Keywords
Black cumin, Nigella sativa, Black seed, Thymoquinone, Prostate.
This article is included in the Oncology gateway.
A growing amount of research is shedding light on functional foods and nutritional supplements’ potential health and disease-preventative advantages. Black cumin (Nigella sativa L.), an esteemed nutraceutical herb, is well-known for its multiple health advantages among health-conscious individuals, researchers, and pharmaceutical businesses. Black cumin and its principal bioactive ingredient, thymoquinone (TQ), have been found to lower oxidative stress and inflammation, while also enhancing immunological function, cellular viability, and energy metabolism. They protect against metabolic, cardiovascular, digestive, hepatic, renal, pulmonary, reproductive, and neurological diseases, as well as cancer. Black cumin works as a countermeasure to minimize the toxicity and side effects of pharmaceuticals. Furthermore, the possible effects of black cumin on prostate health and disorders like benign prostatic hyperplasia and prostate cancer are not well understood. This narrative review seeks to reveal knowledge gaps. This study intends to guide future research into the possible uses of black cumin and TQ in prostate health and illness.
Black cumin, Nigella sativa, Black seed, Thymoquinone, Prostate.
The prostate, a small gland in the male reproductive system, plays a vital role in the production of seminal fluid, which nourishes and transports sperm. However, prostate-related issues, including benign prostatic hyperplasia (BPH) and prostate cancer (PCa), are common concerns in older men.1–3 As conventional