of ZIKV dependency factors with ZIKV proteins.
Extensive data mining revealed there are currently only 22 known ZIKV dependency factors in neuroblastoma cells, identified via a shRNA screen in paediatric SK-N-BE2 neuroblastoma cells.30 In an attempt to supplement this limited pool of ZIKV dependency factors, we sourced known factors from genome-wide CRISPR/Cas9 screens performed in glioma stem cells (GSCs),33 hiPSC-NPC,34 HEK293FT33 and HeLa cells.35 All can ZIKV dependency factors are shown in Table 4. A combination of DAVID GO and pathway analysis, STRING interaction and literature mining approaches were employed to identify relationships between host factors to inform dependencies of ZIKV on host protein complexes and cell machinery.
ZIKV infects and significantly reduces the cell viability of a multitude of neuroblastoma cell lines from both primary tumour and metastatic sites (Table 1). ZIKV can significantly reduce neuroblastoma cell viability at multiplicity of infection (MOI) as low as 0.001.36 The cell viability of 11/15 neuroblastoma cell lines is significantly reduced to approximately 20% or less following ZIKV infection and these observations are apparent despite the differences in the cell line, ZIKV strain, viral MOI and the type of assay performed (Table 1). SK-N-BE1 and SK-N-BE2 cells are from bone marrow metastasis from the same patient before and after treatment, respectively, and are both highly susceptible to ZIKV. SK-N-AS, T-268 and JFEN are highly resistant (cell viability >80%) to ZIKV infection. Susceptibility is independent of patient sex, cell line origin, morphology and MYC-N status (Table 1). The non-sympathetic nervous system and non-paediatric origin of the T-268 and JFEN cells likely explain their resistance to ZIKV infection, as ZIKV has a tropism for paediatric nervous system cancer cells.11 The resistance of the paediatric SK-N-AS cell line is governed by CD24 expression, which regulates the basal antiviral state of these cells.13,37 Whilst LA-N-6 shows partial resistance to ZIKV infection (Table 1), analysis of bulk mRNA and protein show cell LA-N-6 to express CD24.13 Potential reasoning for this partial resistance is that subpopulations within LA-N-6 may be CD24- or a CD24-independent mechanism may be employed to infer resistance. From Table 1 we conclude ZIKV to be a promising oncolytic virotherapy candidate to employ against paediatric neuroblastoma since it can target neuroblastoma cells originating from the primary tumour, metastatic sites, and metastatic sites that are resistant to standard neuroblastoma therapy.
Cell lines are ranked by the degree to which ZIKV infection reduces their cell viability. Cell viability assay reagent/marker used in the original assay is stated with its accompanying reference. Note: ZIKV, Zika virus; MYCN, MYCN Proto-Oncogene; NA, not applicable.
| Cell line | Cell viability | Viability Reagent/Marker | Patient Age/Sex | Cancer type | Cell line origin | Morphology | MYCN status |
| SH-SY5Y | <20% | Annexin V,63 Annexin V/7-AAD,68 Eosin-Y69,70 | 4/F | Neuroblastoma | Bone marrow metastasis (thorax) | Epithelial | non-amplified |
| SK-N-SH | <20% | Reliablue,39 Giemsa71 | 4/F | Neuroblastoma | Bone marrow metastasis (thorax) | Epithelial | non-amplified |
| SK-N-BE2 | <20% | Eosin-Y69,70 | 2/M | Neuroblastoma | Bone marrow metastasis | Neuroblast | amplified |
| SK-N-BE2-M17 | <20% | Eosin-Y70 | 2/M | Neuroblastoma | Bone marrow metastasis | Neuroblast | amplified |
| SK-N-DZ | <20% | Eosin-Y69,70 | 2/F | Neuroblastoma | Bone marrow metastasis | Epithelial | amplified |
| IMR-32 | <20% | MTS,13 Eosin-Y69,70 | 1/M | Neuroblastoma | Abdominal mass primary tumour | Neuroblast, Fibroblast | amplified |
| SMS-KAN | <20% | MTS13 | 3/F | Neuroblastoma | Pelvic primary tumour | Neuroblast | amplified |
| SMS-KCNR | <20% | Eosin-Y69 | 1/M | Neuroblastoma | Bone marrow metastasis (adrenal) | Neuroblast | amplified |
| SK-N-FI | <20% | Eosin-Y69 | 11/M | Neuroblastoma | Bone marrow metastasis | Epithelial | non-amplified |
| CHLA-42 | <20% | MTS13 | 1/NA | Neuroblastoma | Bone marrow metastasis | Epithelial | non-amplified |
| SK-N-BE1 | ~20% | MTS13 | 2/M | Neuroblastoma | Bone marrow metastasis | Neuroblast | amplified |
| LA-N-6 | ~60% | MTS13 | 5/M | Neuroblastoma | Bone marrow metastasis (adrenal) | Neuroblast | non-amplified |
| SK-N-AS | >80% | MTS13 | 6/F | Neuroblastoma | Bone marrow metastasis (adrenal) | Epithelial | non-amplified |
| T-268 | >80% | Eosin-Y69 | 22/F | Olfactory neuroblastoma | Metastasis (paraspinal mass) | NA | NA |
| JFEN | >80% | Eosin-Y69 | 22/M | Olfactory neuroblastoma | Metastasis (chest wall) | NA | NA |
Independent studies have demonstrated inherent differences in the ability of varying ZIKV strains to infect, replicate, and kill neuroblastoma cells.38,39 Here, we assess published data concerning ZIKV infection of neuroblastoma cells and ranked the viral strains based on their ability to infect neuroblastoma cells, produce fresh viral progeny and reduce cell viability (Table 2). Data mining showed the PRVABC59 Asian and Uganda #976 African strains as the top two candidates (Table 2). The PRVABC59 Asian strain induces significantly more DEGs and splice events of immune and inflammatory response genes in SH-SY5Y cells compared to the African MR766 strain, which has 99.95% sequence identity to Uganda #976.14 Brain metastases develop in 5–11% of patients with neuroblastoma and are correlated with poor prognosis.40 The ability of the Asian lineage to access the brain may enhance the therapeutic potential of ZIKV by targeting these brain metastases. Population level data has shown the epidemic Asian ZIKV lineage to rarely cause anything other than mild symptoms in children and adolescents, thus providing evidence for the safety of employing an Asian strain.41 Consequently, from those tested to date, we propose that the PRVABC59 Asain strain holds the greatest promise for development as oncolytic virotherapy against paediatric neuroblastoma.
ZIKV strains are ranked by their ability to infect (Degree of Infection), replicate within (Viral Titer) and significantly reduce the cell viability (Cell Viability) of a multitude of neuroblastoma cells. The Data accordance is a qualitative measure which we employed to describe the degree of similarity of the results between publications that performed ZIKV infection assays of neuroblastoma cells using the same ZIKV strain. Data accordance of five denotes that the findings of one publication closely support the findings from another, a data accordance of one denotes publications reporting vastly contrasting results. When a viral strain is published in only one paper, it is allocated a data accordance of NA. ZIKV, Zika virus; NA, not applicable.
