Keywords
chemotherapy, anticancer, drug use pattern, Najran, Saudi Arabia.
This article is included in the Oncology gateway.
Effective cancer management include comprehensive evaluation of treatment patterns to ensure optimal resource utilization. This is a single center study review anticancer drug utilization, with an emphasis on adherence to the World Health Organization Essential Medicines List (WHO EML) and regional prescribing trends amid increasing cancer incidence.
We conducted a retrospective cohort study of 512 adult patients with histologically confirmed malignant neoplasms that were managed with anti-cancer therapy at King Khaled Hospital, Najran, from 2014 to 2022. Data extraction included demographic characteristics, treatment regimens, and supportive medications, analyzed using IBM SPSS Statistics (version 21). Prescription quality was assessed against WHO EML criteria and Saudi Food and Drug Authority (SFDA) standards.
The study revealed slight male predominance (56.4%), with a mean age 55.2 ± 17.0 years. Gastrointestinal (29.7%) and breast cancers (25.8%) accounted for the majority of cases, and 46.7% of patients presented with metastatic disease. First-line regimens predominantly included doxorubicin-cyclophosphamide (20.1%) and FOLFOX (13.5%). Notably, 86.7% of prescribed agents were listed on the WHO EML, surpassing the 85.3% benchmark, and 90.1% were generics. Supportive care commonly involved metoclopramide-based antiemetics (76.1%). Medication shortages occurred in 8.4% of cases, predominantly involving BCG.
Our findings demonstrate an optimal utilization of WHO Essential Medicines List, reflecting evidence-based, cost-effective prescribing practices that exceed international benchmarks. Despite the observed supply chain vulnerabilities, the study reinforces the relevance and applicability of the WHO model at the regional level.
chemotherapy, anticancer, drug use pattern, Najran, Saudi Arabia.
We sincerely appreciate the reviewers' feedback, which has significantly improved our manuscript. Below, we summarize the key revisions made in response to reviewer comments:
1. Title & Scope:
- Removed references to survival analysis, as it was not conducted. The revised title is now: “Anticancer Drug Utilization and Prescription Practices in a Saudi Cancer Center (2014–2022).”
2. Methodology:
- Clarified that logistic regression was not performed and adjusted the Methods section accordingly.
- Added prescribing protocols (NCCN/SFDA guidelines) and specified the use of SPSS v21 for analysis.
- Corrected inconsistencies in exclusion criteria (e.g., patients with incomplete records).
3. Results & Presentation:
- Added a new figure (Figure 1) showing annual prescription trends.
- Removed incorrect references to logistic regression in tables and text.
- Clarified patient numbers (N=512, with 2 excluded due to unidentified cancer sites).
4. Discussion & Context:
- Expanded comparisons with regional/international studies.
- Discussed socioeconomic and supply chain influences on prescribing patterns.
- Added evidence-based recommendations for improving treatment strategies.
5. Language & Compliance:
- Replaced informal language with passive voice.
- Standardized keywords per MeSH terms.
6. Supplementary data:
- Added a new supplementary file as Extended data
See the authors' detailed response to the review by Pouyan Ebrahimi
See the authors' detailed response to the review by Shouki Bazarbashi
Cancer remains a formidable global health challenge, accounting for approximately 10 million deaths annually attributed to malignancies.1 While therapeutic advances have reduced age-standardized mortality rates by 33% since 1991, significant disparities persist across healthcare systems.2 Such disparity is particularly evident in Middle Eastern countries like Saudi Arabia, where reported cancer incidence increased from 20,000 cases in 2018 to over 27,000 by 2020.3,4 The region faces unique challenges, including higher mortality-to-incidence ratios compared to Western nations, potentially reflecting limitations in early detection and treatment access.5
While a remarkable expansion of therapeutic options, including cytotoxic chemotherapy, targeted agents, and immunotherapies, has been observed in recent years, pronounced disparities in cost-effectiveness and accessibility persist, particularly in resource-limited settings.6 The WHO Essential Medicines List (EML) serves as a critical tool in this regard, providing evidence-based guidance for optimal medication selection.7 Despite the significant value of WHO EML, studies demonstrate substantial variability (up to 61%) in adherence to therapeutic guidelines across institutions, influenced by factors ranging from drug availability to clinician training.8,9 This was attributed, in part, to the limited availability and affordability of several therapeutic agents in resources-limited regions.10
The Saudi healthcare system has made significant strides in cancer care standardization through initiatives like the Saudi Food and Drug Authority's (SFDA) essential medicines program.11–14 However, regional treatment patterns remain understudied, particularly in southern provinces like Najran. Clarifying local prescribing behavior is vital for detecting gaps between national policy and day-to-day practice, guiding resource allocation, and ultimately improving patient outcomes. In addition, assessing how closely these practices align with the WHO EML also sheds light on the suitability of global standards across diverse settings.
