Keywords
breast cancer, survivors, prior history, racial disparities, major adverse cardiac and cerebrovascular events (MACCE), cardiovascular, mortality
This article is included in the Health Services gateway.
This article is included in the Oncology gateway.
breast cancer, survivors, prior history, racial disparities, major adverse cardiac and cerebrovascular events (MACCE), cardiovascular, mortality
With the improving survival of breast cancer patients, cardiovascular adverse events in this population group have been gaining increasing attention. For women diagnosed with breast cancer between 2010 to 2016, the 5-year relative survival rate for nonmetastatic invasive breast cancer was reported to be 84%.1 Of note, the prognosis of breast cancer has been improving over the years. Since 2007, the number of women 50 years and older who have died of breast cancer has continued to decline. The number of women younger than 50 who have died of breast cancer has remained consistent. From 2013 to 2018, the death rate for women with cancer dropped by 1% each year.2 Racial disparities in cardiovascular morbidities among breast cancer survivors are not well studied.3
We conducted this national population-represented sample-based study to investigate major cardiovascular and cerebrovascular adverse events in breast cancer survivors and how they might differ among the various ethnicities. Breast cancer and its treatment has been shown to have an association with cardiovascular morbidity and mortality. Anthracycline is used in both early and advanced stages. Anthracycline-related irreversible cardiomyopathy has been of concern since the initial years of its use as a part of chemotherapy regimens.4 Black and Hispanic patients have a higher incidence of heart failure following anthracycline treatment compared to other ethnic groups.5 Ethnic variation in the incidence of breast cancer has been well-established for decades.2 Incidence is known to be higher among white women. However, survival rates have been reported to be varying among different races, and black women are known to have poorer outcomes.6 The choice of categories in our study was based on the previous studies, as noted above, that evaluated the racial differences in the incidence and survival of breast cancer. As the differences are not studied for major cardiovascular and cerebrovascular adverse events, we decided to conduct this study. There were no funding agencies involved and no rules of human categorization were required for this study.
We used National Inpatient Sample datasets (NIS) from October 2015 to December 2017. These datasets are publicly available under the Healthcare and Utilization Project of the Agency for Healthcare Research and Quality (AHRQ).7 Datasets of each year contain 20% stratified sample of discharges from community hospitals (excluding rehabilitation and long-term acute care institutions). Statistical weighted analysis of these datasets yields national estimates of inpatient hospitalizations, utilization, access, costs, and outcomes for the United States. We did not need Institutional Review Board approval because NIS contains de-identified data. To minimize the potential for bias, comprehensive multivariable regression analysis was performed.
We used ICD10 codes to identify adult hospitalizations with breast cancer survivors (i.e., with prior history of breast cancer - Z85.3) and with and without a history of chemotherapy (CT, Z9221) or radiotherapy (RT, Z923). The Clinical Classifications Software Refined (CCSR) aggregates ICD-10-CM/PCS diagnostic and procedural codes into clinically meaningful categories.8 Using CCSR outcomes of interest were generated. AHRQ comorbidity software-generated comorbidities measures (binary variables) were used in the analysis.
Using the algorithm as shown in Figure 1, all adult admissions with history of breast cancer, above the age of 18 years, were identified. This cohort was further divided into two sub groups; patients who had received some form of chemotherapy (CT) and/or radiotherapy (RT) and those without exposure to CT/RT. A subsequent sub-cohort of the population whose racial data was available were identified. These cohorts were then further stratified by race to study outcomes of interest, including cardiovascular disease (CVD) burden, major adverse cardiovascular and cerebrovascular events (MACCE), and healthcare resource utilization. The patient population whose data pertaining to race was not available were excluded from the study.
Age in a continuous fashion and other variables (race, admission type, hospital characteristics including hospital bed size (small, medium, or large), location/teaching status (rural, urban non-teaching or urban teaching), region, median household income quartiles, and payer status, disposition type, death as an outcome) in categorical fashion were used for statistical analysis.