| ZIKV strain | Lineage | Cell Viability | Viability Reagent/Marker | Degree of infection | Viral titer | MOI Range | Data accordance | Infection Conditions |
| PRVABC59 | Asian | <20% | MTS13, Eosin-Y69 | >80% | >107 per ml | 0.5-10 | 5 | All cell lines in Table 1, excluding SK-N-BE2-M17.13,69,72 |
| Uganda #976 | African | <20% | Reliablue39 | >60% | 106-107 per ml | 0.01-10 | 4 | SH-SY5Y51, SK-N-SH38,39 |
| Brazil PE/243 | Asian | 20-40% | MTT46, Annexin V/7-AAD68 | >60% | >107 per ml | 0.5-10 | 4 | SH-SY5Y46,68,73 |
| MR766 | African | 20-40% | Annexin V63, Giemsa71 | >60% | >107 per ml | 0.01-10 | 3 | SH-SY5Y56,63,74,75, SK-N-SH38,71,72,76 |
| SZ01/2016/China | Asian | <20% | Annexin V63 | NA | >107 per ml | 1 | NA | SH-SY5Y63 |
| French Polynesia/2013 | Asian | 20-40% | Reliablue39 | >20% | 106-107 per ml | 0.01-10 | 4 | SH-SY5Y51,77, SK-N-SH38,39 |
| HS-2015-BA-01 | Asian | 40-60% | WST-178 | NA | >107 per ml | 0.01-1 | 4 | SH-SY5Y36,78 |
| Paraiba/2015 | Asian | 20-40% | Giemsa71 | NA | 106-107 per ml | 0.1-10 | 2 | SK-N-SH71,79 |
| BR/800/16 Brazil 2016 | Asian | 40-60% | Reliablue39 | >20% | 106-107 per ml | 0.1-1 | NA | SK-N-SH39 |
| PLCal_ZV | Asian | >80% | WST-178 | NA | <104 per ml | 0.01-1 | NA | SH-SY5Y78 |
Differential gene expression analysis identifies 453 and 256 significantly upregulated and downregulated genes (fold change > 1.5), respectively, in ZIKV-infected paediatric neuroblastoma SH-SY5Y cells (Figure 1A). GO, Reactome and KEGG pathway analysis identifies nine significantly upregulated and 12 significantly downregulated terms (Figure 1B-C). Upregulated processes include “TNF signalling pathway”, lipid metabolism (“Cholesterol biosynthesis”, “Cholesterol biosynthetic process”, “Activation of gene expression by SREBF (SREBP)”), endoplasmic reticulum (ER) stress (“Response to endoplasmic reticulum stress”, “XBP1(S) activates chaperone genes”) and transcription (“BMAL1:CLOCK, NPAS2 activates circadian gene expression”, “Positive regulation of transcription from RNA polymerase II promoter”). The downregulated terms are predominantly cell cycle- and DNA replication-related processes and this downregulation is apparent when the “Cell Cycle” KEGG pathway is plotted for all DEGs (fold change > 0) (Figure 2B). A potential explanation for this observation is that ZIKV can disrupt the cell cycle by targeting the centrioles in neuroblastoma cells.42
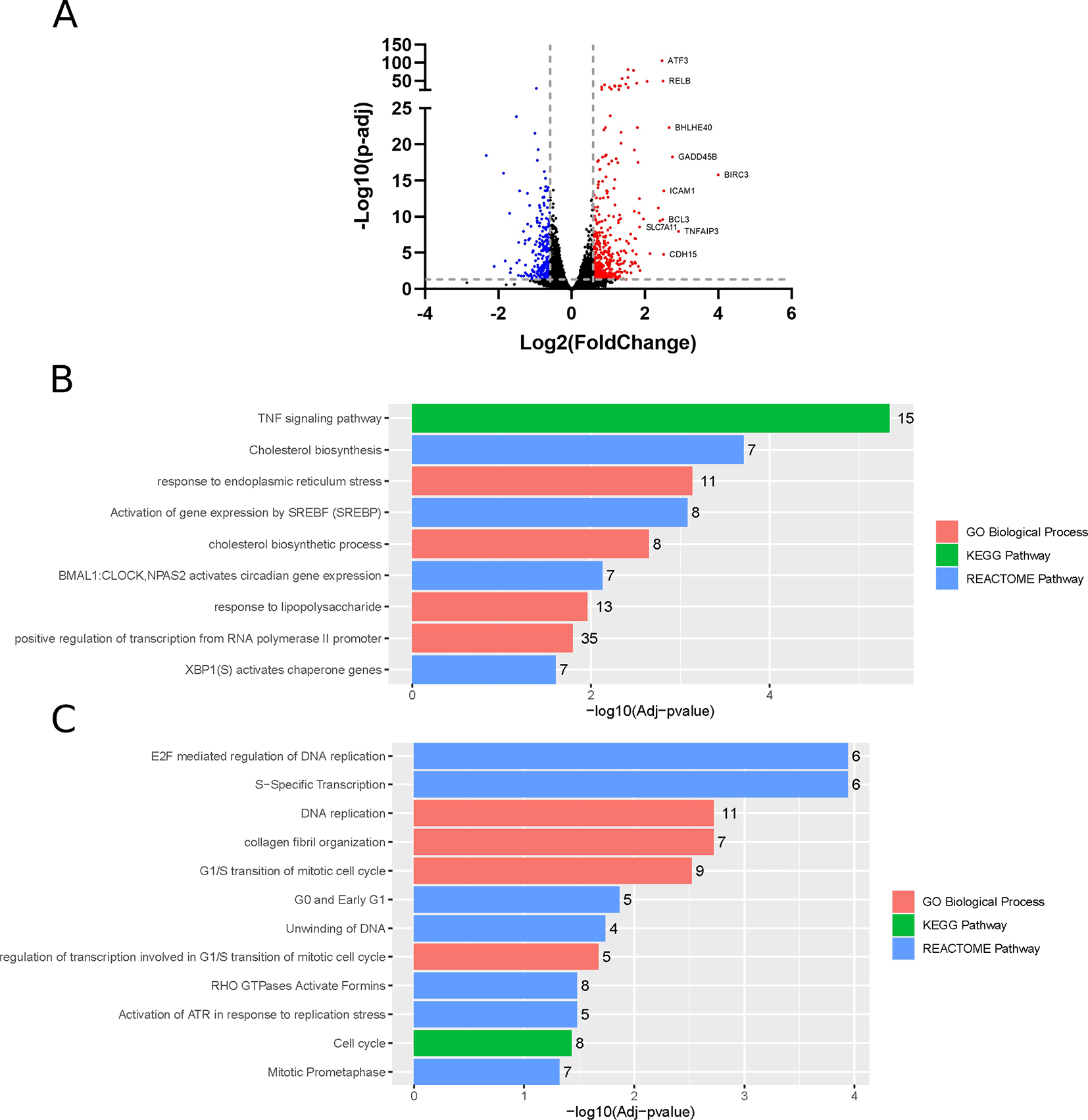
Volcano plot of genes differentially expressed in response to ZIKV infection of SH-SY5Y cells, with the top 10 upregulated genes labelled (A). Significantly upregulated (B) and downregulated (C) GO Biological Processes, KEGG and Reactome pathways in response to ZIKV infection in SH-SY5Y neuroblastoma cells. Significance values are corrected for multiple testing using the Benjamini and Hochberg method (padj < 0.05). GO, Gene Ontology; ZIKV, Zika virus; DEG, differentially expressed gene.
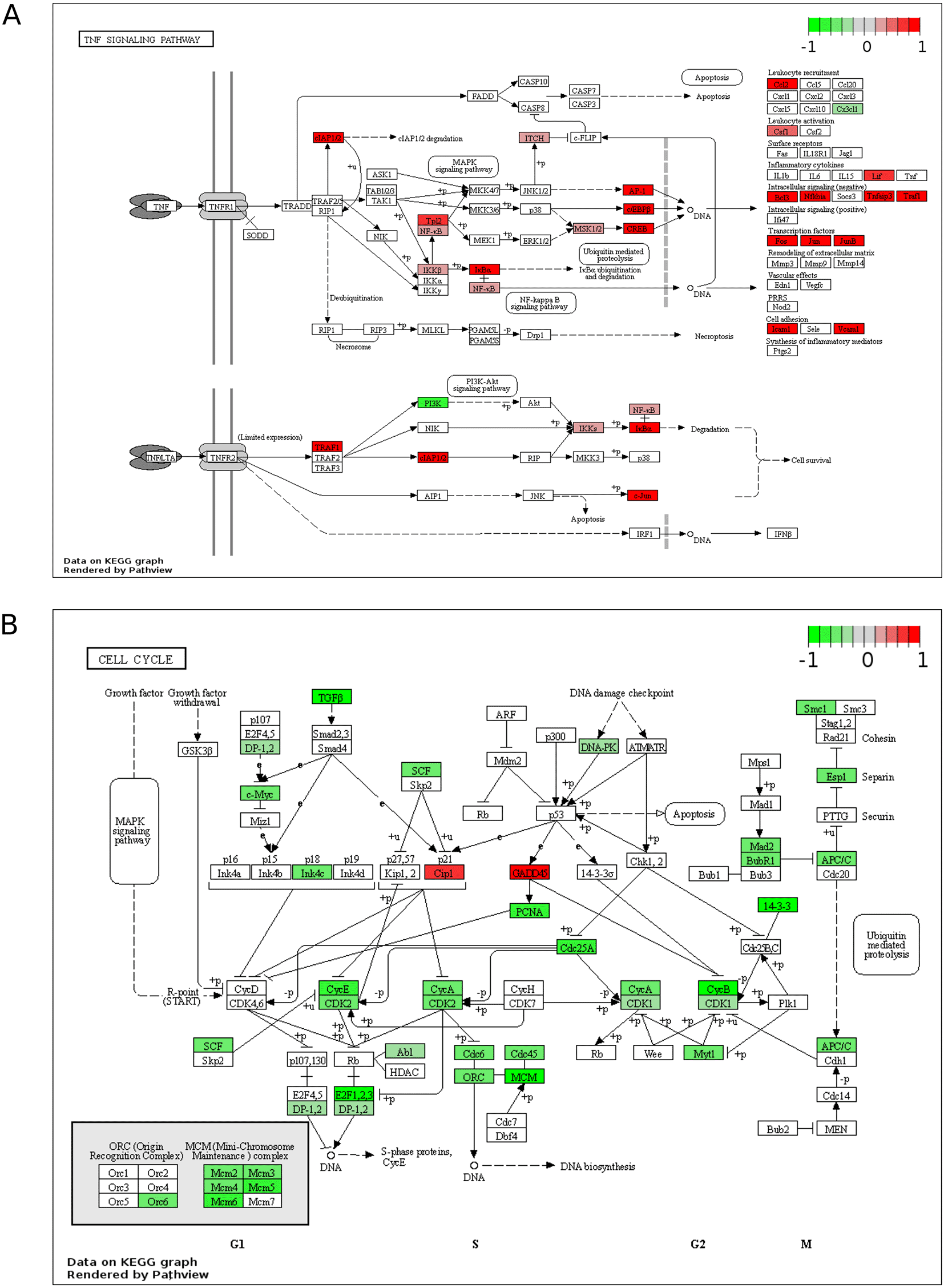
KEGG maps of the up-reulated TNF Signalling Pathway (A) and down-regulated Cell Cycle (B), plotted using all DEGs (fold change > 0). Significance values are corrected for multiple testing using the Benjamini and Hochberg method (padj < 0.05). ZIKV, Zika virus; KEGG, Kyoto Encyclopedia of Genes and Genomes; DEG, differentially expressed gene.
