Keywords
mitochondria, apoptosis, cell death, cell signaling
This article is included in the Cell & Molecular Biology gateway.
This article is included in the Coronavirus (COVID-19) collection.
Mutations in the viral genome of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can enhance its pathogenicity by affecting its transmissibility, disease severity, and overall mortality in human populations. In addition to mutations within the coding region of SARS-CoV-2 structural proteins, there have been reports of mutations in other SARS-CoV-2 proteins that affect virulence, such as open reading frame 3a (ORF3a), which is involved in viral replication. The expression of ORF3a in host cells activates cell death signaling, leading to tissue damage, which affects the severity of COVID-19. The ORF3a-Q57H variant is the most frequent and recurrent variant of ORF3a and is likely associated with increased transmissibility but lower mortality in the 4th epidemic wave of COVID-19 in Hong Kong. Computational structural modeling predicted that the Q57H variant destabilizes the protein structure of ORF3a, which may result in reduced protein expression in human cells. However, it is still unknown how this mutation affects ORF3a protein function and, if so, whether it can change the severity of host cell damage.
Plasmids carrying SARS-CoV-2-ORF3a from Wuhan-Hu-1 strain (i.e., wild-type; WT) and its variant Q57H were transiently transfected into HEK293T cells and used for biochemical and cell biological assays.
SARS-CoV-2-ORF3a-Q57H variant exhibits similar protein expression in whole cell lysates compared to WT, but less expression at the plasma membrane. ORF3a-Q57H expression results in less apoptosis in host cells compared to WT via lower activation of the extrinsic apoptotic pathway.
The relatively mild phenotype of the SARS-CoV-2-ORF3a-Q57H variant may result from alterations to ORF3a function by this mutation, rather than its protein expression levels in host cells.
mitochondria, apoptosis, cell death, cell signaling
Following reviewers’ suggestions, we performed the additional experiments, which includes 1) confirmation of wild type (WT) and mutant ORF3a protein expression using anti-ORF3a antibody, 2) providing the potential molecular mechanism for ORF3a-mediated extrinsic apoptotic pathway activation, and 3) validation of inflammatory signaling activation in H9c2 cells. We revised our manuscript using this new data set.
See the authors' detailed response to the review by Wei Liu
See the authors' detailed response to the review by Julian Leibowitz and Sarisa Na Pombejra
COVID-19 is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is responsible for the global pandemic that began in 2020.1 SARS-CoV-2 can produce 29 proteins, including 9 accessory proteins encoded by open reading frames.2,3 These proteins were originally identified as critical factors for viral entry, viral genome production and replication, virion morphogenesis, and viral release from the host cells.2,4
Mutations in the SARS-CoV-2 genome can alter its pathogenic potential, ultimately affecting the severity and transmissivity of COVID-19 in humans.5 Since 2020, the World Health Organization has been identifying, tracking, characterizing, and labeling some SARS-CoV-2 variants as “variants of interest” and “variants of concern” to prioritize global monitoring and research.6 Thirty-six non-synonymous and 78 synonymous mutations have been reported in open reading frame 3a (ORF3a), which is the largest accessory protein in the SARS-CoV-2 genome.7 The 25563G>T-(Q57H) variant is the most common ORF3a variant (30-40%) reported in COVID-19 patients in the US, and the next most frequent ORF3a variant is 10 times less prevalent than Q57H.8 Q57 is located near the end of the first transmembrane domain of ORF3a, facing the hydrophobic lipid interface,9,10 which changes the amino acid glutamine (Q), which has a non-charged polar side chain, into the positively charged amino acid histidine (H). ORF3a-Q57H was first identified in Singapore in 2020 and has since been observed in the COVID-19 Beta, epsilon, and Mu variants.3 Q57H was the only mutant consistently reported with a high frequency in the entire period of 2020, whereas the frequency of the other ORF3a mutations fluctuated.10 In the fourth epidemic wave of COVID-19 in Hong Kong, this variant was associated with increased transmission and decreased mortality rates.11 Viral samples isolated from patients during this wave did not exhibit enhanced replication kinetics or cytokine/chemokine induction in the host cells. A recent study using computational modeling9 predicted that the Q57H mutation may decrease protein stability and increase the rigidity of the ORF3a protein compared to the original Wuhan-Hu-1 strain (i.e., wild-type; WT), which likely affects downstream signaling in host cells. However, it has not been established whether the Q57H mutant affects the function of ORF3, its role in host cell damage during SARS-CoV-2 infection, and ultimately the severity of COVID-19 phenotypes in patients.
Here, we report that the protein expression of SARS-CoV-2-ORF3a-Q57H variant leads to reduced activation of the extrinsic apoptotic pathway in the host cells compared to WT. Our findings may support the potential molecular linkage between this major mutation and a mild phenotype, but higher transmissibility, in COVID-19 patients.
The antibodies and plasmids used in the experiments are listed in Tables 1 and 2, respectively. All the cells, chemicals and reagents were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA) unless otherwise listed in Table 3.