We included all-cause in-hospital mortality, acute myocardial infarction (AMI), arrhythmia, and stroke as major adverse cardiovascular and cerebrovascular events (MACCE) for the outcomes. All-cause in-hospital mortality was available as NIS has an in-build variable “died” corresponding to in-hospital deaths. Other outcomes were extracted from discharge diagnosis using clinical classification software for all-cause mortality, acute myocardial infarction (AMI, CCS 100), arrhythmia (CCS 106), and stroke (CCS 109). NIS has an in-built variable “race”, with the categories: White, Black, Hispanic, Asian or Pacific Islander, Native American, and other. MACCE was compared among these races. Racial disparities for MACCE were also assessed for breast cancer survivor subgroups with and without prior CT/RT.
In SPSS V24.0 (IBM Corp., Armonk, NY, USA), data on national estimates were examined using sampling weights and complex sample modules. Discharge records with missing data for race (<5%) were excluded from the final analysis. Baseline characteristics including demographics, comorbidities; and MACCE outcomes were compared among races using chi-square (statistical significance determined as a two-sided p-value 0.05) for categorical or student’s t-test for continuous data. Besides extracted breast cancer survivor sample population, these were also compared for sub-populations with and without prior CT/RT. The adjusted odds ratios (aOR) of MACCE were estimated with whites as the reference group using multivariate logistic regression, correcting for relevant confounders such as sociodemographic factors, cardiac and extracardiac comorbidities, and prior CT/RT.
We identified 1,301,320 nationwide hospitalizations with a prior history of breast cancer in women, 83.9% (1,092,280) without, and 16.1% (209,040) with prior CT/RT. Of the admissions with a prior history of breast cancer with exposure to CT/RT (209,040), those whose data pertaining to race was not available, were also excluded, resulting in a final cohort of 201,965 patients. Discharge records with missing data for race (<5%) were excluded from the final analysis. The majority of hospitalizations were distributed between White (151, 510 patients, 75.8%) and Black patients (27, 885 patients, 11.3%), followed by Hispanic patients (12,190 patients, 6.04%), Asian/Pacific Islander patients (4,765 patients, 2.35%), Native American patients (750 patients, 0.37%) and patients of other races (4,865 patients, 2.4%). Breast cancer survivors had a higher mean age for White patients, followed by Asian or Pacific Islander, Black patients, Hispanic patients, and Native American patients at hospitalization (75, 72, 69, 69, 69, and 69 years, respectively Table 1). Breast cancer survivors without prior CT/RT maintained a similar pattern to the overall study population, but breast cancer survivors with prior CT/RT had lower mean age for all races; White, Asian or Pacific Islander, Black, Hispanic, Native American (71, 66, 64, 63, 63 years, respectively Table 2). Non-elective admissions were highest in Black patients (82.9%), followed by Native American patients (80.6%), Hispanic pateints (80.3%), Asian or Pacific Islander patients (78.9%), and White pateints (77.4%), with a similar pattern observable in both subgroups i.e., with and without prior CT/RT (Table 3).
