Keywords
Indonesian seagrass, marine resources, Thalassia hemprichii, Zostera marina, antioxidants, anti-obesity, combating free radicals, marine plant
This article is included in the Plant Science gateway.
Indonesian seagrass, marine resources, Thalassia hemprichii, Zostera marina, antioxidants, anti-obesity, combating free radicals, marine plant
Obesity is a complex problem being faced in almost every country in the world.1 In recent years, the obesity rate in the world has doubled since 1980, as one-third of the world’s population is overweight, and the same figure applidesto the Southeast Asian region.2 Obesity is a complex issue that can be treated with a variety of approaches. One is a biological approach that involves antioxidants, especially those derived from plants. Most natural antioxidant compounds in plants are divided into three types, namely polyphenols, carotenoids, and vitamins.3 Antioxidants work by inhibiting the oxidation of other compounds, and they have extensive benefits such as anti-inflammatory, neuroprotective, and cardioprotective properties. This means that antioxidants have a broad role in overcoming obesity and abnormalities of fat metabolism. Some of the antioxidants’ important roles include anti-inflammatory4 and inhibiting lipid anabolism in the body.5
The anti-inflammatory properties in antioxidant compounds serve to reduce obesity complications. This is because most complications stem from chronic low-grade systemic inflammation that often occurs in individuals with obesity. Such systemic inflammation can trigger insulin resistance, vascular endothelial remodeling, and other complications in obesity.6 While the inhibition of lipid anabolism is caused by the activation of (AMP)-activated protein kinase, AMPK reduces fat tissue formation which causes obesity. Antioxidants have also been shown to have an inhibitory effect on cholesterol formation through the inhibition of HMG-CoA reductase, thereby reducing the risk of dyslipidemia.7
Seagrass is a flowering plant that can form vast seagrass meadows in temperate and tropical waters (marine environments) on all continents except Antarctica.8 Some types of seagrasses are widespread and invasive, such as the seagrass Halophila stipulacea, a type of tropical seagrass native to the Gulf of Aqaba (GoA; Northern Red Sea), which has invaded the eastern Mediterranean basin, south of the Adriatic, south of the Tyrrhenian Sea, the Atlantic Ocean, and even the French Riviera, far from its normal distribution.9 These plants can also dominate local species and potentially reduce ecosystem resilience, especially in areas that have been affected or changed due to human activities.10 However, seagrass has a lot of potential such as its high level of antioxidants.11 Apart from the potential waste and bioactive effects on the life of marine organisms, seagrass has several nutritional and pharmacological benefits that are sometimes overlooked. Seagrass is an important natural biological source of metabolites with diverse bioactivity. This plant has a biochemical composition (carbohydrates, proteins, ash, phenols, flavonoids, tannins, lipids) and photosynthetic pigments (chlorophyll A, chlorophyll B, and carotenoids) that function as nutrient-filled foodstuffs and have several medical effects on the body.12
Seagrasses Thalassia hemprichii and Zostera marina contain substances including cinnamic esters of tartaric acid which are also contained in Halophila stipulacea.13,14 These metabolites are known to work as antioxidants through the mechanism of free radical scavenging. Free radical scavenging is the ability of the phenol group (-OH) to provide hydrogen ions or electrons that will form stable phenoxy radicals to reduce free radicals circulating in the body. In addition, structural modifications, especially the substitution of aromatic rings, can be carried out to increase antioxidant activity. This metabolite is also able to reduce body weight and angiotensin-converting enzyme (ACE) levels in serum.15 A decrease in ACE results in a decrease in the formation of angiotensin II and a decrease in bradykinin metabolism, which leads to systematic dilation of arteries and veins and a decrease in arterial blood pressure. Therefore, pancreatic lipase inhibitors are considered valuable therapeutic reagents for treating diet-induced obesity in humans because fats will not be absorbed by the body, and the inhibitors reduce the lipogenic effects which results in weight loss, observed in our study. In addition, cinnamic acid also plays a role in lowering leptin levels, a precursor to hunger, in the human body; leptin levels increasewith increased body weight.16 Therefore, it is strongly suspected that two Indonesian seagrasses – T. hemprichii and Z. marina – have promising antioxidant and anti-obesity activities that have not yet been reported.
Another substance found in the seagrasses T. hemprichii and Z. marina is flavone glycosides.13,17 The basic characteristics of flavonoids, such as polarity, solubility, and steric conformation, will affect their ability to bind to cell membranes. Compounds with hydrophilic groups that are more polar interacting with the membrane surface via hydrogen bonding will protect cell membranes from oxidative stress.18 In addition, flavonoids inhibit glucose digestion in the gut and glucose production in the liver, increase glucose uptake in skeletal muscle, and protect against pancreatic islet damage resulting in weight loss in obese patients.19 Judging from both seagrasses’ content (T. hemprichii and Z. marina), they have no less potential than H. stipulacea regarding antioxidant and anti-obesity.13,14,18 However, research discussing these two marine plants both in terms of metabolites and biological activity is still very limited and unfortunate considering their abundance and availability. Therefore, this study aims to provide new insights related to chemical constituents profiling and biological activity of two Indonesian seagrasses T. hemprichii and Z. marina in fighting free radicals and obesity via molecular docking and in vitro study.