| Inserted gene | Vector Backbone | Source/Provider/RRID (if available) | Company | Notes | Ref. |
|---|---|---|---|---|---|
| Mitochondrial matrix-targeted DsRed (mt-RFP) | pDsRed1-N1 (Clontech, Mountain View, CA, USA #6921-1) | Dr. Yisang Yoon | 40 | ||
| Empty | pEGFP-C1 (Clonetech, # 6085-1) | Clontech | |||
| Empty | pEGFP-N3 (Clonetech, # 6080-1) | Clontech | |||
| SARS-CoV-2-ORF3a- P2A-eGFP | pcDNA3.1+P2A-eGFP (GenScript) | GenScript | ORF3a was tagged with GFP by bridging “self-cleaving” small polypeptides (P2A) | ||
| SARS-CoV-2-ORF3a-GFP | pcDNA3.1+C-eGFP (RRID:Addgene_129020) | GenScript | |||
| SARS-CoV-2-ORF3a Q57H-GFP | pcDNA3.1+C-eGFP (RRID:Addgene_129020) | GenScript | This construct was generated by PCR-based site-directed mutagenesis using the SARS-CoV-2-ORF3a-GFP constructs as a template. | ||
| SARS-CoV-2-ORF3a-Q57H-P2A-GFP | pcDNA3.1+P2A-eGFP (GenScript) | GenScript | This construct was generated by PCR-based site-directed mutagenesis using the SARS-CoV-2-ORF3a- P2A-eGFP constructs as a template. | ||
| SARS-CoV-2-orf3a-2xStrep | pLVX-EF1alpha-IRES-Puro (Clontech) | Dr. Nevan Krogan/RRID: Addgene_141383 | Addgene, Watertown, MA, USA | Addgene plasmi # 141383 | 41 |
| Mouse MCU-L-GFP | pEGFP-N1 (Clontech) | Dr. Rosario Rizzuto | 42 | ||
| Empty | pLVX-EF1α-IRES-puro | ZAGENO, Cambridge, MA, USA | PVT2308 |
| Name of cells, chemical/reagents | Supplier | Catalog number | Notes | Ref. |
|---|---|---|---|---|
| HEK293T cells | Dr. Keigi Fujiwara, University of Texas, MD Anderson Cancer Center, Houston, TX, USA | N/A | Used in Figure 1. | 13 |
| H9c2 rat cardiac myoblasts | ATCC, Manassas, VA, USA | CRL-1446 | Used in Figures 2-5. | 12 |
| Interleukin 1β (IL-1β)/IL-1F2 recombinant protein | R&D Systems, Minneapolis, MN, USA | 501-RL | Used in Figure 3A. 100 ng of recombinant IL-1β was used for the positive control for the western blotting. | |
| Z-LEHD-FMK | ApexBio, Houston, TX, USA | B3233 | Used in Figure 5D. Z-LEHD-FMK (PubChem CID: 10032582) was dissolved in DMSO and used for the final concentration of 20 μM. | |
| caspase-8 Staining Kit (Red) | Abnova. Taipei City, Taiwan | KA0760 | Used in Figure 4A-C. One μL of Red-IETD-FMK (PubChem CID 25108681) was added to 300 μl of cell culture medium and cells were incubated for 30 min at 37°C incubator with 5% CO2. The caspase inhibitor Z-VAD-FMK (PubChem SID: 404336810) at 1 μl/ml was added to inhibit caspase activation. | 43 |
| FuGENE HD | Promega, Madison, WI, USA and Fugent, LLC, Madison, WI, USA | E2312 and HD-5000, respectively | Used in Figures 1-5. 0.5-3 μg of plasmids and 7 μl of FuGENE HD were added to 100 μL Opti-mem (Thermo Fisher Scientific) at room temperature. The mixture was incubated for 15 min and added to 2 ml cell culture medium in 3.5-cm dishes. In Figure 4D and E, 10 μg of plasmids and 35 μl of FuGENE HD were added to 500 μL Opti-mem at room temperature. The mixture was incubated for 15 min and added to 10 ml cell culture medium in 10-cm dishes. | |
| Cell lysis buffer | Cell Signaling Technology | 9803S | Used in Figures 1-5. Two hundred μl of 1x Cell lysis buffer were used for each 6-cm dish to harvest protein. | |
| Fluorescence-conjugated secondary antibodies | LI-COR Biosciences, Lincoln, NE, USA | 926-32211 and 926-68020 | Used in Figures 1-5. Secondary antibodies were added in 0.05% PBST (1;20, 000 dilution). The nitrocellulose membrane was incubated with secondary antibody-containing PBST for 1 hr at room temperature. | |
| NucView® 405 substrates | Biotium, Fremont, CA, USA | 10407 | Used in Figure 2C and D. PBS containing 2 µM NucView® 405 substrate was treated to the cells at room temperature for 30 min before observation. | 44 |
| Cell Meter™ Caspase-9 Activity Apoptosis Assay Kit *Red Fluorescence* | AAT Bioquest, Pleasanton CA, USA | 22817 | Used in Figure 5D. Five μL of 200X Ac-LEHD-ProRed™ stock solution was added to 1 mL of Assay Buffer provided from the manufacturer to make caspase-9 substrate working solution. Cells were incubated with the working solution at room temperature for 1 hr, before observation. | 45 |
| Nigericin | Adipogen Corporation, San Diego, CA | AGCN20020M005 | Used in Figure 4A and B. Nigericin was dissolved in DMSO and used in the final concentration as indicated in the figure legends. | |
| Pierce™ Cell Surface Biotinylation and Isolation Kit | Thermo Fisher Scientific | A44390 | Used in Figure 4E to isolate the plasma membrane protein from transfected HEK293T cells. |
Study protocol was approved by the Institutional Biosafety Committee at University of Minnesota and University of South Florida (#2305-41075H and #PROTO2025-050, respectively). HEK293T and H9c2 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 4.5 g/L glucose, 1 mM sodium pyruvate, 1% L-glutamine, 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C with 5% CO2 in a humidified incubator, transfected with plasmids (0.5-3 μg/3.5-cm dish, except Figure 4D and E [10 μg/10-cm dish]) using Fugene HD, and used for experiments 48 to 72-hr after transfection.12,13
Mitochondria-enriched fractions were separated from cytosolic fractions by different centrifugation speeds and dissolved with lysis buffer containing protease inhibitor cocktails and 1 mM phenylmethylsulfonyl fluoride.12,13 Whole cell lysates were prepared as we reported.12,13 To collect the plasma membrane (PM) proteins, a cell-surface biotinylation assay was performed using PierceTM Cell Surface Protein Isolation Kit and Pierce™ Cell Surface Biotinylation and Isolation Kit (Thermo Fisher Scientific).14 The immunoreactive bands were visualized, and the whole blotting images for each figure panel46 were obtained using a near-infrared fluorescence imaging system (LI-COR Biotechnology, Lincoln, NE, USA).12,13
Total RNA samples from the transfected HEK293T cells were treated with DNase (Zymo Research, Irvine CA) for the removal of the plasmid DNAs and processed with RNA Clean & Concentrator-5 kit (Zymo Research). RNA quantity and purity were measured using a NanoDrop 2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). cDNA was synthesized from the total RNA using the iScript™ reverse transcription kit (Bio-Rad Laboratories, Hercules, CA) or Superscript™ IV VILO™ Master Mix (Thermo Fisher Scientific [TFS], Waltham, MA, USA), respectively. RT-qPCR was performed using a QuantStudio™ 3 (TFS) with an Applied Biosystems PowerUp SYBR Green Master Mix (TFS). The relative transcript levels for ORF3a were determined by the ΔΔCT method with GAPDH as the endogenous reference gene.15
Cells stained with cell-permeable dyes ( Table 3) were observed by an FV3000 confocal microscope (Olympus, Tokyo, Japan) at room temperature. Localization of GFP-tagged proteins was observed in H9c2 cells stably overexpressing mitochondrial matrix-targeted DsRed (mt-RFP) and the colocalization efficiency was estimated using Pearson’s correlation coefficient.12
We first examined the effect of a Q57H mutation on ORF3a protein expression levels in HEK293T cells by transiently expressing SARS-CoV-2-ORF3a (Figure 1A). ORF3a is tagged with GFP by bridging “self-cleaving” small polypeptides (P2A),16 allowing for bicistronic expression of non-tagged ORF3a proteins and GFP (Figure 1A and Ref. 46). When the high amounts of plasmid (≥1.0 μg/well) were transfected, ORF3a-WT and -Q57H constructs produced similar levels of ORF3a protein, as assessed with ORF3a-specific antibody (Figure 1B and C). However, under lower amounts of plasmid transfection (0.5 μg/well), We found significantly higher protein expression of Q57H compared to WT-ORF3a (Figure 1B and C). No significant differences were observed in mRNA levels between WT-ORF3a and Q57H mutant transfection in all transfection conditions (Figure 1D).