| Characteristics | White (986690) | Black (147055) | Hispanic (69525) | Asian/Pacific Islander (24180) | Native American (4280) | Other (26130) |
|---|---|---|---|---|---|---|
| Admitting Characteristics | ||||||
| Age (years) | 75 [67-84] | 69 [59-78] | 69 [58-79] | 72 [61-81] | 69 [59-77] | 72 [61-81] |
| Non-elective admissions | 77.4 | 82.9 | 80.3 | 78.9 | 80.6 | 76 |
| Primary expected payer (uniform) | ||||||
| Medicare | 78.1 | 68.2 | 61.7 | 61.5 | 67.1 | 64.9 |
| Medicaid | 3.3 | 10.9 | 15.5 | 10.5 | 13.3 | 9.7 |
| Private Insurances | 16.7 | 17.8 | 18.6 | 24.7 | 15.9 | 21.6 |
| Self-pay | 0.6 | 1.3 | 2.5 | 1.3 | 1.5 | 1.8 |
| No charges | 0.1 | 0.2 | 0.3 | 0.1 | 0.2 | |
| Others | 1.1 | 1.6 | 1.5 | 1.8 | 2.2 | 1.8 |
| Median household income national quartile for patient ZIP Code (percentiles)# | ||||||
| 0-25th | 20.7 | 51.3 | 35.4 | 12.2 | 41.3 | 23.4 |
| 26-50th | 25.7 | 20.9 | 24 | 16.1 | 25.7 | 18 |
| 51-75th | 26.8 | 16.2 | 22.2 | 27.1 | 18.2 | 24.7 |
| 76-100th | 26.9 | 11.6 | 18.4 | 44.6 | 14.8 | 34 |
| Bed size of the hospital§ | ||||||
| Small | 19.8 | 17.6 | 18 | 16.2 | 21.4 | 17.3 |
| Medium | 29.2 | 29.2 | 31.1 | 26 | 22.4 | 28 |
| Large | 50.9 | 53.2 | 50.9 | 57.9 | 56.2 | 54.7 |
| Location/teaching status of the hospital~ | ||||||
| Rural | 8.9 | 4.5 | 2.8 | 2.7 | 22.2 | 2.9 |
| Urban non-teaching | 26.3 | 16.9 | 25 | 22.2 | 19.3 | 23.4 |
| Urban teaching | 64.7 | 78.6 | 72.2 | 75.1 | 58.5 | 73.7 |
| Region of hospital | ||||||
| Northeast | 21.4 | 18.6 | 17.8 | 16.1 | 7.2 | 35.7 |
| Midwest | 25.7 | 22.6 | 7.6 | 9.1 | 19 | 11.6 |
| South | 35.2 | 50.5 | 42.1 | 15.2 | 36.4 | 35.5 |
| West | 17.7 | 8.4 | 32.5 | 59.6 | 37.3 | 17.2 |
| Comorbidities | ||||||
| Hypertension | 71.3 | 83.3 | 68.8 | 69 | 70.4 | 67 |
| Diabetes Mellitus | 22.6 | 37.8 | 34.9 | 34.1 | 41.1 | 28 |
| Smoking | 34 | 33.2 | 20.9 | 14.2 | 36.8 | 24.1 |
| Dyslipidemia | 45 | 42.3 | 40.2 | 44.8 | 36.9 | 41.1 |
| Obesity | 11.9 | 18.8 | 14.1 | 6.2 | 13.6 | 11.5 |
| Renal failure | 13.1 | 22 | 13.5 | 15.3 | 17.3 | 11.3 |
| Chronic pulmonary disease | 22.5 | 22.1 | 17.6 | 13.3 | 24.6 | 17.3 |
| Congestive heart failure | 12.5 | 15.7 | 10.1 | 9.5 | 13.2 | 11 |
| Pulmonary circulation disease | 0.8 | 1.1 | 0.8 | 0.5 | 1.4 | 0.9 |
| Peripheral vascular disease | 5.4 | 5.3 | 4.4 | 5.3 | 3.9 | 4.2 |
| Coagulopathy | 5.1 | 5.2 | 6.2 | 6.8 | 7 | 5.7 |
| Congestive heart failure | 12.5 | 15.7 | 10.1 | 9.5 | 13.2 | 11 |
| Depression | 15.9 | 10 | 13.1 | 6.5 | 15.5 | 12.3 |
| Outcomes | ||||||
| Acute Myocardial Infarction | 3.2 | 3.1 | 2.7 | 3 | 4.1 | 2.8 |
| Arrhythmia | 29.6 | 21.6 | 19.2 | 23 | 18 | 23.9 |
| Stroke | 3.3 | 3.8 | 2.9 | 3.9 | 2.8 | 3 |
| Died during hospitalization | 2.4 | 2.6 | 2.6 | 3.2 | 2.8 | 3 |
| Disposition of patients | ||||||
| Routine | 47 | 51.6 | 57.2 | 56.7 | 57.7 | 52.2 |
| Transfer to short term hospitals | 1.8 | 1.8 | 1.6 | 1.6 | 2.8 | 2 |
| Other transfers include SNF, ICF, etc. | 26.9 | 20.7 | 17.3 | 18.7 | 19.7 | 21 |
| Home health care | 21.5 | 22.5 | 20.4 | 19.5 | 16.4 | 21 |
| Length of stay (days) | 3 [2-6] | 4 [2-6] | 3 [2-6] | 3 [2-6] | 3 [2-6] | 3 [2-6] |
| Hospitalization charges (USD) | 9076[5376-15331] | 8833[5309-15275] | 9416[5626-16297] | 11199[6655-19760] | 8702[5461-15339] | 10812[6304-18630] |
# Represents a quartile classification of the estimated median household income of residents within the patient’s zip code, https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
§ The bed size cutoff points divided into small, medium, and large have been done so that approximately one-third of the hospitals in a given region, location, and teaching status combination would fall within each bed size category. https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nrdnote.jsp.
