Keywords
Stereotactic Radiotherapy, Stereotactic Body Radiotherapy, micro multileaf collimator(mMLC), Multi leaf collimator (MLC), Treatment planning, Volumetric Modulated Arc Therapy, Dynamic Conformal Arc Therapy
This article is included in the Manipal Academy of Higher Education gateway.
The Elekta micro-multileaf collimator (mMLC) known as apex for planning stereotactic radiosurgery (SRS)/stereotactic radiotherapy (SRT)/stereotactic body radiation therapy (SBRT) provides excellent dose distribution; however, it offers disadvantages such as prolonged treatment duration and technical errors in terms of mMLC and gantry calibration, which adds to the total treatment duration. Hence, we aimed to compare the treatment planning performed with the 2.5mm mMLC and 5mm MLC known as Agility for brain and lung targets treated with SRS and SBRT in Elekta Versa high definition (HD).
The study included 10 patients, five each with brain and lung targets. Two treatment plans were performed for each case using Elekta’s Monaco (5.11.03) treatment planning system (TPS) with 2.5mm and 5mm MLC. An X-ray photon beam of energy 6FFF was used for planning purposes with various gantry, couch, and collimator combinations. These two plans were compared using target coverage (TC), conformity index (CI), homogeneity index (HI), gradient index (GI), and organ at risk (OAR) doses.
No significant differences were found in the target coverage, CI, HI, or OAR doses in either MLC design. Volumetric modulated arc therapy (VMAT) with a 5 mm MLC provided equivalent tumor coverage with an additional number of monitor units. OAR doses were comparable in both MLC widths for brain targets, whereas for lung targets, OAR doses were slightly lower with 2.5mm mMLC. GI was superior in the 2.5mm mMLC compared to the 5mm MLC giving a steep falloff in the dose distributions (p = 0.158).
The TC, CI, HI, and OAR doses were similar in both 2.5mm and 5mm based VMAT plans. The gradient index was better in the 2.5mm mMLC resulting in steep dose gradients, which further reduced the isodose volumes. Therefore, a 5mm MLC (agility) can also be used for SRS/SBRT treatment planning, with a further reduction in the gradient index. However, the study must be extended further with more samples and multiple comparison parameters.
Clinical Trials Registry - India, registration number CTRI/2021/11/037842, registration date. 8th November, 2021.
Stereotactic Radiotherapy, Stereotactic Body Radiotherapy, micro multileaf collimator(mMLC), Multi leaf collimator (MLC), Treatment planning, Volumetric Modulated Arc Therapy, Dynamic Conformal Arc Therapy
The manuscript underwent several revisions based on expert suggestions to improve clarity, accuracy, and focus on the primary topic of stereotactic body radiotherapy (SBRT) for lung cancer. The introduction was revised to include clinical data on the incidence of lung cancer, addressing the prevalence and significance of lung cancer treatment. References to brachytherapy and liver treatment were removed to maintain focus solely on lung cancer and SBRT. The sentence regarding the better tumor response provided by Stereotactic Radiosurgery (SRS) was clarified to specify its relevance to benign tumors. The discussion on the complexity of SRS/SBRT procedures was made more specific, distinguishing them from fractionated external beam radiotherapy (EBRT). The statement on smaller error margins in SBRT was revised to emphasize high precision machines and immobilization devices. The multileaf collimator (mMLC) term was spelled out initially and its specifications were corrected to report the accurate 2.5mm leaf width. Vendor names and countries were cited after each medical device listed in the introduction for transparency.