treatments evolve, complementary and alternative approaches are being explored to improve prostate health. One such approach involves the use of Black Cumin, scientifically known as Nigella sativa, which has gained attention because of its potential effects on prostate health.4–7
Black cumin is a plant belonging to the family Ranunculaceae. It is native to certain regions of Asia and the Middle East and has a long history of use in traditional medicine and culinary practices. Black cumin is also referred to by various other names, including black seed, black caraway, kalonji, and the Habitat of Al-Baraka or Al-Habba Al-Saouda.6,8–11 This plant produces small, black seeds that have been used for medicinal purposes for centuries (Figure 1).12 These seeds have a distinct flavor, aroma and are often used as spices for cooking. The oil extracted from black cumin seeds is used in various culinary and herbal preparations.8,9
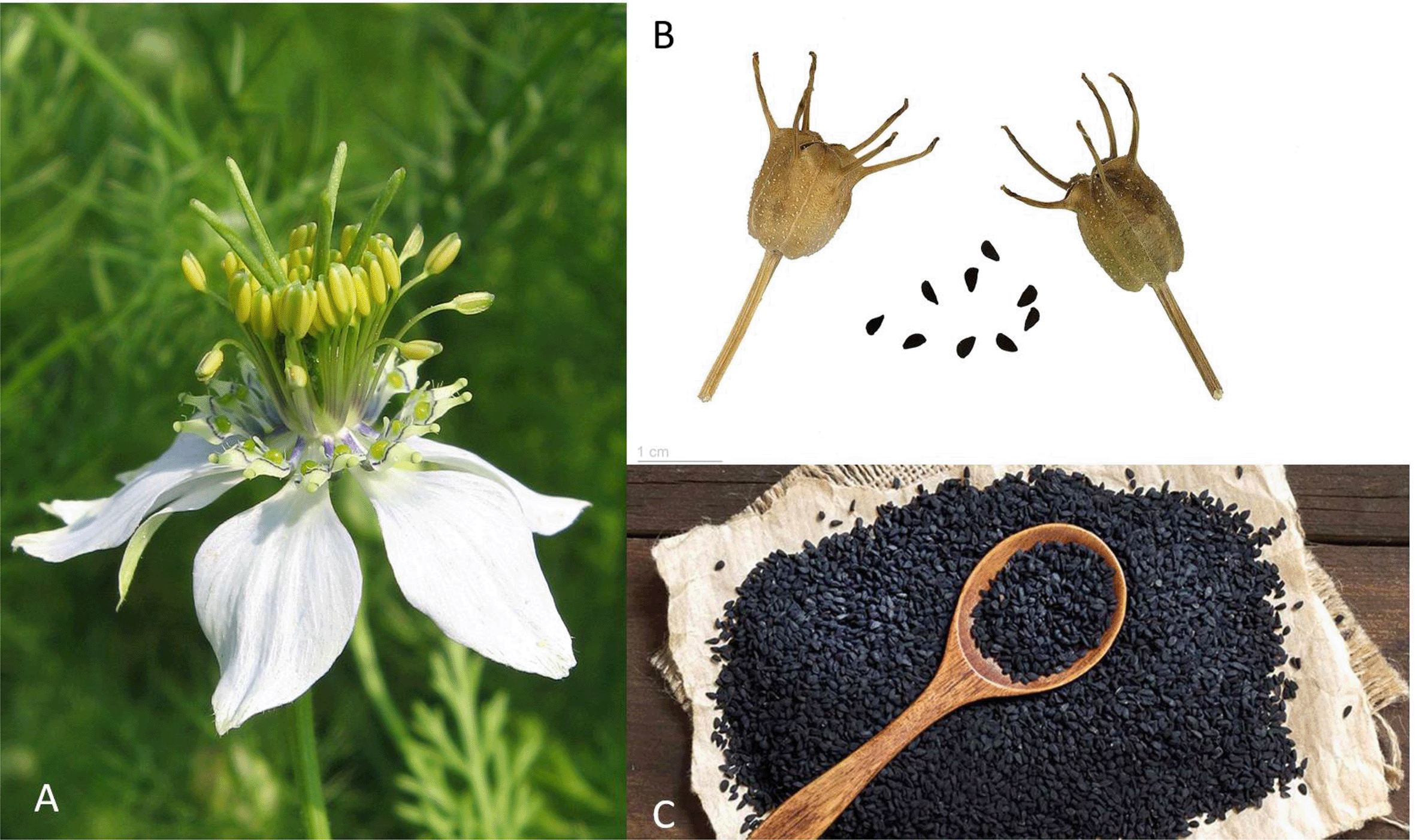
This figure has been reproduced with permission from Ref. 12.
Black Cumin is historically revered for its medicinal properties and has been used in traditional medicine for centuries. The seeds are a rich source of bioactive compounds with various health benefits.9 Recent studies have highlighted Black cumin’s potential effect on the prostate, raising interest in its role in mitigating prostate-related issues.6,9,13 Thymoquinone (TQ) (2-isopropyl-5-methyl-benzoquinone)14 is the primary bioactive component found in the volatile oil extracted from black seeds (Figure 2).15
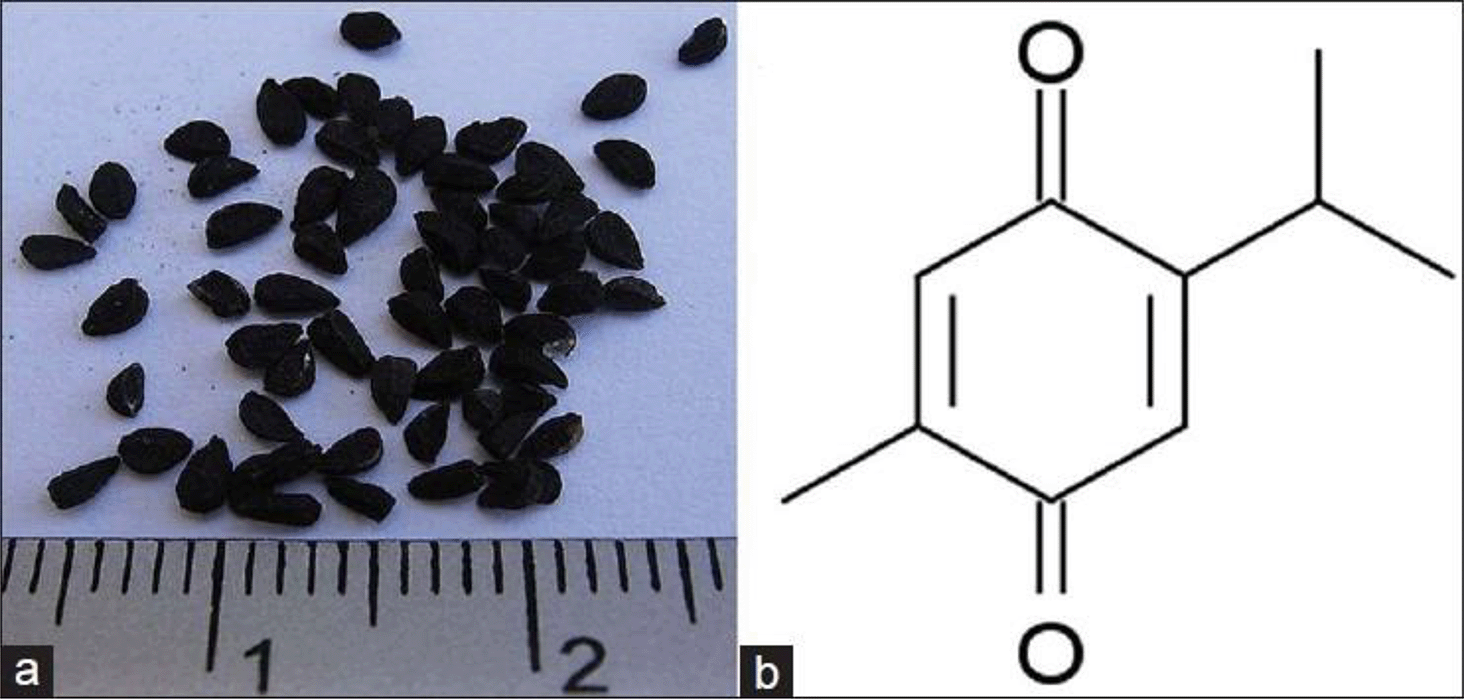
This figure has been reproduced with permission from Ref. 15.