~ A hospital is considered to be a teaching hospital if it has an American Medical Association-approved residency program. https://www.hcup-us.ahrq.gov/db/vars/hosp_ur_teach/nrdnote.jsp.
The prevalence of comorbidities was assessed. Hypertension was the most common condition in the two sub-populations of breast cancer survivors, and among all races; with Black patients having the highest prevalence (83.3%), followed by White patients, Native American patients, Asian/Pacific Islander patients, and Hispanic patients (71.3, 70.4, 69. 68.8 percent, respectively). Overall and in both cohorts, diabetes mellitus (DM) was prevalent in Native American patients (41.1%) followed by Black patients (37.8%), and it was lowest in White patients (22.6 percent). In the whole breast cancer survivor population and prior CT/RT subgroup, White patients had a higher prevalence of dyslipidemia (45%, 42.2%), followed by Asian or Pacific Islander patients (44.8%, 39.3%) and Black patients (42.3%,37.8%). Smoking was prevalent in the whole breast cancer survivor sample population and two subgroups, with Native American patients smoking the most, followed by White and Black patients. Obesity, renal failure, congestive heart failure, and pulmonary circulation disorder were the most common conditions in Black patients; chronic pulmonary illness and coagulopathy were the most common conditions in Native American patients; and depression was the most common condition among White patients.
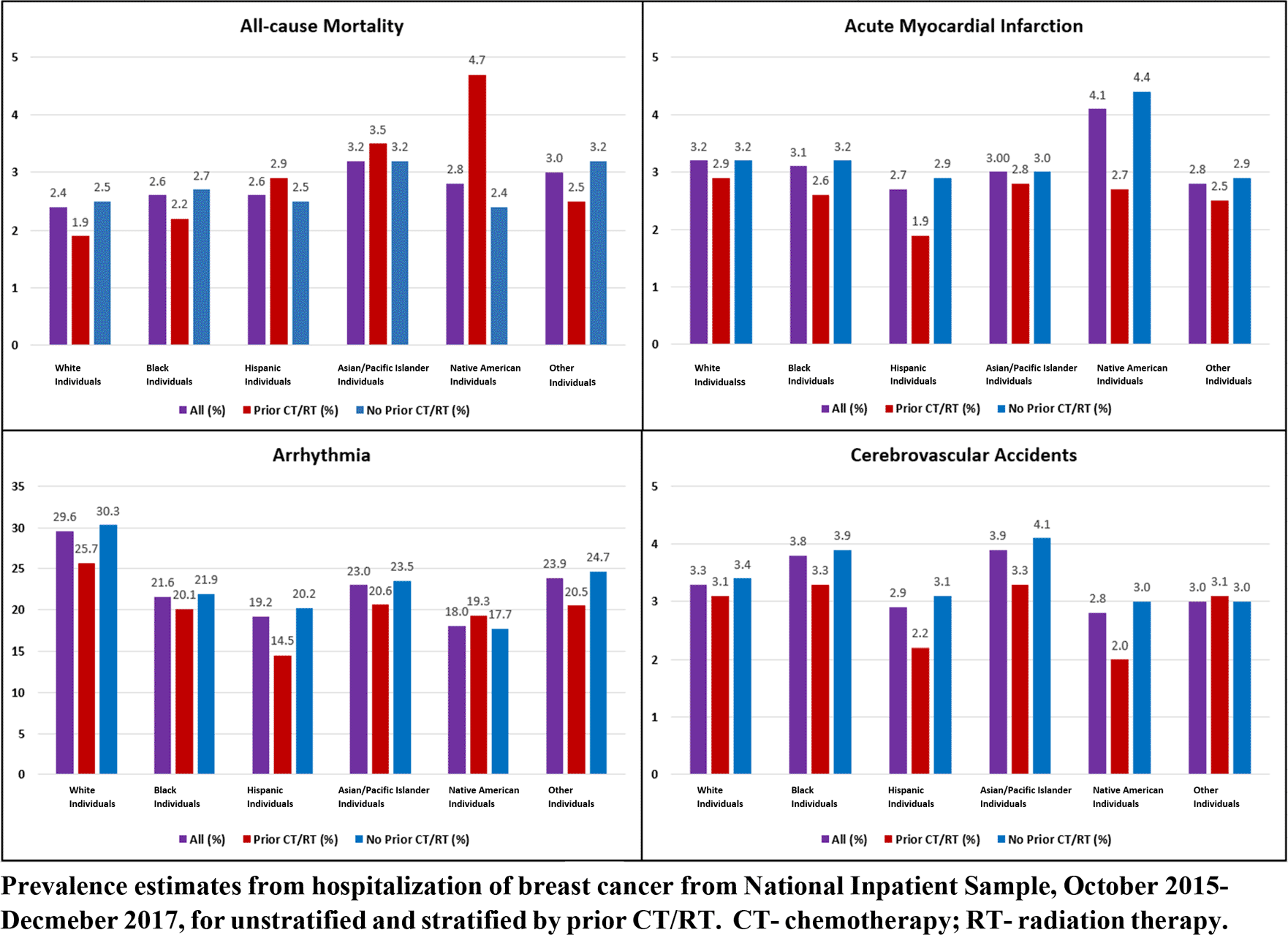
The prevalence and adjusted odds ratios of MACCE among racial groupings were our outcomes of interest (Figure 2). Prevalence of in-hospital deaths was the highest among Asian or Pacific Islander patients in the whole breast cancer survivor population (3.2%) and in those without prior CT/RT (3.2%), followed by Native American patients (3%) whereas Native American patients (4.7%) had higher in-hospital deaths as compared to Asian or Pacific Islander patients (3.5%) in those with prior