Of the top 10 upregulated DEGs in SH-SY5Y cells, four (BIRC3, TNFAIP3, ICAM1 and BCL3) are components of the TNF signalling pathway (Figure 1A). The TNF pathway is particularly noteworthy to consider for oncolytic virotherapy since it may play a role in both oncolysis (direct cell death) and the anti-tumoral immune response. Here, mapping the “TNF Signalling” KEGG pathway for ZIKV-infected SH-SY5Y cannot deduce if ZIKV may activate CASP-mediated apoptosis or CASP-independent necroptosis (Figure 2A). However, ZIKV-infected SH-SY5Y cells clearly show significant upregulation of transcription factors (AP-1, cEBPβ and CREB), leukocyte recruitment and activation (CCL2 and CSF1), intracellular signalling (BCL3, NFKBIA, TNFAIP3 and TRAF1) and cell adhesion genes (Icam1 and Vcam1) (Figure 2A). ZIKV significantly upregulates the expression of multiple Activator protein 1 (AP-1) transcription factors, including members from all four AP-1 subfamilies (ATF, JUN, FOS and MAF) in SH-SY5Y cells (Figure 3A). AP-1 can regulate the expression of a diverse set of genes in response to nutrients, cytokines, stress or pathogen infection, and is involved in innate and adaptive immunity, differentiation, proliferation, survival and apoptosis.43 AP-1 transcription factors can regulate the immune response of tumours, and significant AP-1 upregulation by ZIKV infection potentially identifies AP-1 as a mechanism through which ZIKV could yield an anti-tumoral immune response against neuroblastoma in vivo.44
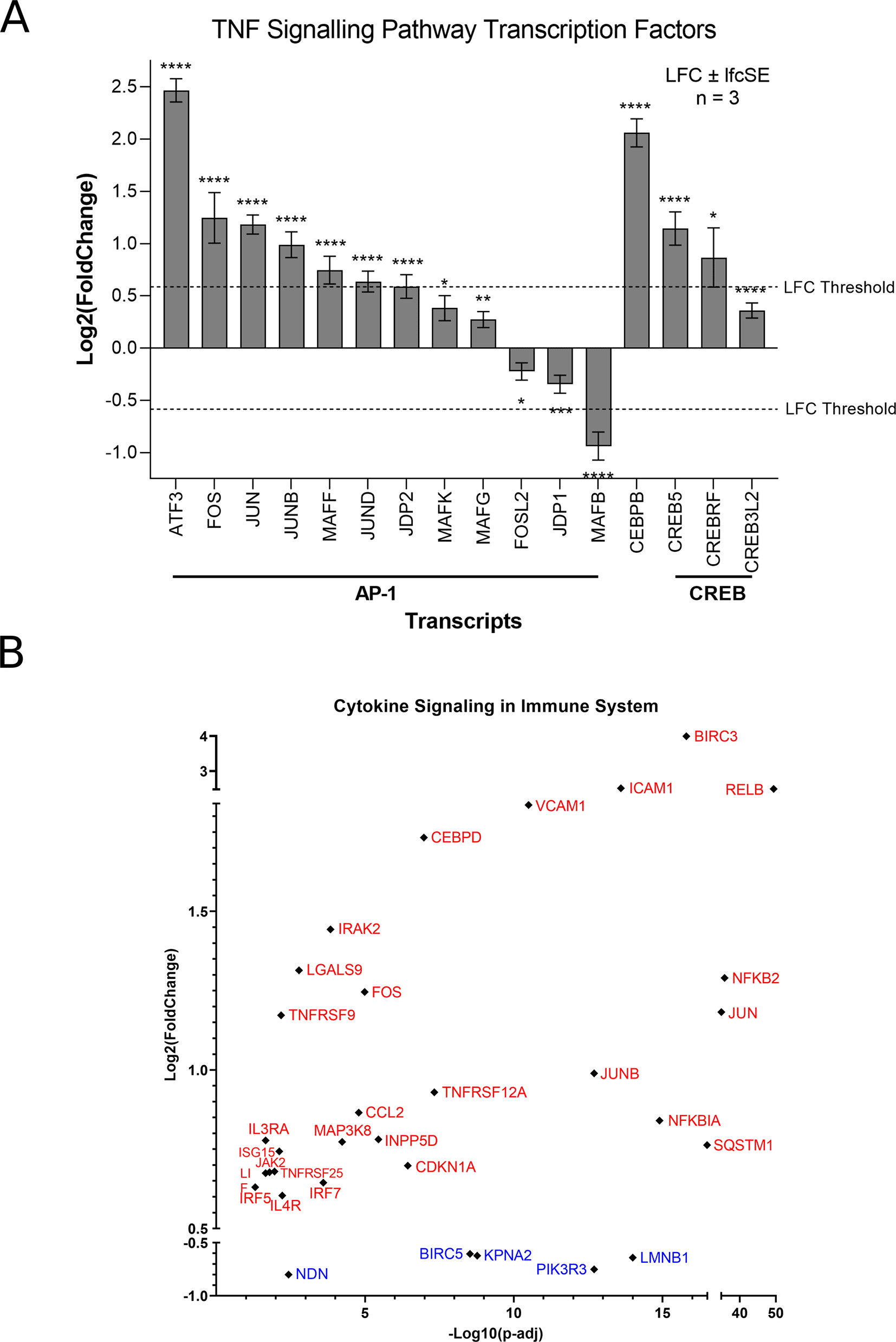
Expression levels of TNF Signalling Transcription Factors in SH-SY5Y cells in response to ZIKV infection (A). Expression levels of cytokine signalling in immune system genes in SH-SY5Y cells in response to ZIKV infection (B). Significance values are corrected for multiple testing using the Benjamini and Hochberg method (padj < 0.05). A threshold line of Log 2(1.5 Fold Change) has been applied for the expression values. Log 2FoldChange (LFC) ± standard error of the LFC estimate (lfcSE), n = 3. ZIKV, Zika virus; DEG, differentially expressed gene; AP-1, activator protein 1; CREB, cAMP response element-binding protein.
Here, CCL2 (MCP-1) is significantly upregulated at the transcriptome level by ZIKV infection, and two independent studies have shown CCL2 to be secreted by ZIKV-infected SH-SY5Y cells.45,46 CCL2 is a pro-inflammatory mediator that recruits leukocytes via chemotaxis to infiltrate tissues, including the CNS, to stimulate inflammation.47 A non-neurotoxic herpes simplex virus (HSV)-based oncolytic virotherapy, engineered to express physiologically relevant levels of CCL2 (M010), significantly reduced Neuro-2a neuroblastoma growth in the flank of immune-competent mice and recruited CD4+ and CD8+ T-cells to infiltrate the tumour.47 Additionally, CCL2 is secreted by ZIKV-infected cultured canine glioblastoma cells when in the presence of monocytes and is detected in serum and CSF samples of canines bearing spontaneous brain tumours following ZIKV infection.12 We propose here that CCL2 may be capable of inducing an anti-tumoral immune response against paediatric neuroblastoma during ZIKV infection. Supporting the notion of a ZIKV-induced inflammatory response, 32 genes implicated in cytokine signalling in the immune system are significantly differentially expressed in ZIKV-infected SH-SY5Y cells: 27 upregulated and five downregulated (Figure 3B).