Black cumin seeds contain a variety of bioactive compounds other than TQ, including thymoquinones and flavonoids. These compounds are believed to contribute to the health benefits of Black cumin. It has been traditionally used to address a range of health concerns, including digestive, respiratory, cardioprotective, antidiabetic, anti-inflammatory, antiviral effects, immune system function, nephroprotective, and hepatoprotective properties; antioxidant activity; skin problems; renal and prostate conditions.4–9,15–21 It is important to note that while black cumin has a long history of traditional use and promising preliminary research, more extensive studies are needed to fully understand its potential effects on health and establish its safety and efficacy. Among the various options, Black cumin has emerged as a promising candidate for health enhancement owing to its minimal toxicity and multifaceted modes of action.21
This review explores the emerging evidence regarding the effects of Black cumin on prostate health. While conventional medical treatments remain the cornerstone of managing prostate conditions such as BPH and PCa, investigating the potential benefits of natural compounds, such as Black cumin, could pave the way for novel therapeutic approaches. This article delves into the current state of knowledge regarding the effects of Black cumin on prostate health, highlighting both its traditional use and the scientific research that underpins its potential benefits. As our understanding deepens, a more comprehensive picture of the role of Black cumin in prostate health will emerge, shedding light on its potential as a complementary strategy alongside established medical interventions.
The literature search was conducted in scientific databases MEDLINE/PUBMED, by using keywords such as “black cumin” and “black seed,” “N. sativa” and “active compounds,” “N. sativa” and “Thymoquinone” and “prostate cancer,” “benign prostatic hyperplasia, “prostate cancer.” All the included articles published in English till 2023 were selected. Two senior authors (M.A.A. and R.M.) were individually tasked with gathering the relevant information. These details included author identities, year of publication, subjects involved, type of intervention, and the main outcomes of studies reporting the potential effect of black cumin on prostate health. If disagreements arose, a third reviewer reached a consensus. Our approach did not impose any constraints on the publication dates of the articles. We eliminated non-English literature, articles lacking relevance, duplicates, abstract-only publications, articles without full-text availability, reviews, and books. After identifying pertinent articles, we scrutinized the reference lists of these articles and recent reviews to ensure comprehensive coverage, avoiding omissions. We identified 2 articles related to our search. Following the predetermined inclusion and exclusion criteria, 13 articles were excluded because they did not meet our requirements. Ultimately, 11 articles fulfilled our criteria, reporting on Black cumin and its effects on the prostate, which formed the basis for our review. A flowchart of the article selection process is shown in Figure 3.
Eleven relevant articles were published between 2007 and 2021. Ten articles reported the effect of Black cumin on PCa, while one article was on BPH. Most of the studies were experimental in vivo and in vitro. The extracted data on the potential benefits of Black cumin on the prostate based on human and animal models are summarized in Table 1 and Table 2.