In the methods section, references to the RTOG and SABRT guidelines were added for patient selection, along with clarification on the MRI type and rigid image registration process used for patient immobilization. The section on treatment planning was modified to move technical details on slice thickness and structure delineation to the imaging protocol section. Additionally, the evaluation of dose prescription for different MLC plans was clarified, confirming that both 2.5mm and 5mm MLC plans were prescribed to the same isolines. The results were presented with consistent terminology for the mMLC and MLC plans, and a statistical correction was made regarding the use of the t-test, ensuring normality of distributions. Lastly, the discussion was revised to eliminate redundant content, focus on the results, and address potential improvements by including adjacent organs at risk in future studies.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Cancer is considered one of the leading causes of death worldwide, and its incidence and mortality rates may rise to approximately 29 million and 16 million people, respectively, by 2040. In India, the incidence rate of brain or nervous system-related cancers is the leading cause of death and is ranked 13th in males and 11th in females among all cancers.1 Lung cancer is one of the most commonly diagnosed cancers and is considered to be the leading cause of death worldwide. It makes up 5.9% of all cancers and 8.1% of all cancer-related fatalities in India. It was found that smoking is one of the major reasons to cause lung cancer in 80% of the patients.2,3 On average, approximately 20–54% of cancer patients will suffer from lung metastasis in their lifetime and almost 10–30% of all carcinoma patients have a risk of brain metastasis, which can originate from other primary sites.4,5 Radiotherapy is an extremely cost-effective treatment modality, and almost 50% of cancer patients receive radiation therapy during their course of cancer treatment.6 In radiotherapy, high-energy photons such as X-rays, gamma radiation, or high-energy particles are used to deliver treatment externally using external beam radiation therapy (EBRT).7
Stereotactic radiosurgery (SRS) is a radiotherapy treatment technique in which a high radiation dose is delivered in a single fraction, using multiple radiation beams or arcs. If the same treatment is delivered in multiple fractions, it is called stereotactic radiotherapy (SRT).8 For selected intracranial lesions, SRS/SRT is performed as it delivers a high amount of radiation to the tumor volume, and the dose gradient is steep so that the surrounding uninvolved structures are spared.9 Lars Leksell, a Swedish neurosurgeon, initially introduced SRS in 1969 for treating intracranial lesions and has successfully shown positive results in managing intracranial lesions and brain metastasis and provided potential benefits in terms of good tumor control and reduced toxicity. It has increased the tumor control rate, survival rate, and quality of life. An SRS system based on a linear accelerator (LINAC) was developed in 1980, which allowed the treatment of SRS, SRT, and stereotactic body radiation therapy (SBRT) with high precision and accuracy.10,11 Similar to SRS, SBRT is an SRT procedure that is used to treat extracranial lesions where hypofractionated radiation is administered in a fewer number of fractions. SBRT has been regularly incorporated as a better treatment option for metastatic and primary tumors such as lung cancer, liver lesions, and prostate cancer.12
Although surgery is the standard of care for patients with lung cancer who are healthy enough to undergo surgery, SBRT is considered to be one of the best therapeutic alternate options for inoperable patients.13 It was found that SBRT offers three-year local control in 70 to 90% of patients and two-year survival rate of 50 to 70% of patients.14,15 For brain tumors less than 3cm, treatment with SRS given in a single fraction has shown the best results in the management of benign tumors, as it has various advantages such as a greater decrease in tumor size, sparing of normal tissues due to rapid dose fall-off, minimum radiation-related side effects, lesser duration of radiotherapy, and better tumor response. If the tumor size is greater than 3cm, SRS must be delivered in multiple fractions (SRT) so that normal tissue toxicity is minimized.16,17
SRS/SRT/SBRT are complicated treatment procedures that include various steps such as patient immobilization and imaging with breath-hold techniques (specific to SBRT), treatment planning to avoid certain beam angulations especially when non-coplanar beams used, performing patient-specific quality assurance (QA) checks involving small volume ion chambers, and delivering radiation therapy to the patient with the attachment of additional small size multileaf collimator (if required in a particular setup like Elekta) to the LINAC head.18 As high radiation doses are delivered in a smaller fraction of treatments, and more suitable immobilization devices meant for SRS/SBRT are used to deliver radiation precisely to the tumor, very small error margins are given compared to conventional radiation therapy. A small error in the localization of the tumor, even in a single fraction, can lead to an underdosage of the tumor by 20% or more, and at the same time, it can overdose the surrounding normal tissues, thereby resulting in potentially serious injuries to the organs involved.19
The treatment delivery of stereotaxy has generally utilized dedicated specialized equipment such as Gamma Knife and CyberKnife. Medical LINACs have been increasingly adopted as a mode for delivering hypofractionated radiotherapy schedules. There are important advantages to using a LINAC for SRS/SRT/SBRT treatment, including increased versatility, cost benefits, and higher throughput. Technological advances have made use of LINAC and are increasingly accurate in stereotaxic treatment. One such device, designed to improve conformity to the target, is an externally attached additional collimating device with a micro-multileaf collimator (mMLC).20 The mMLC has a high-resolution collimation system with a width of 2.5mm at the isocenter. This would facilitate the delivery of a conformal radiation dose to the tumor with a steep dose gradient beyond the tumor, thus better sparing the surrounding normal tissues. However, there is a disadvantage of reduced clearance between the patient and collimator, which limits the utilization of non-coplanar beam directions.21
Using the Elekta mMLC (Elekta, Sweden) known as apex for planning SRS/SRT/SBRT provides excellent dose distribution, but also offers technical errors in terms of mMLC and gantry calibration, correction of which adds to the total treatment duration. If the same plan with excellent output is available without the use of mMLC but with flattening filter-free (FFF) photon beam energies, then the treatment duration is expected to be reduced compared to Apex-based delivery.