CT/RT. Asian or Pacific Islander patients (aOR 1.19, 95% CI 1.10, 1.28, p0.001), Hispanic patients (aOR 1.14, 95% CI 1.09, 1.20, p0.001), and Black patients (aOR 1.10, 95% CI 1.06, 1.20, p0.001) had significantly higher odds of all-cause mortality than White patients, whereas Native American patients had non-significant difference. The incidence of AMI was the greatest among Native American patients (4.7%) among the whole breast cancer survivor population, followed by White patients (3.2%) and Black patients (3.1%). In the prior CT/RT subgroup, White patients had 2.9% AMI incidence, followed by Asian or Pacific Islander patients (2.8%), Native American patients (2.7%), Black patients (2.6%), and Hispanic patients (1.9%). When compared to White patients, Native American patients had higher odds of having an AMI (aOR 1.31, 95% CI 1.12, 1.53, p=0.001), but other races had statistically significantly lower odds. In the whole breast cancer survivor population, the incidence of arrhythmia was highest among White patients (29.6%), followed by Asian/Pacific Islander patients (23%), and Black patients (21.6%). In both the CT/RT and non-CT/RT subgroups, the incidence of arrhythmia followed a similar pattern. All races had considerably lower odds of developing arrhythmia than White patients, with Native American patients having the lowest odds ratio (aOR 0.62, 95% CI 0.57, 0.68, p0.001) (Table 4). Amongst all the breast cancer survivors, stroke was most common among Asian or Pacific Islander patients (3.9%) and Black patients (3.8%), followed by White patients (3.3%), with a similar pattern observed across both subgroups. With White patients as referents, Black patients had statistically significant odds (aOR 1.12, 95% CI 1.08,1.15, p0.001), while Hispanic patients had significantly reduced odds (aOR 0.89, 95% CI 0.85, 0.94, p0.001) of stroke. Though Asian or Pacific Islander patients had a higher trend toward strokes, the adjusted odds ratio did not reach statistical significance.