| Author/study location/year | Prostate cell experimental model | Subjects | Treatment with Doses |
|---|---|---|---|
| Kou, China (2017)22 | PC-3, DU-15 | Vitro | TQ (0.1–30 μM) for 2 hours. |
| Dirican, Turkey (2015)23 | PC-3 and DU-15 | Vitro | Combination of docetaxel and TQ in DU-15 hormone- and drug-refractory prostate cancer cells and their effects on PI3K and ERK signaling pathways |
| Kus, Turkey (2018)24 | LNCaP | Vitro | N. sativa oil for 2 and 8 h |
| Koka, USA (2010)25 | PC-3 and C4-2B | Vitro | TQ (25-150 micromol/L) for 2-8 h |
| Zubair, India (2013)26 | PC3, LNCaP, DU145 and C42B | Vivo | TQ 25 mM |
| Kaseb, USA (2007)27 | PC-3, LNCaP, DU-15, DU145, and C4-2B | Vitro and in vivo | TQ (0–100 Amol/L) 2 to 8 h |
| Yi, USA (2008)28 | PC-3 | Vitro and in vivo | TQ 6 mg/kg/day |
| Dawaba, Egypt (2019)29 | PC-3 | Vitro | N. sativa oil 10 mg/ml |
| Alshyarba, Saudi Arabia (2021)30 | PC-3, DU-15 and LNCaP | Vitro | TQ (dose not reported) |
| Dirican, Turkey (2014)31 | PC3 and DU-15 | Vitro | TQ (10 μM) |
| Sadeghimanesh, Iran (2021)6 | BPH model | Vivo | 400 and 800 mg/kg N. sativa oil |
| Author/study location/year | Major findings (including molecular changes) | Main outcome |
|---|---|---|
| Kou, China (2017)22 | TQ substantially arrested the proliferation of prostate cancer. TQ inhibited the migrating and invading capability of prostate cancer DU-15 and PC-3 cells. TQ also downregulated the expression of TGF-β. | Antineoplastic activity |
| Dirican, Turkey (2015)23 | TQ promoted apoptosis by modulating the PI3K-AKT pathway in human prostate carcinoma DU-15 and PC-3 cells. TQ blocking of the PI3K/Akt signaling pathway. | Reducing chemotherapy (docetaxel) dosages and its adverse effects in cases with CRPC |
| Kus, Turkey (2018)24 | TQ exhibits a robust concentration and time-dependent impact on diminishing cancer cell viability through apoptosis in LNCaP prostate cancer cells. | Antineoplastic activity |
| Koka, USA (2010)25 | TQ significantly up-regulated the expressions of growth arrest and DNA damage-inducible gene (GADD45a) and apoptosis-inducing factor-1 and down-regulated the expressions of several Bc12-related proteins, such as BAG-1, Bcl2, Bcl2A1, Bcl2L1, and BID. The primary reason for cell death induced by TQ is the heightened generation of ROS and the reduction in levels of GSH. | Antineoplastic activity |
| Zubair, India (2013)26 | TQ causes DNA oxidative damage, counteracted by copper-chelating agents and antioxidants. It affects cellular copper in prostate cancer cells, leading to prooxidant-induced cell death. | Antineoplastic activity |
| Kaseb, USA (2007)27 | Antineoplastic activity | |
| Yi, USA (2008)28 | TQ blocked angiogenesis and tumor growth, even at low doses with minimal side effects. TQ selectively affected endothelial cells, inhibiting proliferation, migration, and inducing apoptosis. TQ suppressed extracellular signal-regulated kinase activation by vascular endothelial growth factor but didn't affect vascular endothelial growth factor receptor 2 activation. | Antineoplastic activity |
| Dawaba, Egypt (2019)29 | Nigella sativa essential oil in vitro cytotoxicity testing revealed that the oil encapsulated in nanoparticles is more effective in reducing cancer cell viability compared to the unencapsulated oil. | Antineoplastic activity |
| Alshyarba, Saudi Arabia (2021)30 | TQ has the capacity to block the metastatic processes induced by IL-7 in prostate cancer cells. | Antineoplastic activity |
| Dirican, Turkey (2014)31 | The synergistic combination of ZA and TQ inhibits cell viability and induces apoptosis in PC-3 and DU-15 prostate cancer cells. | Compared to cytotoxic agents, it exhibits a minimal profile of hematological and non-hematological toxicity. |
| Sadeghimanesh, Iran (2021)6 | Significant decrease in PI, PV, DHT concentration, PSA, and serum MDA level, and also significantly increased serum antioxidant capacity. | Anti-BPH effects |
In Table 1, most studies were conducted in Turkey and the USA, but the majority of continental representations were from Asia. A xenograft human prostate cancer cell line model has been used in most studies. All studies used the active component of Nigella sativa (thymoquinone, TQ), as it is the major ingredient of black seed oil and extract. The treatment dose and duration were variants among studies. Combining TQ with standard PCa chemotherapeutic agents can enhance the efficacy of bone-preventive adverse events in metastatic PCa while reducing toxicity. In BPH, significant reductions in PI, prostate volume (PV) Dihydrotestosterone (DHT) levels, prostate-specific antigen (PSA), and serum malondialdehyde (MDA) were observed along with noticeable increases in serum antioxidant capacity.