This study aimed to compare SRS/SRT/SBRT treatment plans with and without the mMLC, using FFF radiation beams to compare the generated treatment plans in terms of various indices of planning target volume (PTV) coverage and organ-at-risk doses.
Ten patients were retrospectively considered for the study after approval from the Institutional Ethics Committee, Kasturba Medical College and Kasturba Hospital, Manipal Academy of Higher Education Manipal (IEC427-2021) 8th August 2021, Clinical Trials Registry, India; registration number CTRI/2021/11/037842, 8th November 2021. population included five patients each with brain and lung targets who were treated in the year 2021 at our institute. The eligibility criteria for the patient selection was based on the guidelines given.22–24 The selected cases were planned using SRS and SBRT treatment techniques following the guidelines prescribed by the Radiation Therapy Oncology Group (RTOG) and Stereotactic Ablative Body Radiotherapy (SABR) guidelines22,25–27 using the Monaco 5.11.03 (Elekta, 2016) treatment planning system (TPS). For every patient, two plans were generated with the mMLC (2.5mm) and the other without the mMLC (i.e., with the Elekta Agility, 5mm). The gantry, collimator, and couch angles were determined based on the tumor location and kept constant in both treatment plans.
The selected patients were immobilized with a Fraxion patient-specific cranial immobilization device (Fraxion, P10106-103, Elekta) consisting of a computed tomography (CT) adaptor, table top adaptor, vacuum cushion, thermoplastic mask, and Fraxion stereotactic frame. Vacuum cushions from Fraxion are specially used as headrests for patients, and they provide precise positioning of the head and reproducibility of the treatment setup. Each cushion was molded individually for each patient and used throughout the course of treatment.28 Each patient was immobilized using a thermoplastic mask. A CT scan of the patient was performed with the Fraxion stereotactic frame and marking sheet. A stereotactic frame was used to locate the tumor in SRS/SRT. It has three Z-shaped radiopaque markers that are visible in the axial cut CT image as nine dots that act as fiducials for the identification of the target coordinates. The marking sheet has three coordinates, that is, x, y, and z, which are used to locate the tumor in the coordinate system. This makes it convenient to position patients according to the treatment isocenter. The acquired CT images were exported to the Monaco TPS. Magnetic resonance imaging (MRI) is considered superior to CT in soft-tissue discrimination of the brain. The rigid image registration was carried out between MRI (Philips Achieva, 1.5 Tesla) and CT (Philips, Brilliance 16 Big Bore) and mapping of the structures was performed to avoid any misinterpretation. T1 weighted contrast-enhanced and T2 weighted FLAIR MRI images were used for registration purpose. Contouring of the target and critical structures such as the normal brain, optic nerves, optic chiasma, brainstem, eyes, lens, cochlea etc. was performed after image registration.29
Selected patients were immobilized using a thermoplastic mask. An active breathing coordinator (ABC) (Elekta limited, 201510) was used to implement effective respiratory motion management throughout the treatment.30 An ABC is used to control respiratory motion by applying simple and efficient breath-holds at an applicable threshold level. A CT image of the patient was obtained with the same setup using a thermoplastic mask and ABC.31–33 The rigid image registration was carried out between MRI (Philips Achieva, 1.5 Tesla) and CT (Philips, Brilliance 16 Big Bore) and mapping of the structures was performed to avoid any misinterpretation. T2 weighted arterial phase or portovenous phase MRI images were used for registration purpose to properly delineate the tumor volume and critical structure, where appropriate.