ZIKV infection significantly upregulates lipid metabolism-related terms in SH-SY5Y cells; specifically, “Cholesterol biosynthesis” and “Activation of gene expression by SREBF (SREBP)” (Figure 1B). Cholesterol and lipids are essential cellular components and there are complex systems that function to regulate their intracellular abundance and localisation. These systems include regulation of cholesterol biosynthesis by the sterol regulatory element binding protein (SREBP) pathway, intracellular cholesterol trafficking, and cholesterol efflux by the liver X receptor (LXR) pathway. Cholesterol and fatty acids are required for multiple stages of the flavivirus life cycle, including regulating viral entry, the formation of viral replication complexes in the ER membrane and viral egress.48 ZIKV elevates lipogenesis and remodels the composition of the lipid classes in infected SK-N-SH cells.49 Our data mining of published literature identified several approaches to regulate ZIKV infection of neuroblastoma cells through modification of intracellular lipid levels (Table 3). These include supplementation with pathway regulators (PF-429242, fenofibrate, lovastatin, U18666A and LXR 623) or exogenous lipids (oleic acid, docosahexaenoic acid (DHA) and cholesterol).
List of compounds that regulate lipid homeostasis and are capable of restricting or enhancing ZIKV infection in paediatric neuroblastoma cells. ZIKV, Zika virus; LXR, liver X receptor; SREBP, sterol regulatory element binding protein; DHA, docosahexaenoic acid.
| Compound | Cell line | Mechanism of action | Effect on ZIKV infection | References |
| Bafilomycin A1 (V-ATPase inhibitor) | SH-SY5Y | Impairs acidification of endosomal-lysosomal compartments | Restrict | 51 |
| U18666A | SH-SY5Y | Cholesterol accumulation impairs late endosomes & lysosomes | Restrict | 51 |
| LXR 623 (LXR pathway agonist) | SK-N-SH | Induces cholesterol efflux | Restrict | 52 |
| PF-429242 (SREBP pathway inhibitor) | SK-N-SH | Reduces intracellular lipid levels | Restrict | 49 |
| Fenofibrate (SREBP pathway inhibitor) | SK-N-SH | Reduces intracellular lipid levels | Restrict | 49 |
| Lovastatin (SREBP pathway inhibitor) | SK-N-SH | Reduces intracellular lipid levels | Restrict | 49 |
| Oleic Acid | SK-N-SH | Increases lipid droplet abundance | Enhance | 49 |
| Cholesterol | SK-N-SH | Increases lipid droplet abundance | Restrict | 49 |
| DHA | SH-SY5Y | Anti-inflammatory and neuroprotective effects against ZIKV infection | Restrict | 46 |
Three SREBP pathway inhibitors (PF-429242, fenofibrate and lovastatin) reduce the capability of ZIKV to infect SK-N-SH neuroblastoma cells (Table 3). The SREBP pathway is a principal regulator of fatty acid and cholesterol biosynthesis. The SREBF1 and SREBF2 transcription factors control this pathway, and although they share a small degree of redundancy, they primarily regulate the expression of fatty acid biosynthesis and cholesterol biosynthesis target genes, respectively.50 Both SREBF2 and SREBF2-AS1 are significantly upregulated in ZIKV-infected SH-SY5Y cells, and the most highly upregulated SREBF downstream gene (HMGCS1) is a SREBF2 responsive gene (Figure 4A). Pathway analysis identifies significant upregulation of “Cholesterol biosynthesis” (Figure 1B) and here we observe significant upregulation of multiple enzymes of the SREBF2 cholesterol biosynthetic pathway (HMG-CoA synthase (HMGCS1), Mevalonate Diphosphate Decarboxylase (MVD), CYP51 (CYP51A1), Mevalonate Kinase (MVK), squalene synthase (FDFT1), Squalene Epoxidase (SQLE), Lanosterol Synthase (LSS), Lathosterol Oxidase (SC5D)) (Figure 4A). The SREBP pathway is essential for ZIKV infection, and we propose that it may contribute, through the SREBF2 transcriptional pathway, to the upregulation of cholesterol biosynthesis in neuroblastoma cells.
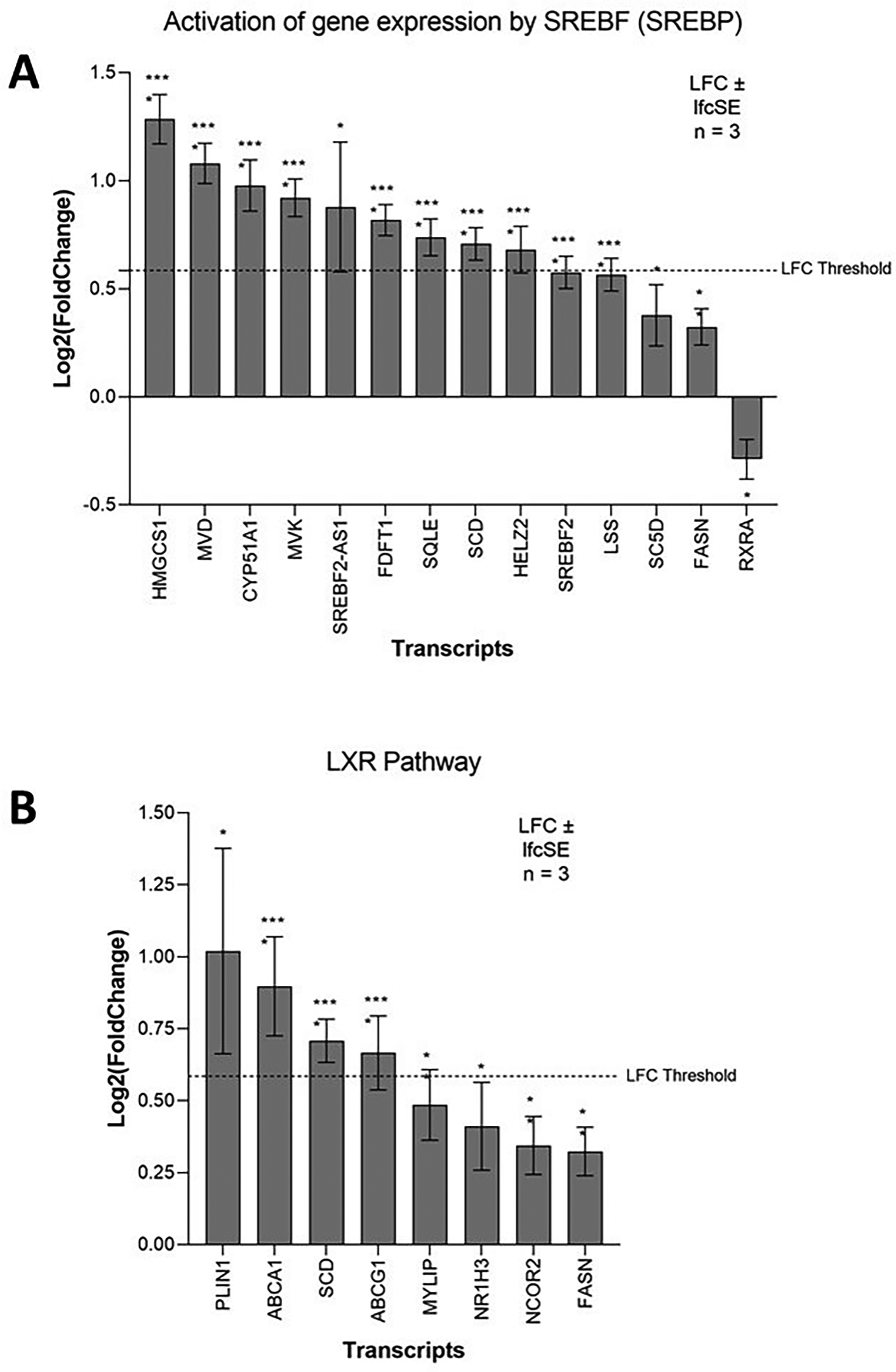
Expression of SREBP pathway (A) and LXR pathway (B) genes in neuroblastoma cells in response to ZIKV infection. Significance values are corrected for multiple testing using the Benjamini and Hochberg method (padj < 0.05). A threshold line of Log 2(1.5 Fold Change) has been applied for the expression values. Log 2FoldChange (LFC) ± standard error of the LFC estimate (lfcSE), n = 3. ZIKV, Zika virus; SREBP, sterol regulatory element binding protein; LXR, liver X receptor.