Thalassia hemprichii and Zostera marina L. were sourced from the Mantehage Island Waters situated in Manado, North Sulawesi Province, Indonesia (Coordinates: 1.728836, 124.783457). These were collected by a third party and then procured as part of this research. Upon acquisition, both seagrass samples underwent an immediate washing process using clean water to eliminate any impurities. The botanical species’ authentication and identification were conducted at Sam Ratulangi University, Indonesia. This process followed the reference outlined by the National Center for Biotechnology Information (NCBI) Taxonomy ID NCBI:txid55496 (T. hemprichii) and NCBI:txid29655 (Z. marina). To prepare the collected specimens, the entire body was thoroughly rinsed using distilled water, then subjected to drying using a hybrid hot water Goodle dryer (King Ston, Republic of Korea),20 and finally lyophilized using the Lyovapor™ L-200 equipment by BÜCHI Labortechnik AG (Flawil, Switzerland). The resulting dehydrated coarse powder was 0.5 mm in size. The fine simplica powder was carefully packed in aluminum foil and stored at a temperature of -20 °C until further use in subsequent experiments.
To prepare the seagrass extracts from Indonesia for analysis, a dark bottle was used to mix 1 kg of simplica powder obtained from each seagrass species (Thalassia hemprichii and Zostera marina) with 2,000 mL of 96% ethanol (EtOH) solvent in a ratio of 1:2. The residue was then soaked in fresh 96% ethanol (EtOH) solvent, repeating the process. Afterward, the mixture underwent sonication for 30 minutes at 40 °C using an ultrasound sonicator (400 W, Branson 2510 model; Danbury, CT, USA). The resulting extract was concentrated for 90 minutes using a rotary evaporator under low pressure (100 millibars) and evaporated in a 40 °C oven. Subsequently, the extract was partitioned into equal volumes using hexane solvents to yield a viscous extract. The extracts obtained included THE: Thalassia hemprichii—ethanol (polar), THH: Thalassia hemprichii—hexane (non-polar), ZME: Zostera marina—ethanol (polar), and ZMH: Zostera marina—hexane (non-polar), as referenced in previous studies (Figure 1).21,22
To analyze the compounds present in the samples of seagrass extracts, an untargeted metabolomic profiling test was conducted.23 For the analysis, 50 μL of each of the four samples was mixed with ethanol (96%) and vortexed 30 times. Subsequently, the mixture was centrifuged at 6000 rpm for 2 minutes. Before analysis, the supernatants were filtered using a 0.22 μm syringe filter. The liquid chromatography high-resolution mass spectrometry (LC-HRMS) system used in the test consisted of a Thermo Scientific Dionex Ultimate 3000 RSLC Nano high-performance liquid chromatography (HPLC) instrument and a micro flow meter. A Hypersil GOLD aQ 50 column (50 × 1 mm × 1.9 μ particle size) was employed, maintained at a temperature of 30 °C. Solvent A contained 0.1% formic acid in the water, while solvent B comprised acetonitrile. A linear gradient was employed for the separation process, with a flow rate of 40 μL/min for a duration of 30 minutes. Thermo Scientific Q Exact HRMS was utilized for full-scan resolution at 70,000 and data-dependent MS2 resolution at 17,500 in both positive and negative modes.
The ASUS Vivobook M413ia - Ek502t laptop, equipped with an AMD Ryzen 5 4500u processor running at 2.3 GHz, 8 GB DDR4 memory, a 512 GB SSD M.2 storage, and the Windows 10 Home operating system, was utilized in this study. The software tools employed included ChemDraw Ultra 12.0, AutoDock tools (version 4.2), and BIOVIA Discovery software. The Protein Data Bank website and the PubChem structure database were also accessed as resources. The molecular docking simulation protocols followed in this study were based on previous research.23
The seagrass extract profiles revealed some compounds, which were selected as test ligands. The 2D structure of these compounds was sketched using ChemDraw Ultra 12.0 and then converted to 3D and optimized using the MM2 algorithm. The target proteins chosen for the study were human inducible nitric oxide synthase (PDB ID: 3E7G), human reactive oxygen species 1 kinase (PDB ID: 3ZBF), human pancreatic lipase (PDB ID: 1LPB), and fat mass and obesity-associated protein (PDB ID: 3LFM). These protein structures were obtained from the Protein Data Bank.
To validate the molecular docking process, redocking was employed. AutoDock tools (version 4.2) were used to transfer the original ligand into the target binding pocket, utilizing specific grid coordinates. Following the redocking procedure, the root-mean-square deviation (RMSD) of the ligand position had to be less than 2.0 Å. The grid and docking parameters were adjusted based on the results of the docking validation. Each docking’s final conformation structure was recorded in a *dlg file, and the interaction between the ligands and receptors was analyzed using Discovery Studio 2016.
The antioxidant activity was assessed using the 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity (DPPH radical, C18H12N5O6+) test, following the protocol of the previous study.21,24 In this test, THE, THH, ZME, ZMH, and Trolox were added to the DPPH reagent (3 mL) in the testing vial. The mixtures were then cooled to room temperature and left for 30 minutes. The change in DPPH concentration was observed by measuring the absorbance at 517 nm.