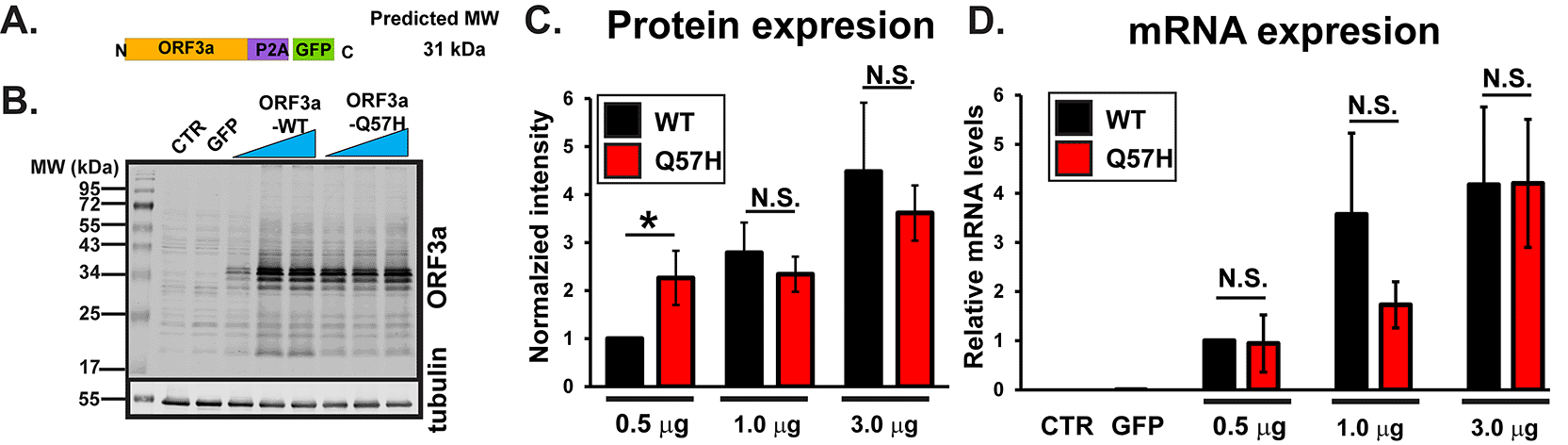
A. Design of SARS-CoV-2-ORF3a-P2A-GFP constructs. B. Expression of a WT and Q57H mutant ORF3a-P2A-GFP in HEK293T cells. The pEGFP-C1 empty plasmid was shown as a control. Each construct was transfected with 0.5, 1.0, or 3.0 μg per 3.5 cm dish. Tubulin was used as a loading control. CTR, cells with no transfection. C. Summary data of B (n =8). In each panel, band intensity was normalized to the value from 0.5 μg of WT-ORF3a/tubulin. * p<0.05. N.S., not significant. D. RT-qPCR analysis of ORF3a in each transfection condition. Expression levels were normalized to the value of 0.5 μg ORF3a-WT/GAPDH. Cells with no transfection (CTR) and those with 3 μg GFP were used as negative controls.
Using a computational model, Wang et al. predicted that the ORF3a protein structure becomes more rigid and less flexible after the Q57H mutation9 and may result in less activation of downstream signaling that causes host cell damage. We tested cellular damage by WT-ORF3a and Q57H-ORF3a proteins in H9c2 cardiac myoblasts because this cell line is more vulnerable to oxidative, apoptotic, and inflammatory signaling than cancer cell lines, including HEK293T cells.12,17 A recent report has shown that SARS-CoV-2-ORF3a expression can activate apoptotic signaling.18 We found that the expression of WT-ORF3a, but not ORF3a-Q57H, increased caspase-3 activity in H9c2 cells, as assessed by the amount of cleaved caspase-3 (Figure 2A and B). The caspase-3 activity was also evaluated by live-cell staining with a fluorogenic DNA dye coupled to the caspase-3/7 DEVD recognition sequence (NucView® substrates). GFP itself produced a population of apoptotic cells as reported,19 but WT-ORF3a expression significantly increased the number of apoptotic cells compared to GFP (Figure 2C and D). The number of apoptotic cells in Q57H cells was similar to that in GFP cells and significantly lower than that in WT-ORF3a cells (Figure 2C and D).