A CT image with a slice thickness of 1mm for the brain and 2mm for the lungs was acquired using suitable immobilization devices. For SBRT cases, CT images with deep inspiration breath-hold (DIBH) were acquired.4 The CT images were then exported to Monaco (5.11.03 version) TPS.34 Contouring of the target volume and organ at risk (OAR) was accomplished by oncologists. A planning target volume (PTV) margin in the range of 2mm to 10mm, depending upon site and immobilization technique, was generated around the gross tumor volume (GTV). Later treatment plan was planned based on the site of the tumor using Monaco TPS.35 For this study, two separate treatment plans for SRS and SBRT were generated retrospectively.
The first plan for SRS/SBRT was developed using the dynamic conformal arc therapy (DCAT) technique with a 6FFF photon beam energy using a 2.5mm mMLC. The mMLC is an additional attachment to the collimator of the Elekta Versa high-definition LINAC consisting of an MLC with a 2.5mm width at the isocenter. This high-resolution collimating device is especially used for SRS treatment delivery to facilitate conformal dose distribution around the tumor. The maximum field size provided by the mMLC is 12 × 14cm2.36 The isocenter was placed at the center of the target volume. The number and direction of the arcs were chosen based on the location of the tumor. Couch movement was restricted in some cases, wherever it was practically impossible to move the gantry with the Apex to reduce the risk of collision of the gantry with the couch and patient. The second plan was generated without using mMLC. The LINAC has an inbuilt MLC with a 5mm width at the isocenter, which is also called Agility. The SRS/SBRT treatment plans in this case were performed with agility using a 6FFF photon beam energy. The treatment plan was implemented using the same gantry couch combinations. Multiple optimizations in both techniques were performed to achieve the prescribed tumor dose and to bring the dose to the OARs within the given limits. The Monte Carlo algorithm was used to calculate the dose distribution.37 For the included cases, SRS was planned with 16Gy in one fraction ( Figure 1) and SBRT with 60 Gy in five fractions ( Figure 2). The dose was prescribed to the 80% isoline for the evaluation purpose.

Red: 100% prescribed dose; Green: 95% prescribed dose; Blue: 50% prescribed dose; Yellow: 115% prescribed dose.
The treatment plans performed using mMLC and agility were compared using Monaco TPS. The quality of the treatment plans was checked using quality indices such as target coverage (TC), conformity index (CI), homogeneity index (HI), gradient index (GI), and organ at risk (OAR) doses. CI was calculated using the formula TVPIV2/ (TV × PIV). Here, TV is the target volume, and PIV is the prescription isodose volume. The ideal value for CI is 1. As the value of the CI decreases from 1, the quality of the plan also decreases. A value greater than 1 indicates that the tumor volume is overirradiated, and a value less than 1 indicates a reduction in the dose to the target volume. The HI was calculated as the ratio of the maximum target dose to the prescribed dose. The ideal value for HI is 1. GI was calculated using the formula PV 50%/PIV. PV 50% represents 50% of the prescribed dose covered by the patient volume. The smaller the GI value, the steeper the dose gradient. If multiple targets are close to each other, then combined GI will be performed for the lowest dose prescribed target in the patient. A clinically acceptable plan will have a lower GI value, higher CI value, and higher TC (>95%). Such a plan will provide better tumor coverage and maximum sparing of the normal brain.16
Jamovi 2.3.2638 statistical software was used for statistical analysis and descriptive data for all continuous variables were presented as mean ± standard deviation (SD). Multivariate ANOVA and independent t-test were performed to compare the treatment plans generated with 5mm and 2.5mm MLC for brain SRS and lung SBRT. A value of p<0.05 was considered as a statistically significant difference between variables.
The volume of the GTV and PTV in the selected brain targets were ranging between 0.6cm3 to 6.0cm3 and 1.2cm3 to 8.5cm3 respectively. Similarly, in the lung targets the GTV and PTV volumes were ranging between 4.3cm3 to 7cm3 and 8.5cm3 to 12.5cm3 respectively. Among the selected ten cases, 50% of the patients were treated at the metastatic sites, and in the remaining cases, treatments were administered at the primary sites. Both treatment planning techniques showed excellent tumor dose distribution and the PTV coverage was comparable in both treatment planning techniques. For brain SRS cases, the mean target coverage in 5mm MLC was 98.8±0.7, and in 2.5mm mMLC, it was 99.2±0.4(p=0.41) ( Figure 3). In lung SBRT cancers, the target coverage in 5mm MLC and 2.5mm mMLC was 97.9±2.0 and 99.1±1.2(p=0.30), respectively ( Figure 4), which shows that the APEX-based treatment plans showed slightly better target coverage, although both planning techniques provided a target coverage of more than 97%.