Both U18666A and exogenous cholesterol restricts ZIKV infection of neuroblastoma cells (Table 3). U18666A is an intracellular cholesterol transport inhibitor that causes the accumulation of cholesterol in lysosomes to hinder the endosomal-lysosomal system.51 Exogenous cholesterol also leads to the inactivation of the late endosomal-lysosomal compartments through a build-up of cholesterol.51 Collectively, this identifies a dependence of the ZIKV life cycle in neuroblastoma cells on intracellular cholesterol for the correct functioning of the endosomal-lysosomal system.
The LXR pathway agonist (LXR 623) promotes cholesterol efflux (Table 3). Flaviviruses require cholesterol for the restructuring of host membranes, and LXR 623 demonstrates this dependence of ZIKV in neuroblastoma by preventing ZIKV-induced vesicle production and ER expansion in SK-N-SH cells.52 The LXR pathway and expression of its downstream lipid homeostasis genes are regulated by the transcription factors LXR-α (NR1H3) and LXR-β (NR1H2). LXR-α protein is significantly increased by ZIKV infection of SK-N-SH neuroblastoma cells from 48 hr.52 Although LXR-α mRNA is only marginally upregulated in our study, two major cholesterol efflux factors that are downstream of the LXR pathway, ATP-Binding Cassette A1 and G1 (ABCA1 and ABCG1), are significantly upregulated (Figure 4B). Interestingly, exogenous addition of oleic acid enhances ZIKV infection in neuroblastoma cells (Table 3). Oleic acid is one of the main monounsaturated fatty acid synthesised by the LXR pathway, which is achieved through induction of FASN and SCD.53 We observe significant upregulation of both FASN and SCD in ZIKV-infected neuroblastoma cells (Figure 4B) and propose that this may induce oleic acid synthesis to aid ZIKV infection in neuroblastoma cells. Collectively, our data mining identifies a dependence of ZIKV on the LXR pathway and suggests that ZIKV may manipulate this pathway in neuroblastoma cells to upregulate cholesterol efflux and/or induce oleic acid syntheis.
We propose that lipid abundance, localisation, trafficking and metabolism regulate ZIKV infection of neuroblastoma cells, and that remodelling of the cellular lipid composition within the host cell may produce a favourable environment for efficient replication.
ZIKV upregulates ER-stress-related terms in SH-SY5Y cells, principally “Response to endoplasmic reticulum stress” and “XBP1(S) activates chaperone genes” (Figure 1B). The Unfolded Protein Response (UPR) dictates the ER-stress response. The UPR is normally inactive due to the ER chaperone binding immunoglobulin protein (BIP) sequestering three ER stress sensors (IRE1, PERK and ATF6). Under stress conditions BIP releases IRE1, PERK and ATF6 to assist protein folding, allowing them to activate their respective UPR-mediated ER-stress pathways.
Activation of the IRE1-mediated UPR leads to IRE1 splicing a 26 bp region from the ubiquitously expressed XBP1 mRNA. The active transcription factor XBP1(S) then drives the expression of genes to help alleviate ER stress, primarily chaperone and ER-associated protein degradation (ERAD) genes. ZIKV infection significantly upregulates 15 genes of the IRE1-mediated “XBP1(S) activates chaperone genes” Reactome pathway in SH-SY5Y cells (Figure 5A). XBP1 is the most highly upregulated gene and others include the endoplasmic-reticulum-associated protein degradation (ERAD) gene SYVN1 and the chaperones DNAJC3 and DNAJB9 (Figure 5A). Multiple IRE1-mediated UPR genes (EDEM1, SYVN1, SSR1, SRPRB, ATP6V0D1, and EXTL3) are ZIKV dependency factors in hiPSC-NPCs (Table 4). Chemical inhibition of IRE1 by 4μ8C impairs ZIKV infection in vivo.54 Our data mining and re-analysis shows that ZIKV significantly upregulates the IRE1-mediated UPR in SH-SY5Y cells and that ZIKV is dependent on this for efficient infection, likely as a means to regulate and combat viral replication-induced ER stress.
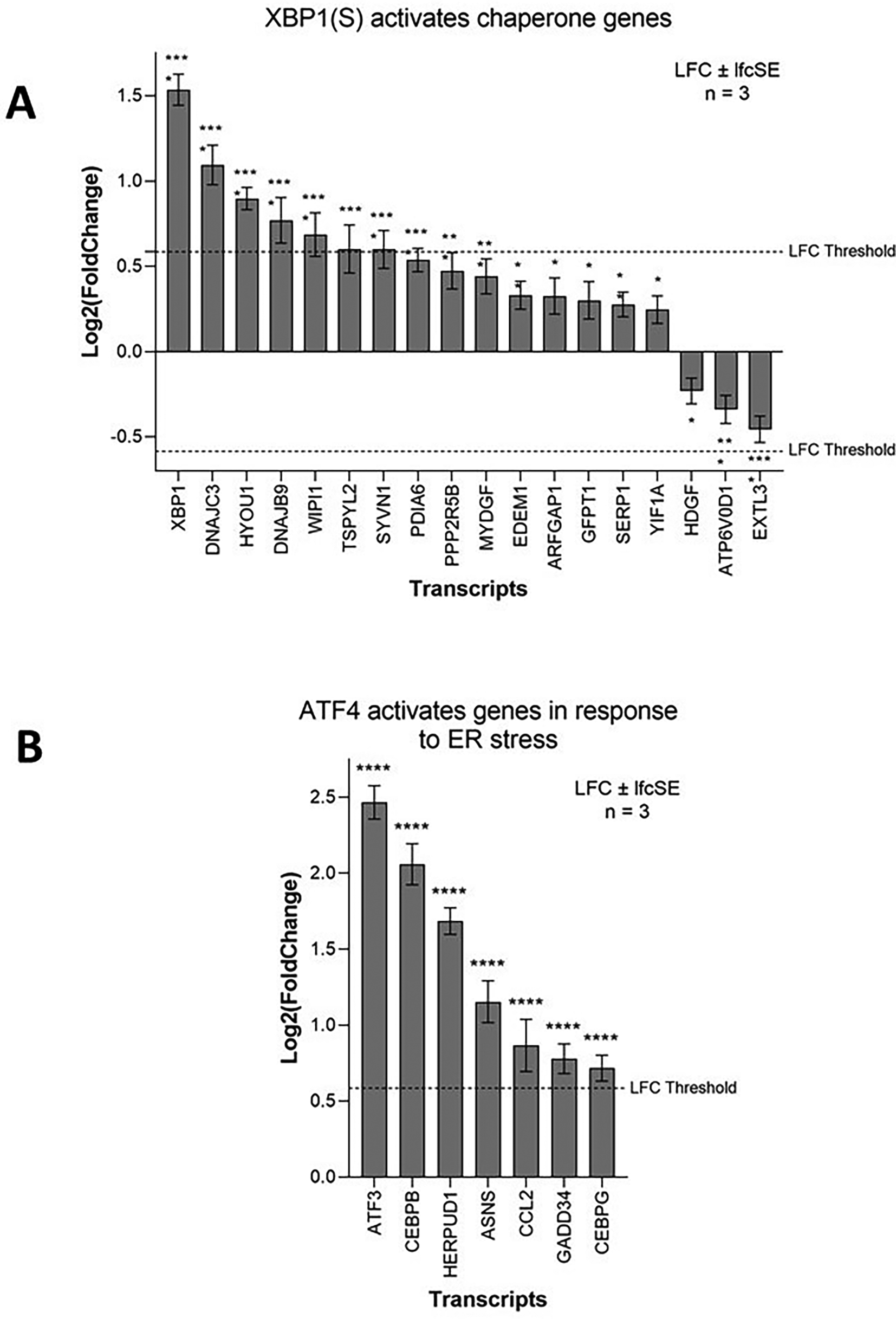
Expression levels of the XBP1(S) activates chaperone genes (A) and ATF4 activates genes in response to endoplasmic reticulum stress (B) genes in neuroblastoma cells in response to ZIKV infection. Significance values are corrected for multiple testing using the Benjamini and Hochberg method (padj < 0.05). A threshold line of Log 2(1.5 Fold Change) has been applied for the expression values. Log 2FoldChange (LFC) ± standard error of the LFC estimate (lfcSE), n = 3. ZIKV, Zika virus; UPR, Unfolded Protein Response; ER, endoplasmic reticulum.
Lists of known ZIKV dependency factors across SH-SY5Y (NB), GSC, hiPSC-NPC (NPC), HEK293FT and HeLa cells. ZIKV, Zika virus; GSC, glioma stem cell; NB, neuroblastoma.