Hospitalizations were greater in large bed-size and urban teaching institutions in the whole breast cancer survivor population and subgroups with and without CT/RT (more than 50 percent across all racial groups) than smaller-size and other non-teaching institutions. Black patients, Hispanic patients, and White patients had higher hospitalizations in the Southern region of the US, whereas Western region hospitals had higher hospitalizations for Asian or Pacific Islander patients and Native American patients. Though Medicare was a major primary payer across all races, it covered hospitalization costs of 78% White patients versus 68% Black patients and 67% Native American patients. In the whole breast cancer survivor population, Black patients (51.3%) were highest in the lowest quartile (0-25th) median household income, followed by Native American patients (41.3%) and Hispanic patients (35.4%); the pattern was comparable in two subgroups. The median length of stay in the hospital was four days for Black patients, compared to three for the other races. Routine discharges were lowest among White patients, whereas transfers to skilled facilities were highest. For the whole breast cancer survivor population and subgroups, Asian or Pacific Islander patients had the highest mean total expenditure, followed by Hispanic patients and White patients.
Previous research has found racial disparities in the incidence of breast cancer, anthracycline-related cardiac outcomes, and cardiovascular risk factors.2,5,6 This research looks at the racial disparities in MACCE in breast cancer survivors along with the results being reported for two subgroups, i.e., with and without prior CT/RT, from the largest nationally representative national database. We discovered that three-quarters of the sample population was White, and about three-eighths was Black, followed by other races. Asian or Pacific Islander patients had the highest all-cause in-hospital mortality in the whole breast cancer survivor population whereas Native American patients had the highest all-cause in-hospitality in the prior CT/RT subgroup. Native American patients had the highest incidence of AMI in the overall population, but White patients had predominated in the prior CT/RT subgroup for AMI. White patients also had the highest incidence rate of arrhythmia regardless of treatment status. For strokes, Asian or Pacific Islander patients and Black patients had a higher prevalence than others. All-cause mortality, AMI, arrhythmia, and stroke had the highest odds in Asian or Pacific Islander patients, Native American patients, White patients, and Black patients respectively. Black patients had a lower socioeconomic position and a longer median stay than the others. White patients had a higher transfer to nursing facilities, whereas Asian or Pacific Islander patients had the highest mean hospital expenditures.
Cardiovascular disease is common among patients with cancer and coexists with other risk factors such as diabetes, obesity, etc.9 This can be attributed to underlying systematic inflammation however, cardiovascular disease also results from anti-cancer therapies.9,11 With the introduction of personalized medicine and providing patient-centered care, there is a trend of developing strategies for early diagnosis and treatment of disease and treatment-related complications such as cardiovascular disease and cerebrovascular disease. The ethnic disparities among patients continue to be a challenge to providing optimal care as race, a complex variable, could act as a proxy for various factors like socioeconomic status, culture, and discrimination.6 It is also implicated to act as a surrogate for differences in tumor and host biology impacting hormone receptor status, tumor grade, and S-phase fraction, which could lead to more aggressive tumor in certain races making treatment challenging.6