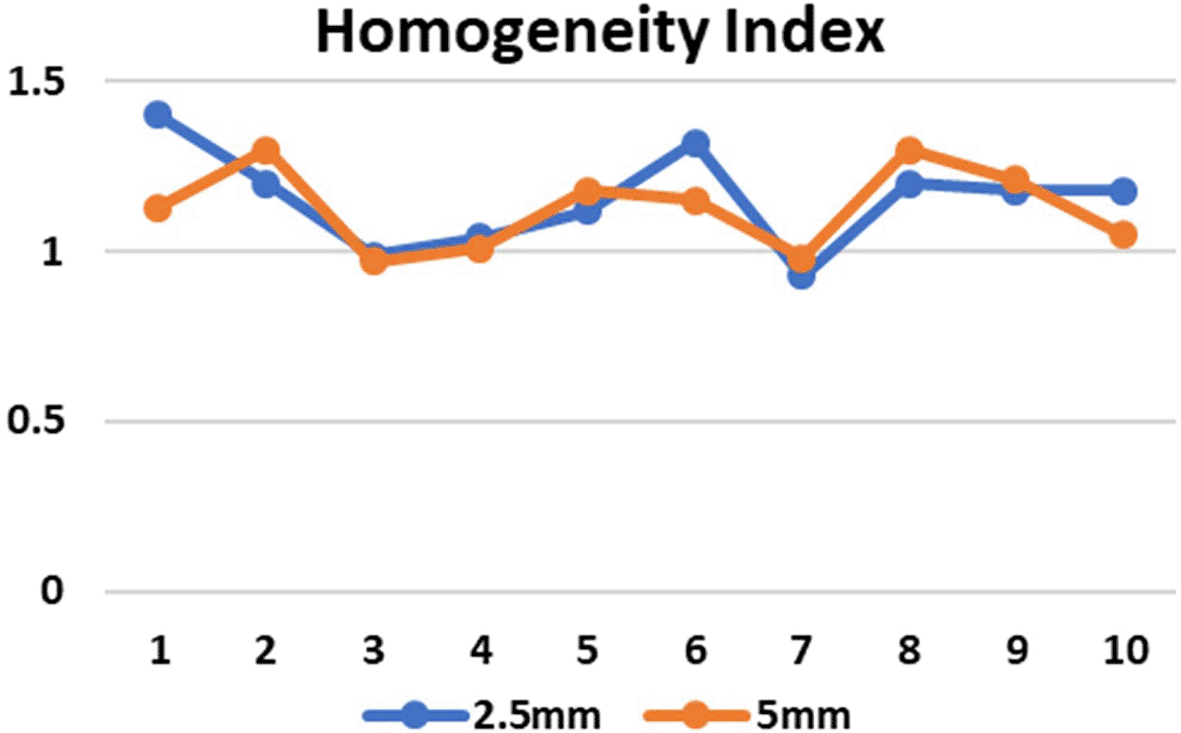
CI in both the SRS and SBRT plans was good in comparison. These values were found to be similar for both the planning techniques. In the 5mm and 2.5mm width MLC the mean CI for the brain targets were 0.9±0.01 and 0.9±0.04 respectively (p=0.80) and for the lung targets were 0.9±0.01 and 0.9±0.04 respectively (p=0.07) which indicates that the target area is optimally covered ( Figure 5) and there is no significant difference in the CI for brain and lung targets in both MLC designs. The HI values in case of 2.5mm and 5mm MLC in brain targets were 1.1±0.2 and 1.1±0.1 respectively (p=0.74) and for the lung targets the values were 1.1±0.1 and 1.1±0.1 respectively (p=0.78). The HI values were also found to be close to unity, indicating a homogenous dose distribution across the tumor volume ( Figure 6). The GI values for 5mm and 2.5mm MLC in brain targets were 5.1±0.4 and 4.3±1.0, respectively (p=0.15), and for the lung targets 4.6±1.3 and 4.5±1.1, respectively (p=0.89). Although there was no significant difference between the GI values, the obtained results showed that GI was better in the 2.5mm APEX based plans compared to 5mm MLC ( Figure 7). The mean and maximum doses to the OARs in both treatment planning techniques for both 2.5mm and 5mm MLC based plans were found to be close to each other. Although there was no significant difference between the two, it has been shown that the doses were slightly lower in most of the OARs in lung targets, whereas in brain targets, we received mixed results, indicating that both techniques are comparable ( Tables 1 and 2). Data on the same have been made available online.39 The treatment duration was assessed by calculating the monitor units (MU) for the 2.5mm mMLC and 5mm MLC plans. It was found that the MU for brain targets with 2.5mm mMLC and 5mm MLC were 2482.6±75.8 and 5060.8±415.04 respectively and for lung targets the MU with 2.5mm mMLC and 5mm MLC were 1886.2±44.28 and 3646.1±506.7 respectively with p value less than 0.05. this indicates that the treatment duration with 2.5mm mMLC is longer compared to the 5mm MLC.