The scavenging activity of the 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+, C18H24N6O6S4) or the diammonium salt radical cation was determined as follows. A mixture of potassium persulfate (K2S2O8; 2.4 mM) and ABTS (7 mM) was prepared at a 1:1 ratio, protected from light using aluminum foil, and allowed to react at 22 °C for 14 hours. The resulting stock solution was diluted by adding 1 mL of the stock solution to 60 mL of ethanol (EtOH, C2H6O) to obtain a working solution with an absorbance of 0.706 at 734 nm. A fresh working solution was prepared for each test. THE, THH, ZME, ZMH, and Trolox samples were diluted with the ABTS working solution. The absorbance was measured at 734 nm after 7 minutes.
To ensure data validity, each sample was tested in triplicate (n = 3) for both ABTS and DPPH assays. Trolox (C14H18O4), a known antioxidant molecule, was used as a positive control in both ABTS and DPPH tests. The half-maximal effective concentration ratio (EC50) was used to indicate the radical scavenging capability of all tested samples as well as Trolox. The EC50 represents the concentration of a sample that causes a 50% decrease in the initial radical concentration. The inhibition of DPPH and ABTS assays was expressed as a percentage and calculated using the formula described as follows. A0 represents the absorbance of the blank while A1 is the absorbance of the standard or sample.
The determination of the lipase inhibition potential of two Indonesian seagrasses was performed by adhering to previous studies.22,25 Initially, crude pig pancreatic lipase (PPL) at a concentration of 1 mg/mL was dissolved in a 50 mM phosphate buffer with a pH of 7. The solution was then centrifuged at 12,000× g to eliminate any insoluble components. To create an enzyme stock solution at a concentration of 0.1 mg/mL, the supernatant was diluted 10-fold with the buffer. The lipase inhibition potential was evaluated based on previous research. In a transparent 96-well microplate, 100 μL of THE, THH, ZME, ZMH, and Trolox were mixed with 20 μL of 10 mM p-nitrophenyl butyrate (pNPB) in a buffer and incubated at 37 °C for 10 minutes. The results were compared to Orlistat (C29H53NO5), a well-known inhibitor of PPL or lipase. Measurements were taken at 405 nm using a DR-200Bc ELISA microplate reader. The unit of activity was determined based on the rate of reaction, specifically the yield of 1 mol of p-nitrophenol (4-nitrophenol) per minute at 37 °C. To assess the lipase inhibition activity, the PPL activity in the test mixture was reduced by 50%. To ensure the reliability of the study results, each sample was tested in triplicate (n = 3). The inhibitory data were calculated using the equation as follows. A represents the activity without an inhibitor; B represents the activity with an inhibitor; Ac and Bc represent the negative control without and with the inhibitor, respectively.
Statistical analyses for the data were conducted using the MacBook version of GraphPad Prism 9 Premium Software. The results are presented as mean values with their corresponding standard deviations (means ± SD). Multiple analysis of variance was performed to compare the lipase inhibition activity of THE, THH, ZME, ZMH, and Trolox at various concentrations. To analyze the EC50 values obtained from in vitro experiments, such as antioxidant inhibition of DPPH, ABTS, and anti-obesity assays (Lipase inhibition), a statistical analysis package called “non-linear regression (log [inhibitor] vs. normalized response)–variable slope” from GraphPad Premium was employed. All experiments were performed three times to ensure reliability and reproducibility.
A total of 9 and 11 metabolites were detected from T. hemprichii and Z. marina, respectively. However, they were classified further based on the extraction solvent. Some of the detected compounds were in the form of acid. Table 1 highlights the observed selected metabolite compounds from both seagrasses. The metabolites that were successfully observed were then continued with studies in silico or molecular docking on selected receptors for antioxidants and obesity.
According to Table 2, the molecular tethering assays focused on specific receptors such as human inducible nitric oxide synthase (iNOS), human reactive oxygen species (ROS) 1 kinase, human pancreatic lipase, and fat mass and obesity-associated (FTO) protein. All of these receptors were validated successfully with a judgment accuracy of less than 2 Å.
Based on Table 3, the results of molecular docking on THE showed that 4 substances had higher ΔG values than orlistat (control) in terms of anti-obesity potential. Luteolin-O-sulphate and luteolin – which are known as beneficial-rich flavonoids – also have a ΔG value exceeding antioxidant control and, interestingly, have an almost threefold greater ΔG compared to Trolox ΔG. THH has substances whose ΔG values are higher than THE’s in general, with 4 substances having an ΔG value above all controls used in this study. In the sea seagrass Z. marina, there were 11 substances studied, with 6 substances coming from ZME and the rest being components of ZMH, all of which have a greater ΔG value than orlistat or, in other words, have better anti-obesity potential than orlistat. 2 substances from ZMH, namely apigenin and diosmetin, also have better ΔG values when compared to controls, meaning that seagrass’s substance quality can compete with Trolox and S-ibuprofen. By doing molecular docking to the 4 components of the control, it was proven that the compound components in T. hemprichii and Z. marina have the potential to have antioxidant and anti-obesity properties in silico.
Amino acid interaction visualization of the active compounds of two Indonesian seagrasses against human pancreatic lipase, ROS1 Kinase, porcine pancreatic lipase, and FTO proteins can be found in Table S1.74 This subsequent part will present the results concerning the health-benefiting potential of the two Indonesian seagrasses based on in vitro biological assessment.