PERK-mediated UPR regulates the expression of genes involved in apoptosis, redox, amino acid transport and autophagy through eIF2 phosphorylation and the transcription factor ATF4. ZIKV infection significantly upregulates seven genes of the Reactome pathway “ATF4 activates genes in response to endoplasmic reticulum stress” (Figure 5B), including the ERAD gene HERPUD1 and the transcription factors ATF3, CEBPB and CEBPG. GADD34 (PPP1R15A), which usually dephosphorylates eIF2α in a negative feedback loop, is significantly upregulated here by ZIKV infection, and ZIKV induces eIF2 phosphorylation in SK-N-SH cells.55 ZIKV likely upregulates GADD34 to combat ER stress-induced translational repression, as fresh virions require de novo protein synthesis. C/EBP homologous protein (CHOP) (DDIT3) is a pro-apoptotic protein downstream of the PERK UPR pathway that others have observed to be significantly upregulated in SH-SY5Y and SK-N-SH cells in response to ZIKV infection.14,55,56 CHOP induces apoptotic markers, including Caspase 3, leading to cell death. Notably, multiple PERK-mediated UPR genes (ATF4, EIF2AK1, EIF2AK2, EIF2AK3 and EIF2AK4) are ZIKV dependency factors in hiPSC-NPCs (Table 4).
Our data mining, integration and re-analysis suggests that ZIKV specifically upregulates and is dependent on the IRE1 and PERK branches of the UPR ER stress response in SH-SY5Y cells; observations that are supported by others.55,56
To determine which host mechanisms ZIKV may be dependent on, we cross-referenced the 22 known proteins that ZIKV requires to infect neuroblastoma cells with ZIKV dependency factors from four cell lines (Figure 6A and Table 4). Between 72–94% of the dependency factors identified across the five different cell lines are cell-specific, highlighting how ZIKV utilises differing host factors across different cell types for its life cycle. The sparse overlap of ZIKV dependency factors identifies only one factor common to all five cell types. MMGT1 (EMC5) is a key component of the Endoplasmic Reticulum (ER) Membrane Protein Complex (EMC). The EMC is a hetero-oligomer composed of 10 subunits, has chaperone properties by assisting multi-transmembrane protein folding, and is implicated in ER stress, flavivirus infection and lipid trafficking.57 To assess if ZIKV is dependent on additional EMC subunits during infection, we searched for them in our ZIKV dependency factor dataset. Our integration of varying ZIKV-dependency factor datasets show ZIKV to have a strong dependence on the EMC independent of cell type; all 10 EMC proteins are ZIKV-dependency factors in hiPSC-NPC, eight are in HeLa and three in GSC and HEK293FT cells (Figure 6B). The EMC facilitates the expression of ZIKV transmembrane proteins (NS2B, NS4A and NS4B), ZIKV NS4B interacts with EMC subunits, and disrupting the EMC impedes infection by ZIKV and other flaviviruses.58,59 We propose that the EMC stabilises ZIKV proteins through integration into the ER membrane, thus permitting efficient infection in neuroblastoma cells. If investigated, we predict that additional EMC subunits would present as ZIKV dependency factors in neuroblastoma cells.
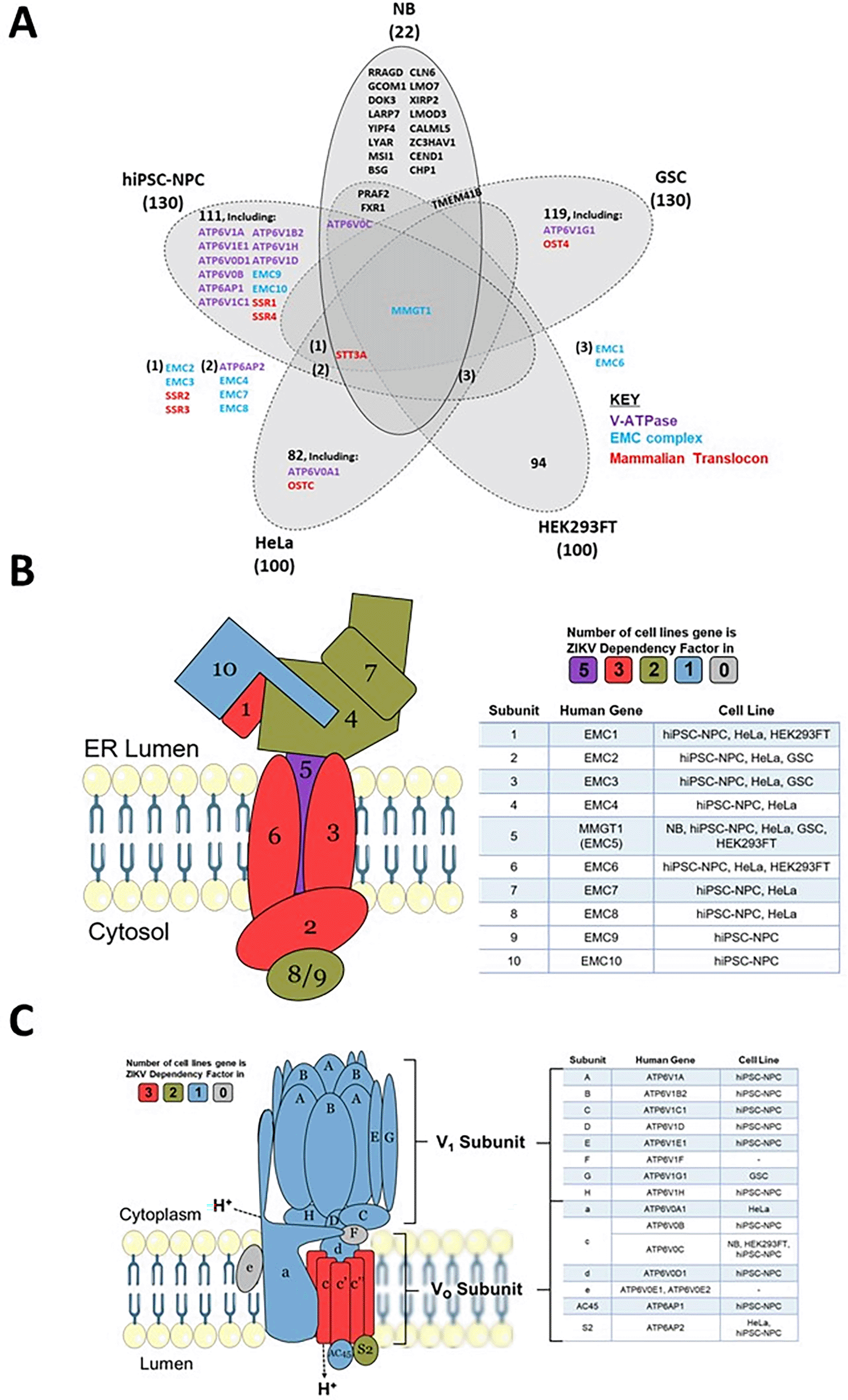
Venn diagram of known ZIKV dependency factors across NB, GSC, hiPSC-NPC (NPC), HEK293FT and HeLa cells, to identify shared and cell-specific factors and protein complexes that ZIKV is dependent on for infection (A). Diagram of the EMC (B) and V-ATPase (C), based on their crystal structures. For the subunits in B and C, colours are allocated based on the number of cell types in which they act as ZIKV dependency factors (cell types stated in the adjacent tables). ZIKV, Zika virus; NB, neuroblastoma; GSC, glioma stem cell; EMC, endoplasmic reticulum membrane protein complex.