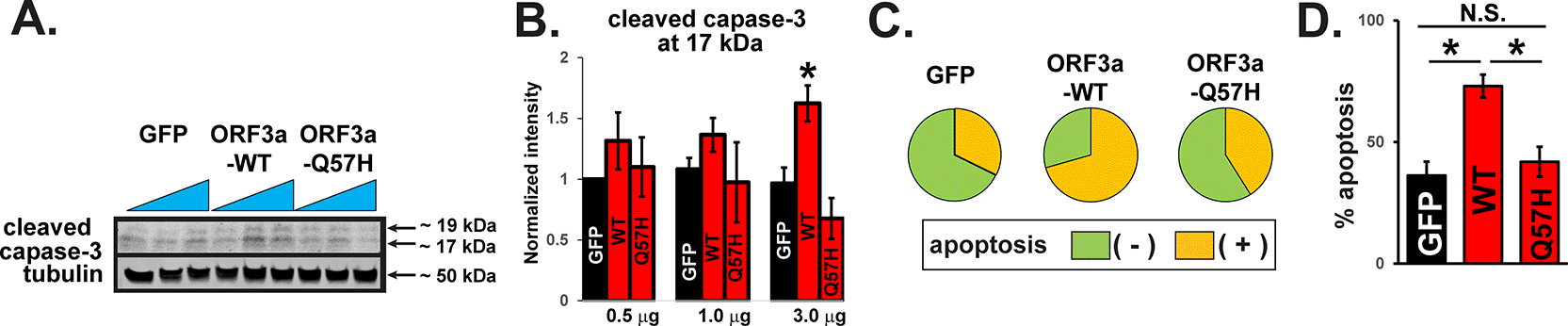
A. Cleaved caspase-3 in H9c2 cells overexpressing WT and mutant ORF3a. GFP was transfected as a control. Each construct was transfected with 0.5, 1.0, or 3.0 μg per 3.5-cm dish. B. Summary data of A (n= 5). All values were normalized to the value of 0.5 μg GFP/tubulin. *p<0.05. C. Detection of caspase-3 activity in live H9c2 cells stained with Nucview 405 caspase-3. GFP-positive cells were selected as transfected cells, and nuclear staining-positive cells by fluorogenic DNA dye were counted as apoptotic cells under the confocal microscopy. D. Summary data of C from three independent experiments. *p<0.05. N.S., not significant.
In addition to apoptotic responses, several groups have shown that the expression of SARS-CoV-2-ORF3a constructs with protein tags activate inflammatory signaling, endoplasmic reticulum (ER) stress, and autophagy flux.20–25 Although nigericin (NG), an activator for nucleotide-binding domain, leucine-rich repeat, and pyrin domain-containing protein 3 (NLRP3), can significantly increase inflammation in H9c2 cells (Figure 2A and B) as previously reported,26 WT-ORF3a and Q57H did not produce significant inflammatory responses, as assessed by the protein expression levels of IL-1β, NLRP3, and cleaved caspase-1 (Figure 3A-D). The expression of ER stress markers, including glucose-regulated protein 94 (Grp94), glucose-regulated protein 78 (Bip/Grp78), and C/EBP-homologous protein (CHOP),27 did not change after the expression of either WT-ORF3a or -Q57H in our system (Figure 3E and F). Finally, both WT-ORF3a and its mutant Q57H showed a similar tendency of increased microtubule-associated protein light chain 3 (LC3)-II/LC3-I ratio, a standard marker indicating the induction of autophagy, but these changes were not significant compared to control cells transfected with GFP (Figure 3G and H). In summary, SARS-CoV-2-ORF3a expression induces apoptotic signaling activation rather than modulating inflammation, ER stress, and autophagic signaling cascades. Importantly, Q57H expression was less involved in apoptotic signaling activation than that of WT-ORF3a.

A. (top) Increased inflammatory activity by 20 μM NG treatment in H9c2 cells, assessed by cleaved caspase-1 and IL-1β. Cells treated with the vehicle (DMSO) were used as controls (CTR). For 24 hr treatment group, Cells were treated with NG for 1 hour and then incubated with standard medium for 24 hrs. (bottom) Summary data. All values were normalized to the value of CTR/tubulin. * p<0.05 compared to CTR. (n= 8). B. (top) Assessment of inflammatory activity in H9c2 cells expressing WT and mutant ORF3a. GFP was transfected as a control. Cell lysates treated with NG (15 μM for 60 min) and a recombinant IL-1β protein were used as positive controls. (bottom) Summary data (n=3). All values were normalized to the value from 0.5 μg GFP/tubulin. C. (top) Detection of the NLRP3 inflammasome in H9c2 cells expressing WT and mutant ORF3a. (bottom) Summary data (n= 4). All values were normalized to the value from the cells transfected with 0.5 μg GFP/tubulin. D. (top) Assessment of the expression of ER stress markers Grp78, Grp94, and CHOP in H9c2 cells transfected with WT and mutant ORF3a. GFP was transfected as a control. (bottom) Summary data (n=4, n =3, n=3, respectively). E. (top) Assessment of autophagic flux by the LC3-II/LC3-I ratio in H9c2 cells expressing WT and mutant ORF3a. GFP was transfected as a control. (bottom) Summary data (n=8). The ratio of LC3-II (low molecular weight) to LC3-I (high molecular weight) was calculated and normalized to the value from 0.5 ug of the transfected GFP control.