Fundamentally, oxidative stress from increased reactive oxygen species production can lead to various complications such as dyslipidemia and obesity.26 Therefore, this study assessed the antioxidant potential of T. hemprichii and Z. marina using in vitro DPPH and ABTS radical scavenging activity (Figure 2). From Figure 2A, it is seen that Trolox (control) had a lower EC50 value compared to the other groups in DPPH radical scavenging activity (EC50 Trolox = 83.85 μg/mL). From the data shown in Figure 2, it can be inferred that T. hemprichii and Z. marina have less potency in DPPH radical scavenging activity compared to the standard drug Trolox. However, between the two species, THE and THH had lower EC50 values (EC50 THE = 97.59 μg/mL, EC50 THH = 99.69 μg/mL) than ZMH and ZME (EC50 ZMH = 116.2 μg/mL, EC50 ZME = 117.7 μg/mL) meaning that T. hemprichii has better potency in radical scavenging activity than the species Z. marina with THE having the highest potency and ZME having the lowest potency respectively (Figure 2A).
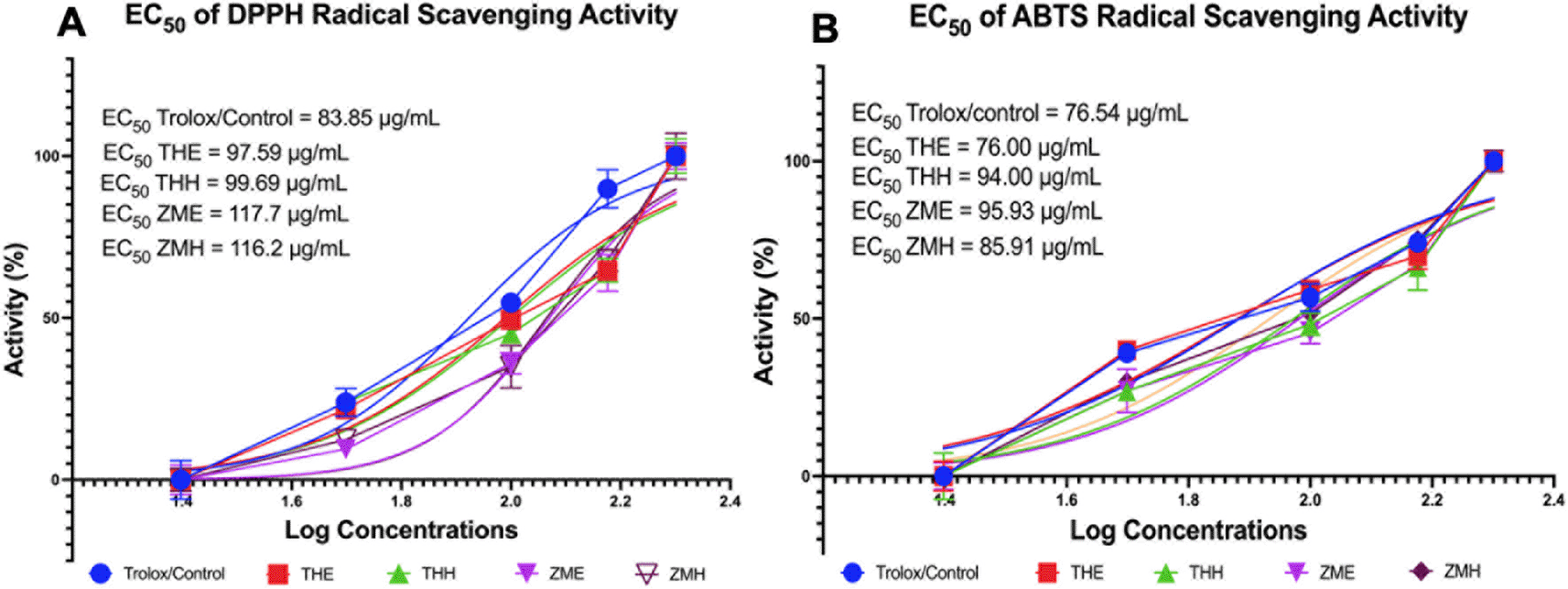
A. EC50 of Antioxidant capabilities via DPPH radical scavenging activity. B. EC50 of Antioxidant capabilities via ABTS radical scavenging activity. THE: Thalassia hemprichii—ethanol (polar); THH: Thalassia hemprichii—hexane (non-polar); ZME: Zostera marina—ethanol (polar); ZMH: Zostera marina—hexane (non-polar).
As for ABTS radical scavenging activity, ZME, THH, and ZMH showed higher EC50 values of 95.93 μg/mL, 94.00 μg/mL, and 85.91 μg/mL respectively, in comparison to Trolox EC50 value of 76.54 μg/mL meaning that THH, ZME, and ZMH performed less potency in radical scavenging activity compared to the standard drug Trolox. Interestingly, THE showed a lower EC50 value (EC50 THE = 76.00 μg/mL) than Trolox (EC50 THE = 76.54 μg/mL) meaning that THE has better potency in radical scavenging activity compared to the control. These results suggested that the ethanol (polar) extract of T. hemprichii (THE) has better antioxidant properties than the standard drug Trolox (Figure 2B).