Acidification of the endosomal-lysosomal system by the V-ATPase is a property that viruses can utilise to drive the release of their nucleocapsid into the cytosol. ATP6V0C, a central component of the V-ATPase, is a ZIKV-dependency factor in neuroblastoma, hiPSC-NPC and HEK293FT cells (Figure 6A). A total of 12 additional V-ATPase subunits are ZIKV dependency factors across GSC, hiPSC-NPC and HeLa cells (Figure 6C). These 13 genes consist of multiple subunits from the Vo proton translocation and V1 ATP hydrolytic domains, identifying a functional dependence of ZIKV on the entire V-ATPase complex. The V-ATPase inhibitor Bafilomycin A1 specifically binds ATP6V0C and through V-ATPase inhibition prevents lysosomal acidification and the autophagy-lysosome pathway.60 Bafilomycin A1 inhibits ZIKV infection of SH-SY5Y cells, supporting our observation (Table 3). siRNA silencing of the V-ATPase significantly impairs ZIKV infection of T98G glioblastoma cells, collaborating its requirement for infection of nervous system tumour cells.61 We propose that loss of V-ATPase function impairs ZIKV infection in SH-SY5Y cells due to a perturbed pH gradient in the endosomal system. This likely prevents fusion of the viral envelope with the endosomal membrane for release of the nucleocapsid, therefore, trapping ZIKV for degradation in the lysosome, as observed in Vero cells.62
ZIKV NS4B protein is principally responsible for the oncolytic effect in SH-SY5Y cells, via activating the mitochondrial apoptotic pathway.63 Determining the interactions and mechanisms underpinning this may yield opportunities to develop a paediatric neuroblastoma therapy based on ZIKV NS4B. ZIKV NS4B has 130 known host interaction partners in SK-N-BE2 neuroblastoma cells.30 Re-analysing this interactome, we observe multiple pathways previously implicated during ZIKV infection of neuroblastoma cells: including mitochondrial-, lipid metabolism- and ER-associated processes (Figure 7). ZIKV NS4B interacts with 10 lipid biosynthesis proteins, three (SCD, SC5D and DHCR7) of which are expressed in response to SREBP pathway activation. This identifies a direct interaction between ZIKV and host lipid metabolism and the SREBP pathway, supporting our previous observations at the transcriptome level.
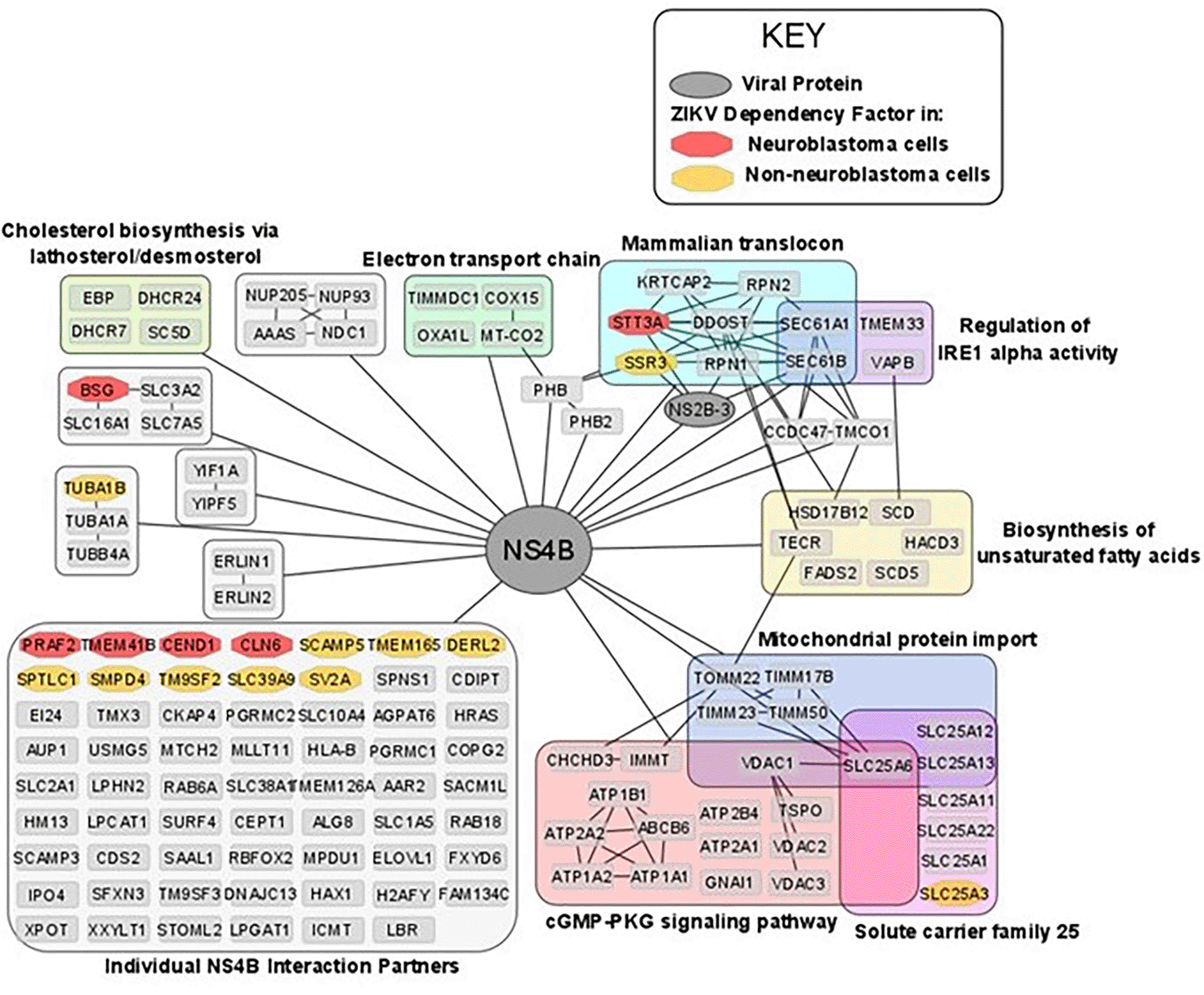
Nodes are grouped and labelled according to any sets of high-confidence interactions between the host proteins, and by pathways they are involved in. Cross-referencing the interactome with the ZIKV dependency factor datasets identifies the interaction of NS4B with dependency factors in neuroblastoma cells (red) and in GSC, hiPSC-NPC, HeLa and HEK293FT cells (collectively termed non-neuroblastoma cells, orange). To aid visualisation, any nodes possessing no high confidence interactions, other than their interaction with NS4B, have been grouped. All nodes within Figure 7 interact with NS4B, thus, to aid visualisation all edges between NS4B and nodes within a group have been condensed into a single edge between NS4B and the grouped set of nodes. ZIKV, Zika virus; NS4B, non-structural protein 4B; GSC, glioma stem cell.
ZIKV NS4B recruits BAX to the mitochondria, triggers its activation, and releases Cytochrome c from mitochondria to induce mitochondrial cell death in SH-SY5Y cells.63 ZIKV NS4B interacts with a multitude of mitochondrial genes (Figure 7). Including, electron transport chain proteins (TIMMDC1, MT-CO2, COX15 and OXA1L), mitochondrial translocases that import proteins into the mitochondrial matrix (TOMM22, TIMM23, TIMM50 and TIMM17B) and Solute Carrier Family 25 members for transport of solutes across the mitochondrial membrane (SLC25A1, SLC25A3, SLC25A6, SLC25A11, SLC25A12, SLC25A13 and SLC25A22). Specifically, MT-CO2, COX15 and OXA1L are conserved catalytic core, assembly and accessory subunits of the Cytochrome c oxidase complex, respectively. The Cytochrome c oxidase complex tightly couples Cytochrome c to the inner mitochondrial membrane. We propose that NS4B interacts with Cytochrome c oxidase to uncouple it from Cytochrome c, causing Cytochrome c release through the BAX pore into the cytosol to drive the mitochondrial cell death pathway in neuroblastoma cells.
ZIKV NS4B interacts with and is dependent on multiple proteins of the Mammalian Translocon (Figures 5A and 6). The mammalian translocon is primarily composed of the Oligosaccharyl Transferase (OST) complex, the Sec61 complex and the translocon-associated protein (TRAP) complex.64 The multimeric OST complex co-translationally N-glycosylates proteins within the ER to assist protein folding, stability and trafficking. The Sec61 complex, a heterotrimer of Sec61α, Sec61β and Sec61γ, co-translationally translocates newly synthesised proteins across the ER and during ER stress can regulate IRE1α activity. TRAP is a heterotetramer of SSR1, SSR2, SSR3 and SSR4 that assists co-translational translocation of proteins into the ER and can prevent aberrant N-linked glycosylation during ER stress.
STT3A, a principal component of the OST complex, interacts with ZIKV NS4B and is a ZIKV dependency factor in neuroblastoma, GSC, hiPSC-NPC and HeLa cells (Figures 5A and 6). Regarding the additional OST subunits, ZIKV NS4B interacts with DDOST, RPN1, RPN2 and KRTCAP2, and OSTC and OST4 are ZIKV dependency factors in HeLa cells and GSCs, respectively (Figures 5A and 6). Two forms of the OST complex exist, the STT3A and STT3B OST paralogs. KRTCAP2 and OSTC are STT3A-specific OST factors, which permit interaction of STT3A with the translocon, whilst TUSC3 and MAGT1 are STT3B-specific OST factors.64 STT3A and both STT3A-specific OST factors are ZIKV interaction partners and/or dependency factors, but neither STT3B nor the STT3B-specific OST factors are. In addition to the OST factors, multiple N-linked glycosylation-related proteins (DPM1, DERL3, SYVN1, UBE2G2 and UBE2J1) are also ZIKV dependency factors in non-neuroblastoma cells (Table 4). The OST complex inhibitor NGI-1 blocks ZIKV infection of Huh7 cells, and disrupting ZIKV prM and E protein N-glycosylation impairs the release of infectious ZIKV particles from Vero cells.65,66 We propose that STT3A functions as a bonafide ZIKV dependency factor in multiple cell types, and propose that efficient infection of neuroblastoma cells by ZIKV is likely dependent on the STT3A OST paralog for N-glycosylation of its viral proteins.