Nigella sativa, also known as black seed or black cumin in English, Habat Al-Baraka in Arabic, and Tikur azmud in Amharic, has a long history of widespread culinary use. It has been employed as a spice and flavoring agent in a variety of dishes, including bread, yogurt, pickles, sauces, and salads.32 This versatile seed has also held a prominent place in traditional remedies across Arabian countries, Far East Asia, Europe, and Africa, earning it the nickname “The herb from heaven” by early herbal specialists.33 The Prophet Mohammed (PBUH) himself praised its healing properties, stating, “Hold on to the use of this black seed, as it has a remedy for every illness except death.”34 Additionally, Avicenna, a renowned physician from the 10th century famous for his work “The Canon of Medicine,” recommended Nigella seeds for boosting the body’s energy and aiding recovery from fatigue and dispiritedness. Notably, Nigella sativa is referenced for its therapeutic qualities in the Holy Bible and is referred to as Melanthion by Hippocrates and Dioscorides.35,36 The medicinal applications of Black cumin seeds in various traditional herbal systems are vast and encompass a wide range of ailments. These include respiratory disorders, pain management (such as chronic headaches and back pain), diabetes, paralysis, infections, inflammation, hypertension, and digestive tract issues, with various preparations used for administration. Furthermore, it has been applied topically for conditions such as blisters, nasal abscesses, orchitis, eczema, and swollen joints.9 Considering the extensive history of traditional medicinal uses of Black cumin and their active components such as TQ, there is a compelling opportunity to explore this valuable herb as an effective herbal medicine with multiple pharmacological actions. Based on the published experimental studies, our review highlights the potential benefits of Black cumin for treating prostate diseases such as BPH and PCa (Figure 4).
In 2019, there were an estimated 9.0 million cases (95% CI 73.2 to 118 million) of BPH cases worldwide. This number marks a significant increase compared to the 51.1 million cases (95% CI 3.1 to 69.3 million) recorded in the year 2000.37 BPH is a prevalent age-related condition of the prostate gland in men and is characterized by symptoms such as urinary tract obstruction, increased urination frequency, urinary retention, diminished urinary tube diameter, altered urine flow pressure, and post-urination dribbling.38 This condition entails enlargement of the prostate gland due to excessive proliferation of cellular components, notably mesenchymal cells. Common therapeutic approaches encompass α-adrenergic antagonists, 5-α-reductase inhibitors, and alternative remedies with natural products.39 Recent research has highlighted the relationship between oxidative stress (OS) and BPH. Notably, patients with BPH exhibit elevated levels of the lipid peroxidation biomarker MDA, coupled with suppressed plasma antioxidant levels.39,40 This underscores the potential utility of antioxidant interventions in the management of BPH. Nonetheless, conventional treatments such as alpha-adrenergic receptor blockers and 5-alpha reductase inhibitors present side effects and economic constraints, prompting the exploration of natural compounds as lead candidates for drug development or adjunctive therapies. Investigative studies have highlighted promising alternatives and complementary options for managing mild BPH, including Serona repens, Pygeum africanum, and Secale cereals. This approach has gained traction owing to factors such as accessibility, cost-effectiveness, and comparatively superior safety profiles compared to prevailing pharmaceutical interventions. Additionally, the global trend towards harnessing natural sources for the treatment of otherwise challenging diseases has bolstered interest in botanicals and other natural reservoirs, with Nigella sativa emerging as a highly endorsed contender within this paradigm.39
A study using the BPH rate model reported that the administration of Black cumin seed oil at doses of 00 and 800 mg/kg resulted in a discernible reduction in DHT levels.6 Intriguingly, these findings indicated that the Black cumin oil, to a certain extent, displayed a more pronounced decrease in DHT levels within the BPH model compared to finasteride.6 Similarly, Hiipakka et al. reported that treatment with polyphenols derived from green tea diminished DHT production and hindered prostate cell proliferation. Given Black cumin’s composition of polyphenolic compounds,41 it stands to reason that the suppression of 5α-reductase activity potentially contributes to the beneficial impacts of this botanical.