Lipase inhibitory activity can determine the anti-obesity properties. It aims to confirm the potential of two seagrasses, T. hemprichii, and Z. marina, as functional food candidates to improve metabolic disorders, especially obesity. It was assessed in this study in a dose-dependent manner using in vitro lipase inhibitory activity (Figure 3B). It was shown that EC50 of T. hemprichii in polar extract (THE) has lower than EC50 of Orlistat, meaning that it has better potency than the positive control (Orlistat). Just as the polar extract, the non-polar extract of T. hemprichii (THH) is close to the EC50 value of Orlistat as a positive control, which suggests it has almost the same potency as the Orlistat. On the other hand, for both Z. marina samples, either extracted in polar (ZME) or non-polar (ZMH) solvent, the EC50 exceeded Orlistat as positive control; this shows that all samples have a lower potential for lipase inhibition activity than T. hemprichii. EC50 values from the smallest to largest values are THH, ZME, and ZMH are 69.97 μg/mL, 78.36 μg/mL, and 119.8 μg/mL respectively, in which Z. marina had larger EC50 than other samples.
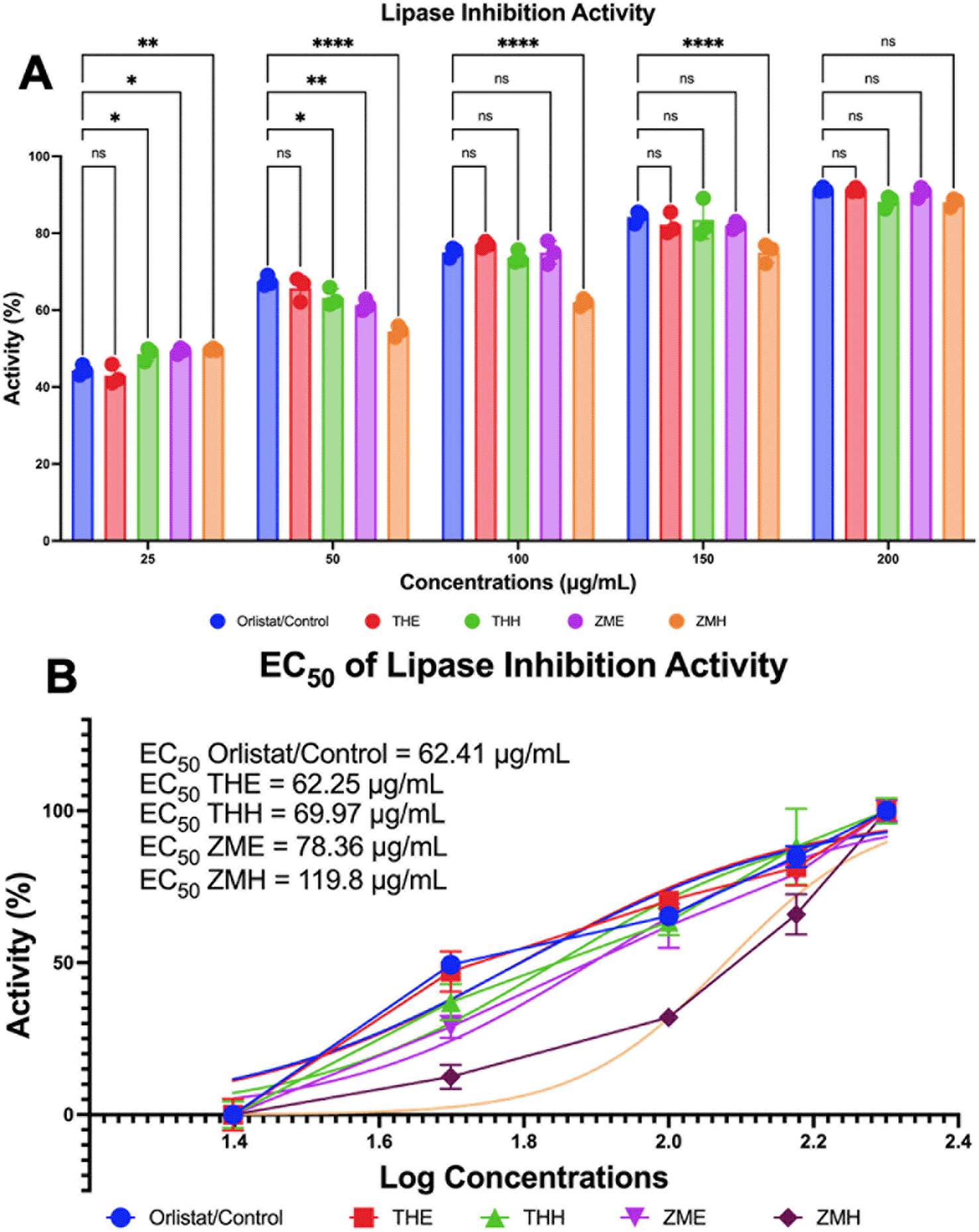
A. Differences in lipase inhibitory activity by Two Indonesian Seagrass compared to control (Orlistat) in different gradients concentration via MANOVA analysis. B. EC50 of lipase inhibition activity. THE: Thalassia hemprichii—ethanol (polar); THH: Thalassia hemprichii—hexane (non-polar); ZME: Zostera marina—ethanol (polar); ZMH: Zostera marina—hexane (non-polar). * p = 0.0417; ** p = 0.0066; **** p < 0.0001; ns p > 0.05 (not significant).