This study examines anticancer drug utilization patterns at King Khaled Hospital, a major oncology referral center in southern Saudi Arabia, from 2014–2022. We focus specifically on: (1) adherence to WHO EML and SFDA guidelines, (2) temporal trends in regimen selection, and (3) supportive care practices. Our findings aim to inform institutional quality improvement initiatives while contributing to broader discussions about equitable cancer care delivery in similar resource-constrained settings.
This retrospective cohort study was conducted at King Khaled Hospital, the main oncology referral center for Najran on region of southern Saudi Arabia. The study spanned an eight-year period (June 2014–April 2022) to capture evolving treatment patterns following updates to national cancer guidelines. Chemotherapy protocols followed National Comprehensive Cancer Network (NCCN) and the European Society of Medical Oncology (ESMO) guidelines, which are updated biennially to reflect contemporary standards.15 Ethical approval was obtained from the Institutional Review Board (Ref: 2022-11 E), with waiver of informed consent granted for this anonymized retrospective analysis.
The study cohort comprised adult patients (>18 years) with histologically confirmed malignancies who received at least one cycle of systemic anticancer therapy at the center. Exclusion criteria were systematically applied to maintain data quality, including: (1) absence of pathological confirmation, (2) incomplete treatment records, (3) referral to other institutions before regimen completion, and (4) loss to clinical follow-up within three months of therapy initiation. These measures ensured analysis of a well-documented patient cohort with verifiable treatment trajectories.
Trained oncology pharmacists abstracted data from electronic medical records using a standardized collection form. Demographic variables included age, gender, and residential district. Clinical parameters encompassed cancer type/stage (classified per AJCC 8th edition), treatment intent (curative or palliative), and metastatic status. Pharmaceutical analysis focused on:
• Chemotherapy regimens (agents, doses, cycles)
• Supportive medications (antiemetics, growth factors)
• Prescription characteristics (generic/branded, EML status)14
• Documented adverse events and treatment delays
To ensure data reliability, 10% of records underwent independent verification by two medical oncologists. Discrepancies (<2% of variables) were resolved through consensus review. Medication classification followed the WHO EML (2021 edition)14,16 and Saudi National Formulary guidelines, with unclear cases adjudicated by the institutional Pharmacy and Therapeutics committee.
• Essential Medicines List Adherence: The proportion of prescribed antineoplastic agents listed in the WHO EML,17 calculated as:
• Generic Utilization Rate: The percentage of medications ordered by international nonproprietary names (INN) rather than proprietary brands, assessed through pharmacy dispensing records.18,19
• Temporal Evolution of Regimens: Longitudinal analysis of protocol selection stratified into three epochs (2014–2016, 2017–2019, 2020–2022) using Mantel-Haenszel trend tests.
• Supportive Care Metrics: Antiemetic prescribing frequency by drug class (5-HT3 antagonists, NK1 inhibitors, corticosteroids) and prophylactic growth factor utilization in myelosuppressive regimens.
• System Challenges: Drug shortage incidence, defined as ≥72-hour unavailability of prescribed agents, and treatment delays (>7 days from scheduled date) attributable to logistical constraints.
To ensure conceptual precision, the following operational definitions were applied:
• Treatment Episodes: A chemotherapy cycle was considered completed if ≥80% of planned doses were administered per RECIST v1.1 criteria.
• Follow-up Continuity: Patients were classified as having adequate follow-up if they maintained ≥3 documented clinical encounters within 12 months post-treatment and ≤90-day gaps between oncology assessments.
• Essential Medicines Compliance: Full adherence required meeting all three criteria: drug listed in WHO EML 2021, prescribed dosage within SFDA-approved ranges, and administration schedule conforming to NCCN guidelines.
• Adverse Events: Toxicity was graded per CTCAE v5.0, with attribution determined by treating oncologists through Naranjo algorithm assessment.
Prescribing Protocols and Guidelines
Anticancer drug prescriptions were guided primarily by the NCCN clinical practice guidelines, adapted regionally in accordance with SFDA formularies. To account for temporal changes in prescribing practices, the study period was divided into three intervals reflecting major protocol updates:
• 2014–2016: Prescriptions adhered to NCCN guidelines version 2.2014, with regional adaptations emphasizing anthracycline-based regimens for breast cancer and fluoropyrimidine-based regimens for gastrointestinal malignancies.
• 2017–2019: Following the 2017 NCCN update and SFDA formulary revisions, targeted therapies such as trastuzumab and bevacizumab were increasingly incorporated. Anthracycline protocols were refined to optimize dosing schedules.
• 2020–2022: The most recent period reflected adoption of immunotherapies (e.g., nivolumab, pembrolizumab) following their regulatory approval in Saudi Arabia, alongside continued adherence to updated NCCN and SFDA guidelines. Annual formulary reviews ensured alignment with the WHO Essential Medicines List (EML).