Among the fatty acids, Black cumin oil contains a substantial amount of essential unsaturated fatty acids, including approximately 1% omega-3, 25% omega-9, and 58% omega-6.8,42 Prior work by Abdel-Rahman et al. posited that fatty acid-enriched compounds might impede the proliferation of prostate cells by reducing testosterone and DHT concentrations43 This aligns with the findings of Liang et al., who demonstrated the inhibitory role of fatty acids on 5α-reductase.42 Increased prostate weight serves as an indicative marker for diagnosing BPH, and the Prostate Index (PI) is frequently used to gauge BPH progression.44 A study on the rate of the model reported that both 00 and 800 mg/kg doses of Black cumin seed oil significantly decreased both PI and PV in the context of BPH.6 Recent insights have revealed the potential of PV and PSA concentrations in predicting the growth of prostate cells. Notably, PSA can serve as a surrogate index for PV and as a diagnostic marker for assessing the risk of prostate carcinoma.44 Hence, an elevated PSA level corresponds to heightened proliferation of prostate cells. A study on the rate of the BPH model reported that treatment with N. sativa seed oil at doses of 00 and 800 mg/kg prominently reduced PSA concentrations compared to the BPH model control group.6 Ren et al. highlighted the ability of polyphenols to repress PSA gene expression,45 suggesting that the ability of N. sativa to reduce PSA levels may be attributed to the presence of polyphenols.6
Consequently, the observed effect of N. sativa oil in hindering lipid peroxidation, and by extension, its potential anti-BPH effects, is likely attributable to its reservoir of antioxidant and free-radical-quenching compounds. A study concerning serum MDA levels, further underscored this assertion, as these parameters were notably elevated in the BPH group compared to the control group.6 Remarkably, in this animal study, within the N. sativa oil-treated groups, a significant reduction in serum MDA levels was evident compared to the BPH group. This reduction strongly signifies the shielding effects of the constituents present in N. sativa oil, thereby potentially enhancing serum antioxidant capacity and concurrently lowering MDA levels. The observed decline in MDA levels appeared to align with the inherent antioxidant potency of plants.6
The increasing global incidence of cancer poses significant medical challenges. In response, there has been a growing endeavor to explore potent natural anticancer treatments as alternatives to existing chemotherapeutic approaches, which often have limited applicability.
PCa is one of the most prevalent malignancies affecting men,46 and recent research has unveiled the noteworthy role of TQ, a prominent bioactive compound in Nigella sativa, in influencing PCa markers (Table 2). TQ, a pivotal constituent of black seed oil, has a wide spectrum of pharmacological effects including potent anti-inflammatory properties47 and notable antioxidant activity.48 Furthermore, TQ was the predominant bioactive compound extracted from Black cumin. Its abundance has been linked to its antineoplastic properties against a diverse array of tumors.49,50 TQ has garnered attention for its antineoplastic properties in various cancers, including pancreatic cancer,48 lung cancer, colon cancer,51 and leukemia.52 Research has shown that TQ not only curbs growth and triggers cell apoptosis and cell cycle arrest, but also deters metastasis and angiogenesis. These multifaceted effects are attributed to its modulation of key signaling pathways such as Akt,53 NF-κB,54 mitogen-activated protein kinase (MAPK), and Signal transducer and activator of transcription 3 (STAT3).55 In this compilation, we have summarized the present scientific knowledge concerning the anticancer properties of Nigella sativa, along with elucidating its mechanism of action, with a specific focus on PCa.
Findings from the study on the effect of TQ on human PCa cell lines DU15 and human prostate cancer cell line (PC3) revealed that TQ effectively curbed the metastatic attributes and restrained the process of epithelial-mesenchymal transition (EMT) within PCa cells through its active downregulation of the TGF-β/Smad2/3 signaling pathway. Furthermore, these outcomes lend support to the proposition that thymoquinone holds promising potential as a therapeutic agent against PCa, functioning by precisely targeting the TGF-β pathway.22 However, the relationship between the TQ and EMT in PCa remains unclear. Furthermore, the precise mechanism underlying TQs inhibition of the metastatic phenotype remains to be fully elucidated.