Marine products hold significant potential as a functional food and drug candidates due to their rich nutritional profiles and bioactive compounds. The oceans are teeming with a diverse range of marine organisms that offer unique health benefits. Fish, shellfish, seaweed, and microalgae are among the key marine products that have garnered attention for their potential therapeutic properties. Seagrasses are rich in essential nutrients, including vitamins, minerals, and dietary fibers. They also contain significant amounts of vitamins C, E, and folate, as well as minerals such as calcium, magnesium, and potassium.12 These nutrients are essential for maintaining optimal health and well-being and incorporating seagrass into the diet can contribute to meeting daily nutritional requirements. Beyond their nutritional value, seagrasses are assumed to contain bioactive compounds with potential health benefits. In this study, a total of 20 metabolites were successfully identified from various seagrass extracts.
Molecular docking is a molecular-level approach to computationally assess the interactions of compounds that have been successfully explored from a natural product to proteins that in this case are disease receptors. In this work, we perform molecular docking on each compound of two Indonesian seagrass T. hemprichii and Z. marina that has the potential to have antioxidant and anti-obesity effects. The control we used to determine the ability of these compounds was to look for receptors from iNOS (3E7G) with ΔG -4.73 found in S-ibuprofen and ROS 1 kinase (3ZBF) with ΔG -5.36 found in Trolox as an antioxidant marker, as well as human pancreatic lipase (1LPB) with ΔG -2.38 and fat mass and obesity-associated protein (3LFM) with ΔG -3.71 used as substances that have anti-obesity effects. Excessive iNOS and ROS 1 kinases in the body have the effect of being free radicals that ultimately damage proteins and induce apoptosis in cells. So inhibition using substances that attach to receptors can provide antioxidant effects and reduce oxidative stress injury.27,28 Orlistat, which targets inhibition of human pancreatic lipase and FTO, is approved by the US Food and Drug Administration for its use as an anti-obesity substance due to its efficacy in reducing the absorption of 30% of fat consumed.29 On the other hand, the enzymatic activity of FTO plays a crucial role in maintaining energy and adipose tissue balance.30 However, when this activity is disrupted, it leads to the dysregulation of genes associated with energy metabolism, thereby affecting the homeostasis of energy and adipose tissue. Therefore, inhibition of FTO is relevant in preventing obesity-related conditions.
So far we have identified four main components of each of the polar and nonpolar extracts of T. hemprichii as well as the polar and non-polar extracts of Z. marina. The results of EC50 measurements provide promising results as the metabolites with potential anti-obesity properties from the two seagrasses have been reported. Of all the samples, only one type of extract, THE, had better EC50 results than Orlistat as a control. However, the entire samples had a much better lipase inhibition profile than the previous study (62.25 μg/mL to 119.8 μg/mL compared to up to 876.30 μg/mL in Ecklonia radiata.31 In addition, another study showed that with a concentration of 10 mg/mL extracts of Ascophyllum nodosum, Laminaria japonica, and Lessonia nigrescens could not even achieve pancreatic lipase inhibition by 40%.32 The comparison of the results of the study above shows Z. marina extract and T. hemprichii has superior lipase inhibition activity.
One of the resistance mechanisms to obesity is the inhibition of lipase enzymes or inhibitors. Exogenous fats obtained from outside the body must go through a hydrolysis process into monoglycerides, glycerol esters, and free fatty acids. This mechanism is mediated by the lipase enzyme found in the mouth, gaster, and pancreas with the pancreas as the main hydrolysate (50 – 70%). Human pancreatic lipase consists of 449 fatty acids with a catalytic center enclosed by an N-terminus. The bonding ability between substrate and lipase depends on the area of the hydrophobic area in the enzyme obtained from changes in lipase conformation. Lipase works together with the coenzyme co-lipase secreted by the pancreas. When co-lipase proteins combine with bile acids in the duodenum, lipase action is activated so that lipid absorption in the intestine is optimal.33 Departing from this concept, the anti-obesity approach using lipase enzyme inhibitors or lipase inhibitors is expected to reduce the number of lipids absorbed to minimize the accumulation of fatty acids, cholesterol, and lipoproteins in the blood and adipose tissue deposition in the body.34 Lipase inhibitors work by binding to residues located at the active binding site of pancreatic and gastric lipase so that the ability of triglycerides to bind to lipase becomes inhibited. Due to its selective nature, weak absorption rate, and low risk of systemic side effects, there is potential to develop lipase inhibitors further into mainstream anti-obesity.35