Hypertension is a modifiable risk factor that has been linked with an increased risk of breast cancer and cardiovascular diseases.12 Soler et al. (1999) reported OR of 1.44 for breast cancer risk associated with treated hypertension in women with BMI >25 kg/m^2.13 This link is not fully understood; however, few mechanisms have been proposed – namely that hypertension and breast cancer share similar pathophysiological pathways leading to a state of chronic inflammation, perhaps from an abundance of adipose tissue, leading to both hypertension and breast cancer. Another mechanism is exposure to steroid hormone factors e.g. estradiol has been implicated in the development of breast cancer and hypertension.14 Furthermore, those with hypertension have a 45% increased risk of mortality in metastatic breast cancer patients. Therefore, hypertension contributes to increased mortality risk in breast cancer, which in part, explains the increased mortality risk in Black patients compared to White patients. Black individuals are known to have a high incidence of cardiovascular risk factors such as HTN, DM, and obesity,12 which can subsequently lead to a higher incidence of cerebrovascular events.12
There are several causes of increased incidence of cardiovascular risk factors and disease in breast cancer patients. There are mainly three explanations. Firstly, breast cancer and cardiovascular disease share similar risk factors – older age, obesity, diet, family history, hormone replacement, physical activity, and tobacco use.9 Our results are aligned with CDC in 2019 reported that the highest incidence of hypertension and obesity were in African American and dyslipidemia in Caucasians.10 Obesity has been linked to the development of CVD, including atherosclerosis, abdominal aortic aneurysm, and heart failure, as well as breast cancer through chronic low-grade inflammation, which accelerates the onset or progression of carcinogenesis.11
White patients were noted to have higher rates of arrhythmias and AMI in this study. This is in accordance with the data reported by Chi. et al. (2020) reported the highest rates of AMI in White patients, followed by African American patients, Hispanic patients, and Asian or Pacific Islander patients.15 This study showed, however, that between 2000 and 2014, the absolute burden (the number of people hospitalized) of AMI declined in the White population. It also showed that the absolute burden of AMI increased for Hispanic population and Asian or Pacific Islander population over the same study period, which is likely explained by rapid growth in population size for these demographics and slower growth for the White population.15 It has been shown in several studies that the White population has an increased risk for arrhythmia's such as atrial fibrillation regardless of concurrent cardiovascular risk factors, particularly when compared to African American population.16 The reasons are unclear as African American patients tends to have higher rates of cardiovascular risk factors, but it could be related to genetic factors or environmental exposures related to race.
As mentioned above, the use of cardiotoxic chemotherapeutic agents such as anthracyclines, alkylating agents, and taxanes is also notorious for causing cardiovascular diseases in cancer patients. Cardiotoxicity is defined as the presence of symptoms of HF with an ejection-fraction reduction ≥5% to <55% or the absence of symptoms with an ejection-fraction reduction ≥10% to <55%.17 There are four main mechanisms of medication-induced damage to the heart 1) direct cytotoxic effects (alkylating agents, anthracyclines, interferon alfa, monoclonal antibodies), 2) cardiac ischemia (antitumor antibiotics, fluorouracil, topoisomerase inhibitors), 3) cardiac arrhythmias (anthracyclines), 4) pericarditis (bleomycin, cyclophosphamide, cytarabine).18 Cardiotoxicity can be reversible/irreversible, acute/chronic, early-onset/late-onset. As per our study, the breast cancer survivor subgroup that received CT/RT has a lower prevalence of acute myocardial infarctions, arrhythmias, and stroke as compared to patients who did not receive CT/RT. It is difficult to ascertain what mechanisms are responsible for our study results as details of chemotherapeutic regimens, dosages, and duration of treatment were not available.
There are disparities in cardio-oncology contributing to the higher morbidity and mortality of African American patients. For example, a retrospective study in 2004 showed that African American patients had a 3-fold higher risk of cardiotoxicity with doxorubicin compared to non-African American patients.19 A second study showed that African American women were more than 2-fold likely to develop cardiotoxicity from trastuzumab and had a much higher likelihood of not completing therapy compared to White women.20 These findings could possibly explain the findings in our study, where Black patients were shown to have higher all-cause mortality.