A study explored the combination of docetaxel and TQ in hormone-refractory prostate cancer cells (DU-15) and its impact on PI3K and ERK signaling pathways.23 The combination of docetaxel and TQ showed a significant increase in cytotoxic and apoptotic effects compared to using each agent separately, with the effect becoming stronger at higher doses. Interestingly, the presence of LY29002 did not substantially alter cell viability when combined with docetaxel and TQ, unlike cells treated with LY29002 alone. - However, introducing FR18020 reduced cell viability significantly when combined with docetaxel and TQ, compared to cells treated solely with the inhibitor. The study suggests that the cytotoxic effect of docetaxel and TQ is connected to the inhibition of the PI3K/Akt signaling pathway in DU-15 cells.23 This combined approach has the potential to offer an alternative to contemporary oncological practices. Additionally, the synergistic use of docetaxel and TQ holds promise for potentially reducing the dosage of docetaxel and mitigating its associated adverse effects while maintaining therapeutic effectiveness for patients with castration-resistant prostate cancer (CRPC).23
In an in vitro study on human prostate carcinoma LnCaP cells, TQ decreased cell viability and enhanced apoptosis by activating caspase-9.24 Furthermore, a study on the mechanism of action of TQ in androgen receptor (AR)-independent (C-2B) and AR naïve (PC-3) PCa cells showed that TQ suppressed the proliferation of human prostate C-2B cancer cells by triggering the activation of JNK and growth arrest and DNA-damage-inducible 45 alpha (GADD45a), concurrently upregulating apoptosis-inducing factor-1 while downregulating Bcl-2-related proteins, including BAG-1, Bcl-2, Bcl2A1, Bcl2L1, and BID25 Furthermore, C-2B and PC3 cells revealed the potential of TQ to suppress proliferation by inducing the accumulation of reactive oxygen species (ROS) and reducing glutathione (GST) levels in both cells.25 In addition, TQ exhibited a pro-oxidant cytotoxic mechanism involving oxidative DNA damage facilitated through a copper-dependent pathway by mobilizing and reducing endogenous cellular copper across various PCa cell lines, including DU15, LNCaP, PC3, and C2B.26 This pro-oxidant cytotoxic mechanism provides a more comprehensive understanding of the anticancer efficacy of plant-derived antioxidants.26 One study reported that TQ, a naturally derived herbal product, holds promise as a potential treatment for both hormone-sensitive and hormone-refractory PCa. Additionally, given its targeted effect on cancer cells, we posit that thymoquinone could be safely employed as a preventive measure against the onset of PCa.27 A study on a human prostate tumor xenograft mouse model27 showed that TQ exerts inhibitory effects on the expression of AR and E2F-1, which are crucial for the proliferation and viability of androgen-sensitive and androgen-independent prostate cancer cells, both in vivo and in vitro. The efficacy of TQ is evident in its ability to diminish the levels of AR and E2F-1 while promoting the activation of pro-apoptotic proteins, including p53, p21Cip1, p27Kip1, and Bax, in androgen-sensitive prostate cancer cells.27 TQ inhibited tumor growth in xenografts originating from androgen-independent C-2B prostate cancer cells in nude mice. Consistent with its effect on cultured cells, this outcome correlated with a substantial reduction in AR and E2F-1 expression and the initiation of apoptosis. Consequently, our perspective is that thymoquinone holds promise as a potential therapy for PCa, particularly in hormone-refractory cases.27 Additionally, TQ dose-dependency increased the inhibitory effect of thymoquinone on DNA synthesis, proliferation, and viability of cancerous cells (LNCaP, C-2B, PC-3, and DU15), but not in non-cancerous prostate (BPH-1) epithelial cells.27 Another study reported that TQ effectively blocked angiogenesis both in vitro and in vivo,28 exhibited preventive effects on tumor angiogenesis in the PC3 mouse model, and curbed human prostate tumor growth at a low dose with minimal chemo-toxic side effects. Notably, this study observed a heightened sensitivity of endothelial cells to TQ-induced phenomena, including apoptosis, suppression of proliferation, and hindered migration, in contrast to PC3 cancer cells. Furthermore, TQ effectively restrained the activation of extracellular signal-regulated kinase induced by vascular endothelial growth factor but did not affect the activation of vascular endothelial growth factor receptor 2.28 These findings led to the conclusion that thymoquinone can efficiently inhibit prostate tumor growth at an early tumor stage (50 mm3) at a dose of 6 mg/kg/day.28 A study on the nanoparticle optimization of Nigella sativa reported the successful development and characterization of a nano-based carrier for Nigella sativa essential oil using optimization techniques, and a methyl thiazolyl-diphenyl-tetrazolium bromide (MTT) assay was performed to compare the in vitro cytotoxicity using two different cell lines (i.e., HCT 116 for colorectal carcinoma and PC3 for prostatic cancer). This study demonstrated