A recent report showed that SARS-CoV-2-ORF3a-ORF3a could activate both the intrinsic and extrinsic pathways of apoptosis.18 Therefore, we examined the activities of signaling molecules from both apoptotic pathways after WT-ORF3a or ORF3a-Q57H expression. The extrinsic pathway caspase-8 was significantly activated by WT-ORF3a expression compared to that in control cells, as assessed by a cell-permeable caspase-8 activity marker, Red-IETD-FMK. However, preincubation with a general caspase inhibitor, Z-VAD-FMK, abolished this change (Figure 4A). Q57H expression did not show significant caspase-8 activation (Figure 4A), and this difference between ORF3a-WT and -Q57H was unlikely based on the expression levels of the constructs, as confirmed by the expression levels of bicistronically expressed GFP (Figure 4B). Caspase-8 is activated by an extrinsic pathway (e.g., cell-surface death receptors) and is known to propagate the apoptotic signal either by directly cleaving and activating downstream caspases (e.g., caspase-3) or by cleaving Bid.28 Cells expressing WT-ORF3a (but only in the lower transfection conditions) showed a significant increase in the truncated Bid (tBid)/Bid ratio, but not by Q57H (Figure 4C). We also found that under the WT-ORF3a (but not Q57H) transfection, there was an increase in the expression of a Fas-associated death domain (FADD), a key adaptor protein that serves as a bridge between death receptors and the caspase-8 for triggering the extrinsic apoptotic pathway29 (Figure 4D). There was no difference between WT and Q57H in the expression levels of a cellular FADD-like IL-1β-converting enzyme (FLICE)-inhibitory protein cFLIP, an intracellular inhibitor of caspase-8 activation29 (Figure 4D). Importantly, Q57H expression at the plasma membrane (PM), where the death receptors and their signaling complex (i.e., death-inducing signaling complex [DISC]) reside,29 was lower than that in WT (Figure 4E). These results suggest that PM-localized ORF3a may increase the expression of FADD, followed by activation of the extrinsic apoptotic pathway; Q57H variant exhibits less activation of this pathway compared to WT, possibly due to its lower expression at the PM and the lower expression of FADD.
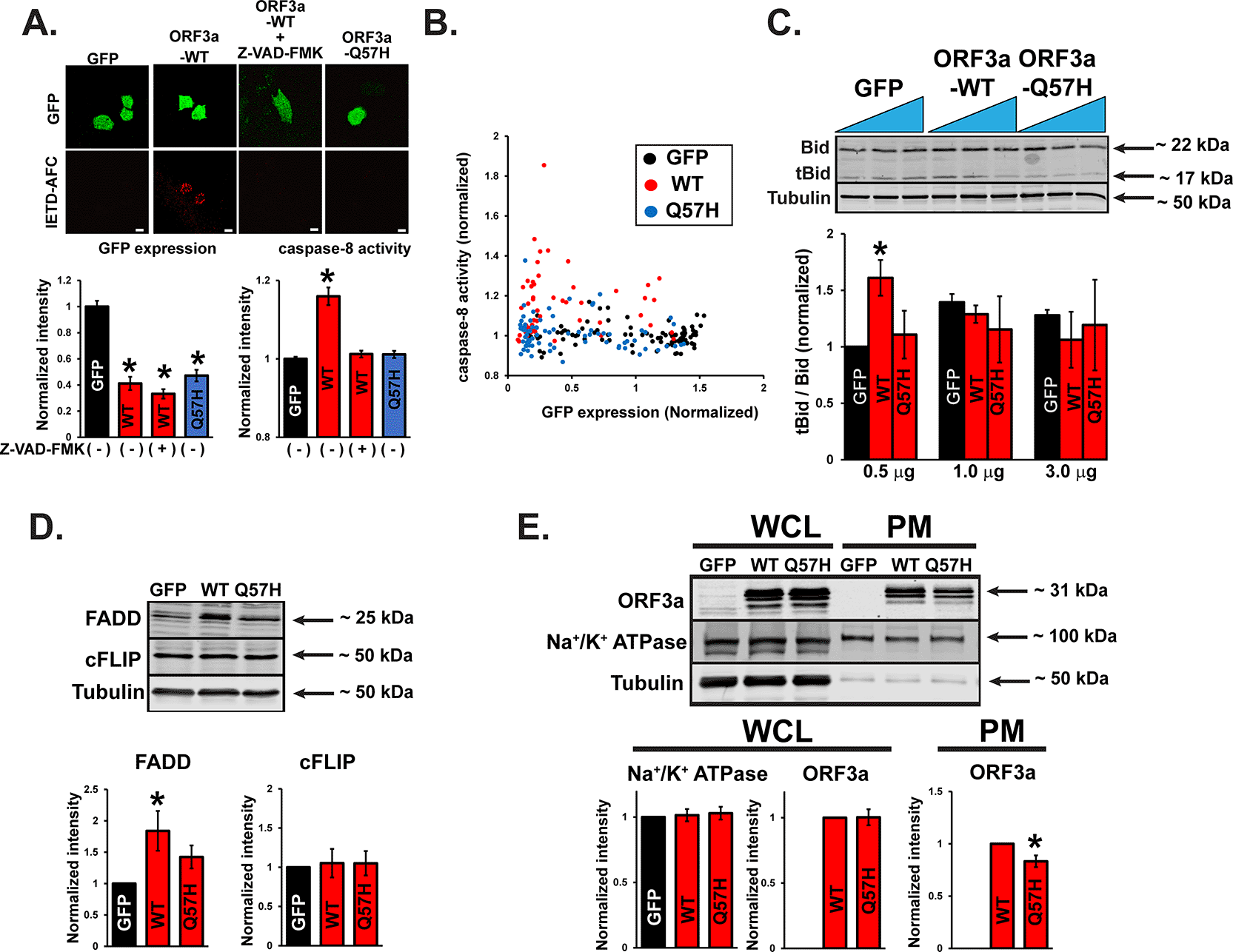
A. (top) Representative confocal images of H9c2 cells transfected with the indicated plasmids and stained with a cell-permeable marker dye for caspase-8 activation, Red-IETD-FMK. Red-IETD-FMK was detected under confocal microscopy with excitation and emission wavelengths of 488 and 570 nm, respectively. ORF3a-WT overexpressed cells pretreated with Z-VAD-FMK for 1 hr were used as a negative control. Scale Bars = 20 μm. (bottom) Summary data. * p<0.05, compared to GFP-transfected cells. Each fluorescence value was normalized to the average fluorescence calculated from GFP-transfected cells. B. Scatter plots of GFP and Red-IETD-FMK measured from individual cells. C. (top) Representative immunoblot of tBid/Bid in H9c2 cells expressing WT and mutant ORF3a. GFP was transfected as a control. (bottom) Summary data. * p<0.05, compared to 0.5 ug of the transfected GFP control. * p<0.05. D. (top) Representative immunoblot of FADD and cFLIP in HEK293T cells expressing WT and mutant ORF3a. GFP was transfected as a control. (bottom) Summary data. * p<0.05, compared to GFP-transfected cells. E. (top) Representative immunoblot of ORF3a in whole-cell lysates (WCL) and PM lysates in HEK293T cells expressing WT and mutant ORF3a. GFP was transfected as a control. Na+/K+ ATPase was used as a loading control for PM. (bottom) Summary data. In WCL and PM, the values were normalized to tubulin and Na+/K+ ATPase, respectively. * p<0.05.