ZIKV NS4B interacts with SEC61A1 and SEC61B of the Sec61 complex in neuroblastoma cells and the Sec61α inhibitor Mycolactone impedes ZIKV infection of HeLa cells.67 ZIKV NS4B interacts with SSR3 of the TRAP complex in neuroblastoma cells and ZIKV is dependent on at least two of the four TRAP complex subunits for infection of GSC, hiPSC-NPC and HeLa cells (Figure 6A). Further supporting our observation of ZIKV interacting and being dependent on the mammalian translocon is its dependence on SRPRB, SPCS3 and TRAM1; subunits of the Signal Recognition Particle (SRP), the Signal Peptidase Complex (SPCS) and the Translocating chain-associated membrane protein (TRAM), respectively. Notably, the viral protease NS2B-3 also interacts with subunits of the OST complex (STT3A, RPN1), Sec61 complex (SEC61B) and TRAP complex (SSR3) (Figure 7). These interactions likely facilitate the co-translational cleavage of the viral polypeptide by NS2B-3 into its individual viral proteins.
From our re-analysis of a published ZIKV interactome we propose ZIKV NS4B and NS2B-3 to interact with the core complexes of the mammalian translocon, and hypothesise that ZIKV infection in neuroblastoma cells may be dependent on these interactions. The dependency of ZIKV likely stems from the mammalian translocon facilitating viral polyprotein co-translational translocation, viral polyprotein cleavage, viral membrane protein insertion and/or viral protein N-glycosylation. Additionally, ZIKV may utilise its protein interactions with the Sec61 complex, TMEM33 and VAPB, to regulate the IRE1- and PERK-mediated UPR ER stress responses, that we observed to be significantly upregulated at the transcriptome level in ZIKV infected-neuroblastoma cells.
Our study highlights the strong therapeutic potential of ZIKV, specifically the PRVABC59 strain, against multiple neuroblastoma cell-lines. Our data mining, integration and re-analysis suggest ZIKV to interact with, and be dependent on, multiple host protein complexes and pathways for its life cycle in paediatric neuroblastoma cells and for inducing oncolysis (Figure 8). Although this area of research is still at an early stage, our extensive survey of neuroblastoma ZIKV infection studies clearly demonstrates the potential of a ZIKV-based therapeutic. There are a few avenues that need to be addressed to progress this area of research, including; (1) assessing ZIKV’s oncolytic effect against neuroblastoma in xenograft mouse models, (2) assessing ZIKV’s capability to induce an anti-tumoral immune response against neuroblastoma in immune-competent in vivo models, and (3) considering the effectiveness and safety of employing different forms of ZIKV-based therapeutics against neuroblastoma. Examples of the latter may include live attenuated ZIKV strains or the construction of a virotherapy that collectively expresses ZIKV NS4B and CCL2, which we observe here to hold elements of ZIKV’s oncolytic and immune activation potential, respectively.
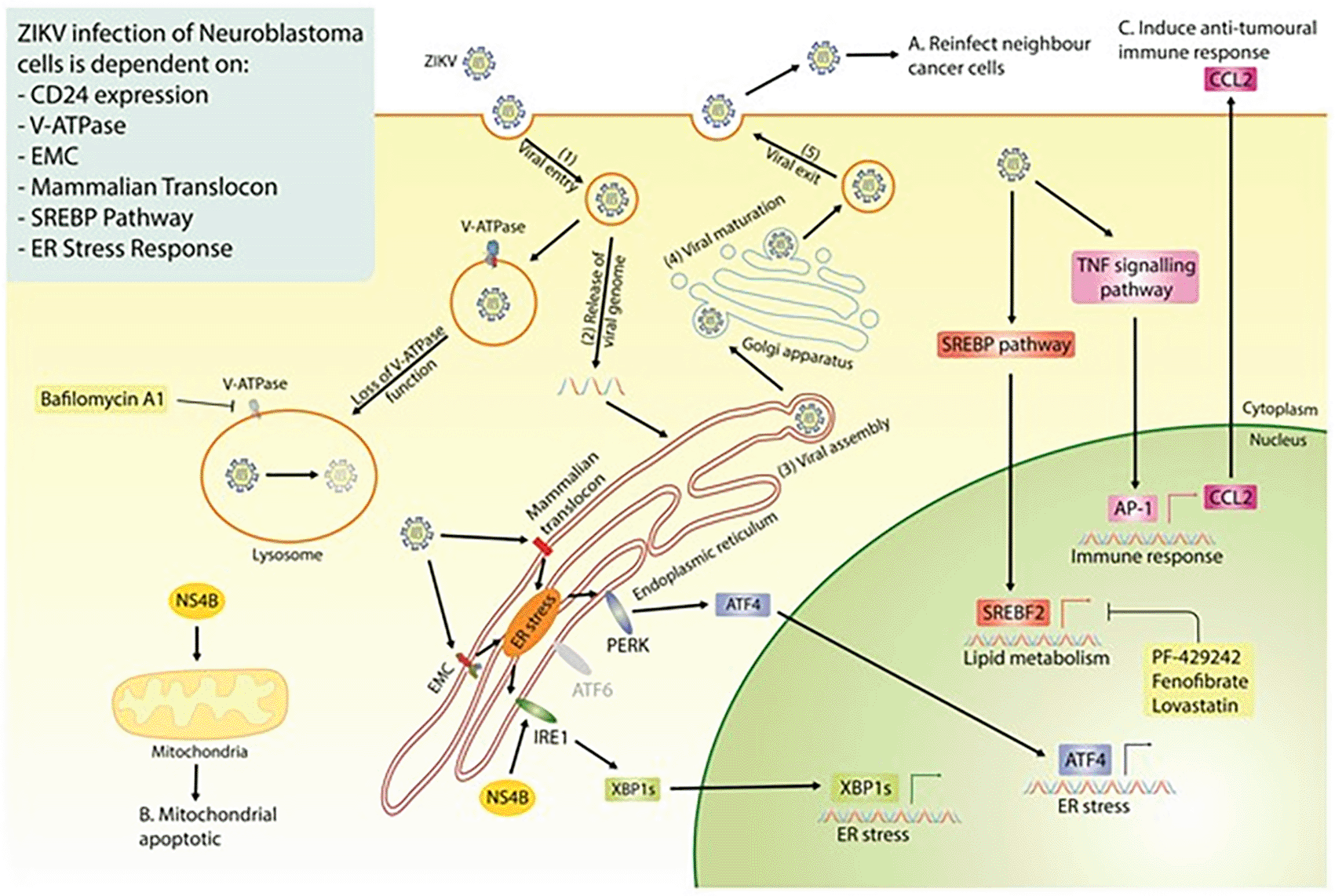
Highlighted are the three essential properties of an oncolytic virus; the production of fresh viral particles to infect additional cancer cells (A), the ability to induce cancer cell death (B) and a mechanism through which ZIKV may induce an anti-tumoral immune response (C). ZIKV, Zika virus; EMC, endoplasmic reticulum membrane protein complex; SREBP, sterol regulatory element binding protein; ER, endoplasmic reticulum; NS4B, non-structural protein 4B.
European Nucleotide Archive: Asian Zika virus isolate significantly changes the transcriptional profile and alternative RNA splicing events in a neuroblastoma cell line. Accession number PRJNA630088 (https://www.ebi.ac.uk/ena/browser/view/PRJNA630088).80
The authors would like to thank the original curators of the datasets used in this study. The abstract can be found on sciety (https://sciety.org/articles/activity/10.1101/2022.11.14.516401) and an earlier version of this article can be found on bioRxiv (https://doi.org/10.1101/2022.11.14.516401).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Proteomics, Cellular Biology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Innate immunity and oncolytic virus research.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: RNA Viruses
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 3 (revision) 21 May 24 |
read | read | ||
|
Version 2 (revision) 20 Nov 23 |
read | |||
|
Version 1 21 Jun 23 |
read | read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)