the enhanced properties of nanoparticulated oil, including improved efficiency in suppressing cancer cell viability compared to free oil.29 Another study reported that TQ can block metastatic processes induced by IL-7 in prostate cancer cells, thereby controlling tumor progression, migration, and invasion.30 An in vitro study found that the combination of TQ and zoledronic acid (ZA) led to increased cytotoxic effects and apoptosis in prostate cancer cell lines resistant to both hormones and drugs. This novel combination approach could serve as an alternative strategy for patients with limited treatment options due to poor performance status and those who are not viable candidates for traditional therapies. In addition, compared with cytotoxic agents, it exhibits a minimal profile of hematological and non-hematological toxicity.31
These studies suggest that Black cumin may play a role in slowing the progression of prostate cancer; however, clinical trials in humans are necessary to determine its efficacy and safety. It is important to approach these findings with caution and consult medical professionals before considering complementary or alternative treatments, particularly for serious conditions such as cancer.
This review has some limitations, including factors that may affect its quality, validity, and reliability. However, this is the first article that summarized in a comprehensive review the growing evidence and insights from in vitro and vivo studies that explored the potential efficacy of Black cumin (Nigella sativa L.) on prostate health and conditions such as BPH and PCa.
Suggestions and prospective pathways for harnessing the advantages of Black cumin (Nigella sativa L.) in promoting prostate health may encourage researchers to conduct further rigorous clinical trials to substantiate the specific benefits of Black cumin on prostate health. These studies should encompass diverse populations, incorporate long-term assessments, and explore in-depth research to unravel the precise mechanisms by which Black cumin compounds interact with prostate cells and influence hormonal balance. This information will aid in a better understanding of their potential therapeutic effects. Furthermore, we determined the optimal dosage and duration of Black Cumin supplementation to maximize the prostate health benefits. This involves investigating both short- and long-term effects at various dosage levels. Additionally, we explored the synergistic effects of incorporating Black cumin into combination therapies with existing treatments for prostate cancer. This could potentially enhance therapeutic outcomes and minimize adverse effects. In addition, we identified and isolated the key bioactive compounds in Black cumin that are responsible for its positive effects on prostate health. This knowledge could lead to the development of targeted supplements and medications. Continued evaluation of the safety profile of Black cumin supplementation, particularly in the context of long-term use, and potential interactions with medications is needed. Future endeavors should focus on optimizing its application through research-backed dosages and combination therapies, and effectively communicating its potential benefits to the public and healthcare practitioners.
Thymoquinone (TQ), a bioactive molecule found in Nigella sativa, is known for its antioxidant, anti-inflammatory, and anticancer effects on prostate tissues. Over the past decade, these effects have been examined both in vivo and in vitro. We anticipate that the findings of this review will be used to advance the possible therapeutic options derived from Black cumin for human prostate health. According to the findings of our review, the TQ component of Nigella sativa revealed interesting prospective advantages for prostate health. TQ is most likely to promote pro-oxidant-induced cell death, reduce tumor angiogenesis, induce apoptosis, and prevent metastasis in PCa experimental models. Combining TQ with standard PCa chemotherapeutic agents can enhance the efficacy of bone-preventive adverse events in metastatic PCa while reducing toxicity. Significant reductions in PI, PV, DHT, PSA, and serum MDA levels as well as significant improvements in serum antioxidant capacity have been reported in BPH experimental models. This natural product can then be utilized to treat various diseases and has become a popular functional food. Future clinical trials are required to explore the safety and efficacy of Black cumin and TQ in terms of their pharmacological advantages.
The authors would like to thank the Deanship of Scientific Research at Majmaah University for supporting this work under Project Number No. [R-2024-1015].
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Partly
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cancer cell biology, multi-omic profiles of cancers specific to different populations. The effect of various compounds on transcriptome ad spliceosome profiles
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 2 (revision) 21 Feb 25 |
read | read | read | |
|
Version 1 27 Mar 24 |
read | |||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)