In the current study, ABC was used for SBRT to manage the breathing of patients, and CBCT was used for verification. Accurate treatment was delivered to make sure that the setup error to be within the margin of ±1mm. Contouring of the brain and lung lesions was performed by the physicians after fusing the CT (Philips, 16 slice) images with MRI to draw accurate tumor volume and the OAR. CT is considered the first-line imaging modality used to diagnose the disease and to determine the true extent of the disease. However, the brain and mass in the lung are soft tissues, and the approximate and true spatial extent of the disease cannot be identified using CT images alone. MRI is superior to CT for the identification of soft tissues, definite tumor size, exact tumor location, number of lesions in the brain, and peritumoral edema. Therefore, both CT and MRI images were combined. The actual tumor volume was contoured on MRI and automatically mapped onto the CT image, which was then used for planning purposes.17
This study identified dosimetric differences in the treatment plans performed with the addition of 2.5mm mMLC and 5mm MLC. It is important to conform the radiation dose to the target volume in SRS/SRT/SBRT because of the size of the lesion and proximity of the OAR so that the dose distribution is bound tightly to the tumor volume. For this reason, dedicated machines are now available, including standard LINAC with added mMLC.40 However, the addition of this device prolongs the total treatment duration and reduces the clearance between the collimator and patient because of restrictions in choosing the appropriate and required gantry angles most of the time. It has limitations in the placement of noncoplanar arc angles.41 The LINAC (Elekta HD Versa) used in the current study was equipped with a built-in 160 MLC with a width of 5mm at the isocenter. To deliver treatment plans performed with a 2.5mm MLC using the same LINAC, an additional collimating device (APEX) must be attached to the machine. APEX consists of 56 pairs of MLCs with a 2.5mm width at the isocenter, which provides a field size of 12 × 14cm2.
The SRS and SBRT treatment plans performed with a 2.5mm MLC were considered as the benchmark in this study, and these plans were compared with a 5mm MLC. The plans with a 2.5mm MLC were optimized to deliver the best possible treatment with more than 95 to 98% coverage of the target volume with other parameters to be within the prescribed limits. In the plans with a 5mm MLC, the gantry, couch, and collimator combinations were kept constant, and the treatment plans were optimized to deliver the same dose distribution by keeping all the dose constraints to the OARs minimum. The resulting PTV coverage was equivalent in the 2.5mm and 5mm treatment plans. The obtained results were comparable to the results obtained by Wu et al., in which three planning techniques were used for the comparison, and the plans were performed using 2.5mm and 5mm MLC. They suggested that the PTV coverage, HI, and CI were better in IMRT-based HD MLC plans; however, as the size of the tumor increased and the OARs were closer to the target, the scope of HD MLC also became less noticeable.41
The treatment plan quality was compared using HI, CI, and GI.42 The HI and CI values for both the plans were comparable. Heather et al. conducted a study in which they compared MLC of three different widths, 2.5, 5, and 10mm, for the SBRT spine. With respect to tumor coverage, CI, and HI, the results of the study were comparable irrespective of leaf width.43 Joablot et al. conducted a study wherein they performed SRS/SRT plans using 2.5mm, 5mm and 10mm MLC. With respect to HI, they found no clinically significant differences in any of the different MLC widths. In addition, there was no difference in the CI between 5mm and 2.5mm for PTV volumes greater than 1cc. However, for PTV volumes lesser than 1cc, the dose conformity was less in the 5mm MLC, but the amount of reduction was clinically acceptable. The OAR doses were found to be comparable irrespective of leaf width. The dose falloff was steep in the 2.5mm MLC which was clinically acceptable.20 This result was also observed in the current study in terms of GI, which showed a steep dose fall off in the 2.5mm MLC. The GI was slightly higher in the 5mm MLC compared in the 2.5mm MLC which indicates a steeper dose fall in the 2.5mm MLC.43 The GI is an important factor that indicates the surrounding normal tissue dose. The lesser the GI, the more the surrounding OARs are spared.44 In a study conducted by Ferrer et al., three SRS cases were planned with a 5mm and 2.5mm MLC. They obtained similar results for conformation indices and OARs. The target coverage in VMAT is also better at the cost of more monitoring units. They found satisfactory results with VMAT, but in order to have more comparisons between VMAT and APEX, planning must be performed with a larger sample size.39