Next, we investigated the effect of WT-ORF3a and ORF3a-Q57H on the intrinsic apoptotic pathway. Since the SARS-CoV-2-ORF3a protein is predicted to possess three transmembrane domains similar to SARS-CoV-1-ORF3a, and its subcellular localization is likely distributed to several membrane structures,30 we next tested whether ORF3a can be expressed in the mitochondria. Indeed, ORF3a protein was found in the mitochondria-enriched fraction compared to that in the cytosolic fraction (Figure 5A). Both WT-ORF3a and Q57H-ORF3a were partially localized in the mitochondrial area labeled by mt-RFP, and their subcellular distribution patterns were not significantly different, as assessed by the values of Pearson’s correlation coefficient (Figure 5B and C). We also found that the Q57H variant was capable of activating caspase-9, and assessed caspase-9 activity, an initiator of intrinsic apoptosis, whose level was comparable to that in WT assessed by Ac-LEHD-ProRed staining (Figure 5D). In summary, these results suggest that the different caspase-3 activation levels in WT-ORF3a and Q57H-ORF3a are mainly due to their different effects on the extrinsic apoptotic pathway.
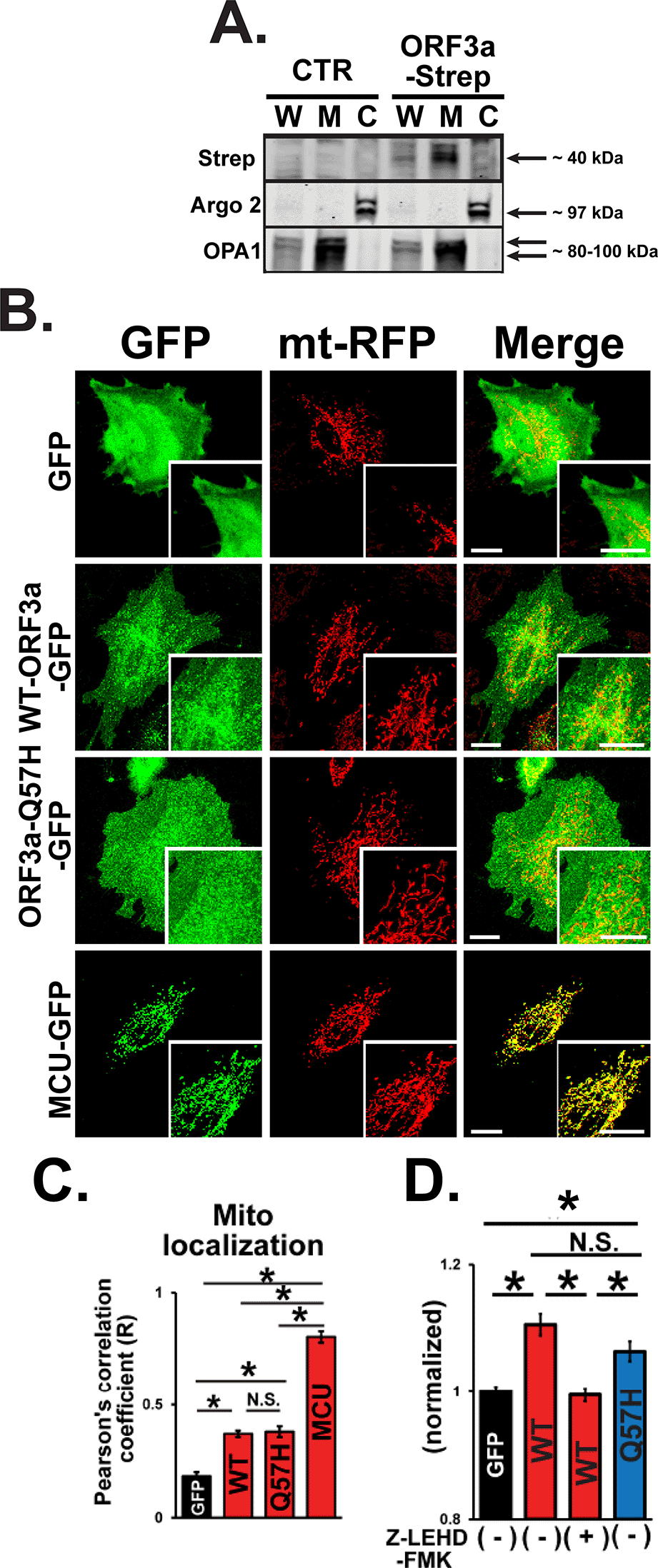
A. Expression of WT-ORF3a-Strep in fractionated proteins from H9c2 cells. Cells transfected with pLVX-EF1α-IRES-puro were used as a control (CTR). Argonaute 2 (Argo2) and optic atrophy-1 (OPA1) were used as markers for the cytosolic fraction (C) and mitochondrial fraction (M), respectively. Whole cell lysates (W) were shown for comparison. B. Representative confocal images of the subcellular localization of GFP, WT-ORF3a-GFP, ORF3a-Q57H-GFP, mitochondrial Ca2+ uniporter (MCU)-GFP (as a positive control) in live H9c2 cells stably expressing mt-RFP. Scale bars = 20 μm. C. Summary data of the mitochondrial localization of GFP constructs estimated by Pearson’s correlation values between the GFP and mt-RFP signals. *p<0.05. N.S., not significant. Cells transfected with a mitochondrial protein MCU-GFP were used as a positive control. D. Assessment of caspase-9 activity in live H9c2 cells transfected with indicated plasmids stained with a cell-permeable caspase-9-specific fluorogenic substrate, Ac-LEHD-ProRed. ORF3a-WT overexpressed cells pretreated for 1 hr with a caspase-9-specific inhibitor, Z-LEHD-FMK, were used as a negative control. ProRed cleaved from Ac-LEHD-ProRed was detected using confocal microscopy with excitation and emission wavelengths of 540 and 620 nm, respectively. The ProRed fluorescence value was normalized to the average fluorescence calculated from GFP-transfected cells.