All-cause mortality, when adjusted for demographic confounders, was found to be higher in Black than in White individuals. This is in line with several published studies and can be explained by the following. African American women have more aggressive forms of tumors – triple-negative breast cancers – when they are discovered.6 African American pateints, in general, have lower socioeconomic status, which translates into less access to healthcare facilities and primary care clinics as well as preventative visits, causing lower rates of screening mammography and late-stage diagnosis.21 Underserved communities are less likely to have equipped facilities for screening or the appropriate expertise available. Lower socioeconomic status also means less access to health insurance; in fact, studies have shown African American women are twice as likely to be uninsured and depend on public insurance as compared to White American women.22 Lower Socioeconomic Status directly correlates to lower educational attainment,6 and therefore patients may not understand the importance of early detection of breast cancer.6 Furthermore, there is a lack of diversity in clinical trials for multiple reasons, such as cultural or language barriers to informed consent, and most cardiotoxicity studies do not report race-specific data on cardiotoxicity prevalence.23
In our study, Native American pateints with breast cancer with and without prior CT/RT were found to have the highest prevalence of diabetes mellitus, smoking, chronic pulmonary disease, and coagulopathy. A 2015 population study estimated the prevalence of diabetes mellitus to be 5.5% in breast cancer patients.24 At present, there is no literature on the prevalence of diabetes in Native American pateints with breast cancer with/without prior CT/RT. The presence of diabetes has been linked to increased mortality and a worse prognosis of breast cancer.24 Multiple mechanisms have been proposed to explain this link. Hyperinsulinemia has been shown to promote cell proliferation, reduced apoptosis, angiogenesis, and metastases of breast cancers by activating insulin growth-like factor 1 (IGF-1R) signalling pathways.25,26 Insulin resistance and hyperinsulinemia are linked to decreased rates of recurrence-free survival.27
Smoking is also a modifiable risk factor contributing to cardiovascular disease in cancer patients.28 In our study, smoking was found to have the highest prevalence (36.8%) in Native American patients with breast cancer with and without prior CT/RT, followed by White patients (34) and Black patients (33.2). The Indian Health Service reported that Native American individuals had the highest smoking prevalence compared to the other racial groups in general, with 23.2% in both Native American men and women.28 This can be contrasted with the lower smoking prevalence in White (21.1% and 17.2%) and African American (22.8% and 15.4%) men and women, respectively.28 There are several possible explanations for the high smoking prevalence in the Native American community. Tobacco is used for ceremonial or medicinal purposes and has a high cultural and spiritual importance. Secondly, the average smoker begins smoking in early adolescence (age 14.7), and thirdly quitting rates are relatively lower compared to other racial groups.29,30 The CDC also reports that tobacco is extensively promoted and marketed to the Native American communities; however, the effect and impact of this are difficult to measure.29 Smoking has been associated with a higher risk of breast cancer-specific and all-cause mortality, as well as poorer prognosis.31 It has also been shown that African American women with ER Negative breast cancer who smoke have higher mortality compared to non-African American women.32
Our study was conducted using the National Inpatient Sample. The study could only be conducted in a cross-sectional manner, without allowing for further analysis of results. The “Others” race cannot be elaborated upon owing to missing information on races in this class. The details of chemotherapeutic drugs or regimes, number of chemotherapy cycles, types of radiation therapy, and details of comprehensive cancer regimes are not known in National Inpatient Sample. Duration since the completion of oncology treatment is not available. Despite this, we provide contemporary data for the racial disparities in the MACCE in the patients who had survived breast cancer which is of large significance in designing comprehensive screening programs for high-risk populations.
Our study shows that there are significant differences in MACCE events in breast cancer survivors of different races. All-cause mortality is lowest in whites even though the incidence of AMI and arrhythmias were higher. There appears to be a complex interplay of factors influencing clinical outcomes in breast cancer patients of various races. These factors are poorly understood, and further basic science research is warranted for a better understanding of why different conditions are more common in different racial populations. This will provide a gateway into major clinical research on cancer therapies oriented based on race, targeting specific morbidities from which that race is more prone to have an adverse outcome. Our results also emphasize the need for tailoring the screening programs differently for different races to prevent cardiovascular events.
Our study examined racial disparities for major cardiovascular and cerebrovascular events in hospitalized breast cancer survivors using discharge data from the National Inpatient Sample and the Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality. We used the 2015-2017 datasets which size over 100 GBs. It is the largest inpatient care database of the United States and contains data on over 7 million hospital stays. Data can be accessed from the website of Agency for Healthcare Research and Quality.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Bryant AK, Banegas MP, Martinez ME, Mell LK, et al.: Trends in Radiation Therapy among Cancer Survivors in the United States, 2000-2030.Cancer Epidemiol Biomarkers Prev. 2017; 26 (6): 963-970 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Health services research, cancer survivorship, endocrinology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: epidemiology, cancer, survivors, racial disparity
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 26 Jul 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)