A study suggests the potential use of H. stipulacea seagrass as an alternative to obesity therapy in extract form. The use of leaf and stalk extracts with hexane, ethyl acetate, and methanol as their solvents on zebrafish showed fat reduction activity in several extracts (EL, ML) with EC50 of 1.2-2.2 μg/mL based on the fat metabolism examination of Nile red zebrafish.36 These results are thought to be a result of the flavonoids cirsmarin (5-hydroxy-6,7-dimethoxy-2-[4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]chromen-4-one), N-acetyl-L-tyrosine, 2,4-dihydroxyheptadec-16-ynyl acetate, and 2,4-dihydroxyheptadec-16-ynyl acetate. Research using Halodule pinifolia extract has been conducted to understand the anti-inflammatory ability of the seagrass. By using ethyl acetal extract (EHP) and methanol (MHP) in vitro models of inflammation triggered by lipopolysaccharides, it was found that EHP has more anti-inflammatory abilities. With modern in vivo endotoxemia, EHP succeeded in reducing plasma IL-6 88.3%, TNF-α (78.2%), and IL-1β (74.5%). Similar results were also seen in carrageenan models of TNF-α edema (69.3%) and IL-1β (43.1%).37 Other studies showed antioxidant, antimicrobial, and antiproliferative properties. Another study observed sulfated polysaccharides from Cymodocea nodosa, finding the results of the examination of total antioxidants (59.03 mg ascorbic acid equivalent/g extract), reducing power (OD = 0.3), DPPH radical scavenging (EC50 = 1.22 mg/mL), and ABTS radical scavenging (EC50 = 1.14 mg/mL).38
Lipase inhibition holds an important key to preventing obesity because lipase inhibition will prevent the absorption of fat in the intestine so that it does not enter the bloodstream. If the amount of fat that enters the bloodstream is high continuously and for a long period, it will result in dysregulation of fat metabolism which will result in dyslipidemia.33 Besides its advantages as a lipase inhibitor, polar extract from T. hemprichii also has better antioxidant activity compared to Trolox as a control on ABTS assay. This makes the extract particularly valuable because it can work as a good antioxidant, where antioxidants are very important to prevent the emergence of obesity complications in people with metabolic syndrome. This is because most of the complications of obesity have a background of oxidative stress mechanisms such as pancreatic beta cell damage, insulin resistance, plaque formation in blood vessels, and endothelial damage to blood vessels.26 Oxidative stress has an important role in the pathophysiology of obesity such as by modifying the concentration of inflammatory mediators associated with the large number and size of adipocytes, promoting lipogenesis, stimulating the differentiation of preadipocytes into mature adipocytes, and regulating energy balance in hypothalamic neurons that control appetite. Fortunately, this can be countered by utilizing plants that have therapeutic antioxidant potential that can scavenge reactive oxygen species (ROS), such as in some types of seagrass plants.39,40
Luteolin appears to show decreased lipogenesis activity, decreased ectopic fat deposits, increased fat thermogenesis, and increased systemic energy expenditure associated with improvements in obesity conditions.41 Betaine may prevent obesity through gut microbiota.42 Another study using experimental rats given oleamide showed a decrease in the incidence of obesity.43 Palmitoleic acid given to rats orally appears to induce satiety through the release of hormones.44 Rosmarinic acid, kaempferol, p-coumaric acid, apigenin, diosmetin, and azelaic acid also appear to be associated with a reduced incidence of obesity or a decrease in metabolic disorders due to obesity.45–50
Antioxidants are one of the most important factors in reducing radical compounds in the body to reduce cell damage and ultimately prevent the occurrence of several diseases.51 Several other types of seagrass such as Enhalus acoroides, Halophila ovalis, Halophila major, and Halophila spinulosa contain flavonoids and phenolics which are commonly known as the largest phytochemical molecules and their antioxidant activity is stronger than vitamins C and E.52–54 Chrysoeriol, one type of flavonoid that is usually studied for its antioxidant effects and is often found in seagrass plants, has a significant effect on increased cell viability, reduced ROS formation, and increased occurrence of antioxidant molecules in H2O2. In addition, this compound has the ability to suppress peroxidation and has lipid interactions with peroxyl radicals.55,56 In phenolic compounds, antioxidant properties are obtained from the presence of hydroxyl groups in the benzene ring of the chemical element. The mechanism of antioxidant activity of this compound is obtained through the mechanism of hydrogen atom transfer by giving H atoms to the free radical substrate and producing non-radical substrate species (RH, ROH, or ROOH) and free radical antioxidants.57,58
There have not been many studies using DPPH substrate to evaluate the antioxidant potential of seagrass plants. Earlier studies used nitric oxide (NO), H2O2, catalase, and glutathione peroxidase to assess the antioxidant potential of this plant.59,60 DPPH can be used as a substrate to evaluate antioxidant activity because its stable nature can also form stable diamagnetic molecules.61 Previous research that has documented antioxidant potential through DPPH radical scavenging activity was obtained in a study that showed that H. ovalis methanol extract showed EC50 scavenging on DPPH radicals at 0.13 mg/mL.61 This was later validated by our in vitro study of DPPH radical scavenging activity in T. hemprichii and Z. marina which showed antioxidant potential as a therapy other than Trolox (Figure 2A).