Although the protein sequences of ORF3a from SARS-CoV-1 and CoV-2 have only moderate homology (72%),3 the expression of both proteins in mammalian cells promotes apoptosis.18 Our results showed that Q57H, the most frequent and recurrent variant of SARS-CoV-2-ORF3a, exhibits equivalent protein expression compared to SARS-CoV-2-ORF3a-WT (Figure 1), but less expression at the PM, and induces less apoptosis in host cells due to a lack of extrinsic apoptotic pathway activation (Figures 2-4, and 6). This property may provide advantages for the SARS-CoV-2-Q57H infection to be relatively mild, thus allowing the virus to have higher transmissivity, as was the case in the fourth epidemic wave of COVID-19 in Hong Kong.11
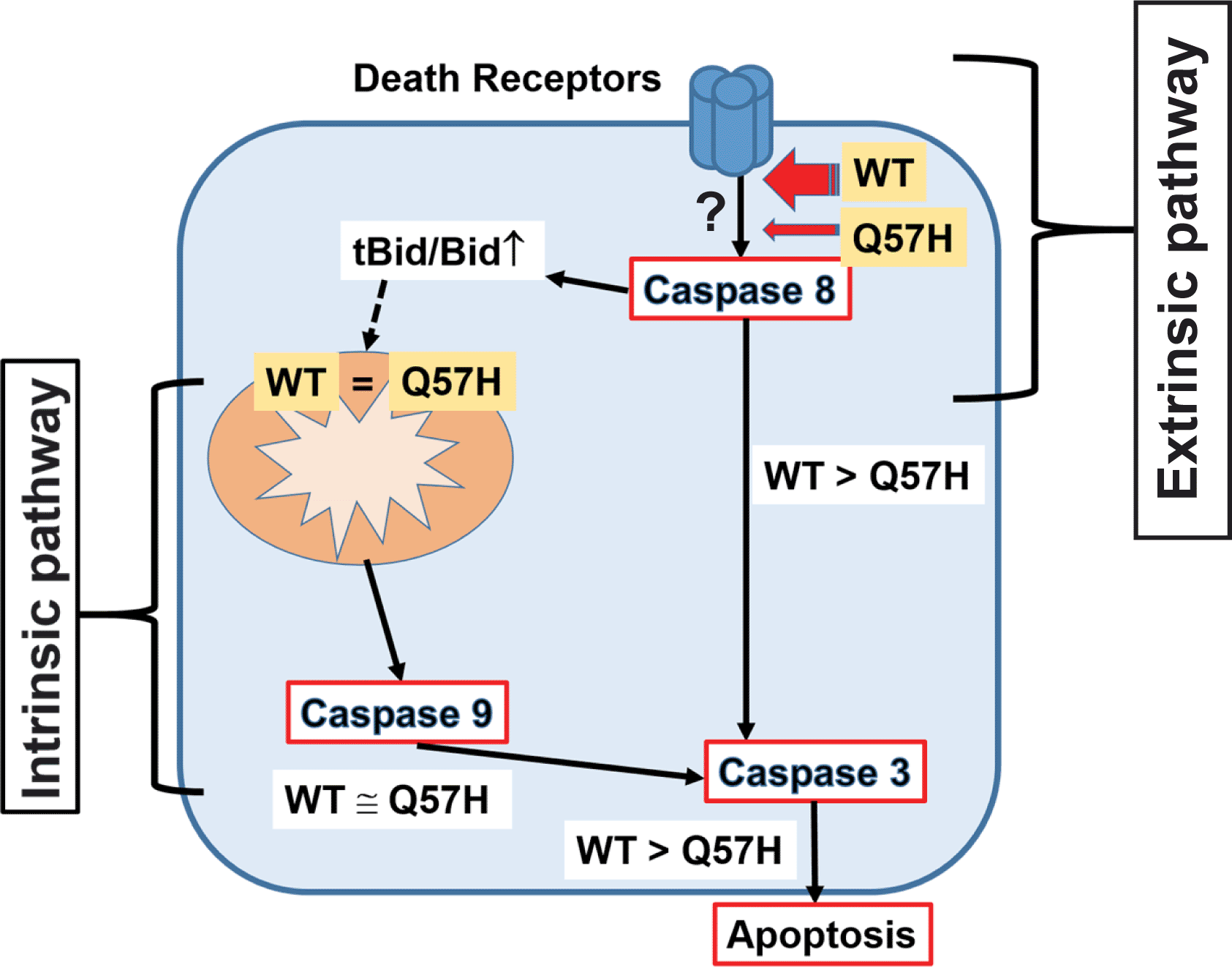
SARS-CoV-2-ORF3a induces apoptosis in host cells via activating both intrinsic and extrinsic pathways. ORF3a-Q57H shows less expression at PM compared to WT, which causes less activation of the extrinsic apoptotic pathway and ultimately less total apoptotic activity compared to WT.
Based on the computational prediction of negative folding stability in SARS-CoV-2-Q57H by Wang et al.,9 we initially hypothesized that the SARS-CoV-2-ORF3a-Q57H variant produces less protein expression in host cells than WT-ORF3a, thus exhibiting less oxidative stress and apoptosis, which may contribute to decreased mortality in COVID-19 patients. However, contrary to computational predictions,9 we did not observe a significant decrease in ORF3a protein expression by Q57H mutation, and ORF3a-Q57H construct produced a comparable amount of ORF3a protein as WT construct did (Figure 1). Rather, we found the higher expression of Q57H compared to WT under the transfection of a lower amount of plasmid, suggesting that Q57H may have higher protein stability than WT. Further studies are needed to clarify what kind of post-translational processes (e.g., protein degradation) are involved in the protein stability in Q57H.