In the current study, the plans with 2.5mm mMLC and 5mm MLC were limited to certain targets which are surrounded by less critical organs. However, it is important to highlight that a fairer comparison could have been made by considering targets located closer to the OARs. In these cases, the use of 2.5mm mMLC with finer leaf spacing is expected to demonstrate superior precision in beam shaping, thereby better sparing OARs compared to conventional 5mm MLC. Such a scenario would likely reveal the full potential of mMLC in minimizing radiation exposure to critical structures adjacent to the target. Including this factor in the study would provide a more comprehensive comparison of the 2.5mm mMLC and 5mm MLC, particularly in cases where the tumor is located near sensitive tissues. Future studies should aim to incorporate such cases to better assess the mMLC vs MLC.
This study adds to the literature by showing that a 5mm MLC may suffice in most cases of SRS and SBRT and will be beneficial in reducing the treatment time. This study was limited by the sample size and its retrospective nature.
There was no clinically significant difference found in the target coverage, CI, HI and OAR doses in ten patients planned for SRS and SBRT using 2.5mm mMLC (APEX) or 5mm MLC (agility) with 6FFF photon beam energy. The target coverage was comparable for both the plans. VMAT with agility provided equivalent tumor coverage with an additional number of MUs. The CI and HI values were almost similar in both plans, resulting in a conformal and homogenous dose distribution to the entire PTV. OAR doses were comparable in both MLC for brain cases, whereas for the lung targets, OAR doses were slightly lower with 2.5mm mMLC. GI was superor in the 2.5mm mMLC compared to the 5mm MLC giving a steep dose fall off in the dose distributions. Hence, a 5mm MLC is also preferred for SRS and SBRT, with GI being an important consideration during planning.
Harvard Dataverse: Underlying data for ‘Comparison of treatment planning for stereotactic radiosurgery and stereotactic body radiation therapy techniques with 2.5mm and 5mm multileaf collimator (MLC): A pilot study’, https://www.doi.org/10.7910/DVN/CFNQEX.39
Harvard Dataverse: STROBE checklist for ‘Comparison of treatment planning for stereotactic radiosurgery and stereotactic body radiation therapy techniques with 2.5mm and 5mm multileaf collimator (MLC): A pilot study’, https://www.doi.org/10.7910/DVN/CFNQEX.39
Data are available under the terms of the Creative Commons Zero “No rights reserved’ data waiver (CC0 1.0 Public domain dedication).
I would like to acknowledge the contribution of Ms. Sneha Ravichandran in editing and reviewing this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I am a Medical Physicist with >15 years of experience in the field of Radiation Oncology. My expertise covers different topics of interest spanning from the implementation of new treatment planning and optimization strategies to the most recent innovations brought by the artificial intteligence in this domain.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Radiological Physics, radiotherapy, treatment planning, dosimetry, radiobiology, quality assurance, research methodology, radiation protection, emergency management
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I am a Medical Physicist with >15 years of experience in the field of Radiation Oncology. My expertise covers different topics of interest spanning from the implementation of new treatment planning and optimization strategies to the most recent innovations brought by the artificial intteligence in this domain.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 17 Mar 25 |
read | |
|
Version 1 18 Apr 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)