In addition to the use of the DPPH method, an interesting discovery was found in measuring antioxidant capabilities using the ABTS radical scavenging activity method which showed that the potential of T. hemprichii in radical scavenging activity was better than the standard drug Trolox with a value of EC50 = 76.00 μg/mL. The results of this study complement the results of previous studies that assessed EC50 in T. hemprichii extracted 50% ethanol plus HCL 1 N at 60 oC with the DPPH method (EC50 = 83.48 μg/mL).62 Research by Jayaprakash et al. in 2017 also explained that compared to other seagrass species, T. hemprichii has the highest free radical flushing activity ability in its habitat.40 The discovery of EC50 values from DPPH and ABTS methods, as well as comparisons with Trolox as a standard antioxidant drug in this study confirm previous findings that have addressed the antioxidant capabilities of the seagrass T. hemprichii and provide comparisons in its potential use as a new antioxidant regimen option developed for humans. Furthermore, computational molecules of each compound showed the inhibition of free radicals by luteolin-O-sulphate, thalassiolin C, thalassiolin A, luteolin, apigenin, diosmetin, and other compounds (Table 3).
Luteolin, kaempferol, and apigenin have previously demonstrated antioxidant activity using DPPH and ABTS methods, with superior antioxidant activity compared to the control (Vitamin C). Among these, luteolin and kaempferol exhibited the highest antioxidant capabilities, followed by apigenin with the lowest antioxidant ability.63 This can explain the antioxidant activity observed in THE, THH, ZME, and ZMH. In a study using radiation-induced liver damage in experimental mice, it was found that treatment with betaine, which was also identified in THE in this study, resulted in decreased oxidative stress, reduced CYP450 activity, increased glutathione levels, decreased caspase-3 activity, and a decrease in fibrotic markers, accompanied by improved kidney function.64 Other studies have also demonstrated the antioxidant abilities of oleamide metabolites in mitochondrial toxicity in experimental mice. The results showed a reduction in lipid oxidation and alterations in glutathione reduction or oxidation.65 Palmitoleic acid, one of the omega-7 fatty acids, found in ZME, appears to exhibit antioxidant activity in HaCaT cells.66 Rosmarinic acid, alpha-eleostearic acid, p-coumaric acid, myristic acid, diosmetin, and azelaic acid have also shown antioxidant activity based on several previous studies.67–72
This study is the first to successfully profile metabolites and perform molecular docking, and the results are promising for anti-obesity and antioxidant functional foods based on in vitro validation and molecular docking results. However, this still needs further research i.e., isolation and purification of each observed compound and continuing in vivo trials in animal models prior to human clinical trials.
New insights have been obtained from this reported study, ranging from the metabolites profile of chemical constituents of two Indonesian seagrasses T. hemprichii and Z. marina, and accompanied by molecular activity against obesity receptors and antioxidant potential via molecular docking simulation. Some of the compounds observed in two Indonesian seagrasses have promising potential as inhibitors of iNOS, ROS1 kinase, human pancreatic lipase, and FTO proteins. These compounds include luteolin observed from THE; 6-hydroxy compounds luteolin O-glucoside, luteolin-O-sulphate, Thalassiolin A, and Thalassiolin C from THH; kaempferol-7,4’-dimethylether-3-O-sulfate from ZME; and apigenin and diosmetin from ZMH. Interestingly, further tests of antioxidant and anti-obesity activity from two Indonesian seagrass extracts showed the same promising potential as the results of a molecular docking simulation. THE’s EC50 value shows antioxidant capabilities via ABTS radical scavenging activity of 76.00 μg/mL, a smaller value than standard antioxidant controls (Trolox, 76.54 μg/mL) and followed by EC50 of lipase inhibition activity by THE which has the same pattern (EC50 THE < EC50 Orlistat). This suggests that the two Indonesian seagrasses have promising biological activity as a candidate for functional food and/or drugs in fighting free radicals and their interlink to obesity. In vivo studies and clinical trials are certainly needed to see the sustained efficacy value of the two seagrasses, which are being planned in the future.
Conceptualization and methodology: B.T.W., F.N., N.A.T. and N.M.; software, investigation, data curation, and visualization: F.N. and B.T.W.; validation, formal analysis and supervision: F.N., N.A.T. and N.M.; Writing, original draft preparation, review, and editing: B.T.W., F.N., W.B.G., M.F.N.A.M., D.G.L., F.R., E.L.B., P.M.D., D.Y.; Validation, Writing-revised-review, and editing: N.S., D.A., C.H., A.A. and R.L.; Molecular Docking Simulation and their visualization: D. A and A.A. All authors have read and agreed to the published version of the manuscript.
figshare: RAW data DPPH, ABTS, and Lipase of Seagrass “Antioxidants and Anti-Obesity Properties of Two Indonesian Seagrass Thalassia hemprichii and Zostera marina”, https://doi.org/10.6084/m9.figshare.22826048.v2. 73
Metabolites data are provided in this manuscript, see Table 1.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
figshare: Fully Visualization of Amino acid interaction of T. hemprichii and Z. marina metabolites against human inducible nitric oxide synthase (iNOS), human reactive oxygen species (ROS) 1 kinase, human pancreatic lipase, and fat mass and obesity-associated (FTO) protein. Available at: Figshare https://doi.org/10.6084/m9.figshare.22826078.v1. 74
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Natural products (Extraction, isolation, analysis and identification), Chromatography, Site Directed Mutagenesis
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: natural product; medicinal chemistry; antioxidant; molecular docking
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 08 Apr 24 |
read | |
|
Version 1 21 Jun 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)