Although the SARS-CoV-2-ORF3a protein can induce apoptotic signaling activation similar to SARS-CoV-1-ORF3a,31 Ren et al. recently reported that SARS-CoV-2-ORF3a has a relatively weaker effect on activating apoptotic signaling than SARS-CoV-1.18 Moreover, they showed that PM localization of ORF3a is required for activating apoptotic signaling in SARS-CoV-2. Ren et al. suggested that 1) SARS-CoV-2-ORF3a mainly activates the extrinsic apoptotic pathway, and 2) the intrinsic pathway is secondarily activated downstream of the extrinsic apoptotic pathway, although the detailed mechanism of how ORF3a activates the apoptotic pathway at the PM has not yet been well clarified.
We found that 1) SARS-CoV-2-ORF3a-WT was able to activate the extrinsic apoptotic pathway in the absence of death receptor ligands (Figures 4 and 6), and 2) ORF3a-WT increases the FADD expression (Figure 4). Our results suggest that ORF3a at PM may enhance the protein stability of FADD beneath PM, which may modulate the caspase-8 activation profile without death receptor stimulation. Although the location of the Q57H mutation in the ORF3a structure is far from the key motifs for PM sorting (i.e., cysteine-rich motif C130/133 and/or tyrosine-based sorting motif Y16018), we found less Q57H expression at the PM compared to WT (Figure 4). The different PM expression pattern between the WT and the mutant ORF3a is likely accountable for the difference in the strength of the apoptotic signaling, but further studies are required to identify the detailed molecular mechanisms by which ORF3a activates DISC, and how the Q57H mutation alters this mechanism.
Our data also showed that WT-ORF3a and ORF3a-Q57H both activated intrinsic apoptotic signaling at similar levels, even though Q57H exhibited less activation of extrinsic apoptotic signaling compared to WT (Figures 4-6). This result indicates that SARS-CoV-2-ORF3a can initiate intrinsic apoptotic signaling independent of extrinsic apoptotic signaling (Figure 6). In both SARS-CoV-1 and -CoV-2, ORF3a has three predicted transmembrane domains3,10 and has been localized in several cellular membrane structures/organelles in host cells, including the plasma membrane, endoplasmic reticulum, Golgi, and lysosomes.32–36 Our protein fractionation and imaging data showed that ORF3a was also localized in the mitochondria (Figure 5A-C), where it likely increased mitochondrial membrane permeability and promoted the release of apoptotic proteins. SARS-CoV-1-ORF3a can form K+-permeable viroporins31,36 that are required to induce ORF3a-mediated cell apoptosis.31 Although still controversial,35 SARS-CoV-2-ORF3a might also form K+-permeable channels at the inner mitochondrial membrane (IMM), which can depolarize the mitochondrial membrane potential similar to the opening of endogenous K+ channels expressed at the IMM, such as the mitochondrial BKCa channel.37 If ORF3a is expressed in the outer mitochondrial membrane (OMM), it is possible that ORF3a may interact with structural proteins that regulate OMM permeability.
Lastly, we tested whether SARS-CoV-2-ORF3a can modulate autophagy flux, ER stress, and inflammatory signaling in addition to apoptosis, but these signaling pathways were not significantly activated in our system (Figure 3). The different results may be partly due to the use of different cell types, which may provide different ORF3a expression levels and/or sensitivity to the stress-signaling pathway. For instance, H9c2 cells may be less competent for inflammasome activation. In addition, the majority of published data20–25,38 were generated from ORF3a constructs with various protein tags, which may alter ORF3a protein function because it is a relatively small protein (~30 kDa). Nevertheless, our results clearly showed a major difference in the activation of apoptotic signaling between ORF3a-WT and Q57H, especially in the extrinsic signaling pathway.
In summary, SARS-CoV-2-ORF3a-Q57H variant expression causes less apoptosis in mammalian cells because of lower activation of the extrinsic apoptotic pathway possibly via its less expression at PM. As our experiments were performed only in cultured cell lines transfected with a part of SARS-CoV-2 (i.e., ORF3a), we still need to consider that our findings cannot be directly applicable to the in vivo situation with SARS-CoV-2 infection. Animal models using SARS-CoV-2 are indispensable for exploring the detailed role of the ORF3a signaling pathway in vivo. Despite these limitations, our results suggest that the relatively mild phenotype of the Q57H variant observed in 4th epidemic wave of COVID-19 in Hong Kong and several COVID-19 variants (i.e., Beta, Epsilon, and Mu) may result from weaker pro-apoptotic signaling. Assessing the cellular effects of ORF3a mutations will improve our understanding of the pathophysiology of COVID-19 and inform the design of new therapeutic strategies to prevent and treat COVID-19 and its long-term symptoms.39
Figshare: Supplementary materials for revised manuscript “SARS-CoV-2-ORF3a variant Q57H reduces its pro-apoptotic activity in host cells”. https://doi.org/10.6084/m9.figshare.30473513.46
This project contains the following underlying data:
Original Western blotting images
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
We thank Ms. Ameneh Ahrari (University of South Florida) for her technical assistance. This study was partially supported by NIH/NHLBI R01HL136757 (to J.O.-U.), R01HL171710 (to J.O.-U.), and R01HL160699 (to B.S.J.), a COVID19 Response Grant (to J.O.-U.) from the Institute of Engineering in Medicine (IEM) at the University of Minnesota (UMN) and COVID-19 Response Grants (to J.O.-U. and B.S.J.) from the Office of Academic Clinical Affairs at UMN, the American Heart Association 18CDA34110091 (to B.S.J), the IEM Annual Conference Pilot Project Grant (to I.P.) from IEM at UMN, and 2024 Summer Undergraduate Research Fellowship (SURF) from American Physiological Society (to B.R.).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Virus infection and immunity; Interaction between viruses and host cells
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Replication and pathogenesis of coronaviruses
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Virus infection and immunity; Interaction between viruses and host cells
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Replication and pathogenesis of coronaviruses
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 22 Nov 25 |
read | read |
|
Version 1 23 Apr 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)