Data analysis was performed using IBM SPSS Statistics Sciences (IBM SPSS Statistics for Windows, version 21.0, Armonk, NY: IBM Corp). Continuous variables were reported as mean ± standard deviation or median (IQR) based on distribution normality assessed by Kolmogorov-Smirnov testing. Categorical variables were expressed as frequencies and percentages. Temporal trends were analyzed using Mantel-Haenszel chi-square tests for ordinal data. The test statistic (χ2MH) evaluated whether prescribing patterns changed monotonically over time. All tests were two-tailed, with p<0.05 considered statistically significant.
The study cohort comprised 512 patients with a mean age of 55.2 ± 17.0 years, demonstrating a male predominance (56.4%, n=289). Gastrointestinal malignancies accounted for 29.7% (n=152) of cases, followed by breast cancer (25.8%, n=132). Metastatic disease was present in 46.7% (n=239) of patients at diagnosis, with two cases exhibiting unspecified primary sites. Comorbidities were prevalent, including diabetes (23.8%) and hypertension (20.1%), while 16.7% of patients required referral to tertiary centers for specialized therapies ( Table 1).
| Characteristic | n (%) or mean ± SD |
|---|---|
| Age, years | 55.2 ± 17.0 |
| Gender | |
| Male | 289 (56.4) |
| Female | 223 (43.6) |
| Cancer Site | |
| Gastrointestinal system | 152 (29.7) |
| Colorectal cancer | 78 (51.3)* |
| Gastric cancer | 32 (21.1)* |
| Pancreatic cancer | 20 (13.2)* |
| Hepatocellular cancer | 7 (4.6)* |
| Other GI cancers | 15 (9.9)* |
| Breast cancer | 132 (25.8) |
| Hematological cancer | 87 (17.0) |
| Lymphoma | 67 (77.0)* |
| Leukemia | 10 (11.5)* |
| Myeloma | 10 (11.5)* |
| Head, neck, and respiratory tract cancers | 53 (10.4) |
| Nasopharyngeal cancer | 15 (28.3)* |
| Lung cancer | 23 (43.4)* |
| Central nervous system cancer | 3 (5.7)* |
| Other cancers | 12 (22.6)* |
| Female reproductive cancers | 39 (7.6) |
| Ovarian cancer | 24 (61.5)* |
| Endometrial cancer | 13 (33.3)* |
| Other female reproductive cancers | 2 (5.1)* |
| Male urologic cancers | 28 (5.5) |
| Prostate cancer | 20 (71.4)* |
| Testicular cancer | 7 (25.0)* |
| Other male urologic cancers | 1 (3.6)* |
| Bladder cancer | 19 (3.7) |
| Distant metastasis | 239 (46.7) |
Analysis of 1,021 prescribed anticancer agents revealed that doxorubicin (14.0%), cyclophosphamide (13.3%), and docetaxel (9.9%) were the most frequently used cytotoxic agents. The Adriamycin–cyclophosphamide (AC) protocol predominated in breast-cancer management (20.1%), whereas FOLFOX regimens were preferred for gastrointestinal malignancies (13.5%) ( Table 2; Figure 1A). Overall, 86.7% of prescribed medicines complied with the WHO EML, exceeding the regional benchmark of 85.3%. Generic prescribing accounted for 90.1% of all orders, and patients received a mean of 1.99 anticancer agents per treatment course (Supplementary Figure 1). The AC regimen was the most common first-line therapy (72.7% of cases), and 54.5% of patients subsequently transitioned to taxane-based regimens ( Figure 1B).
Longitudinal analysis revealed significant shifts in prescribing patterns across the study period (2014–2022) ( Figure IC). Mantel-Haenszel tests demonstrated a marked increase in antimetabolite utilization (χ2=9.27, p=0.002), rising from 27.9% in 2014–2016 to 41.6% in 2020–2022, coinciding with regional adoption of oral capecitabine-based regimens. Targeted therapies similarly showed progressive uptake (12.4% to 22.1%, χ2=6.53, p=0.011), reflecting growing availability of trastuzumab and bevacizumab. Immunotherapies exhibited the most dramatic relative growth (3.8% to 8.9%, χ2=5.91, p=0.015), though from a smaller baseline. In contrast, alkylating agent use remained stable (32.1–33.2%, χ2=0.84, p=0.359) despite protocol updates, suggesting persistent reliance on cyclophosphamide and platinum analogs for core regimens ( Table 3).
Metoclopramide-based antiemetics were administered to 76.1% of patients, with dexamethasone co-prescribed in 90.1% of cases. Documented adverse events occurred in 18.9% of treatment courses, primarily comprising neutropenic fever (2.5%) and hypersensitivity reactions (1.3%) ( Table 4, Figure 1D). Medication shortages affected 8.4% of patients, most frequently involving BCG (0.8%) and gemcitabine (0.6%), resulting in a median treatment delay of 7 days (IQR: 5–11) when shortages occurred.
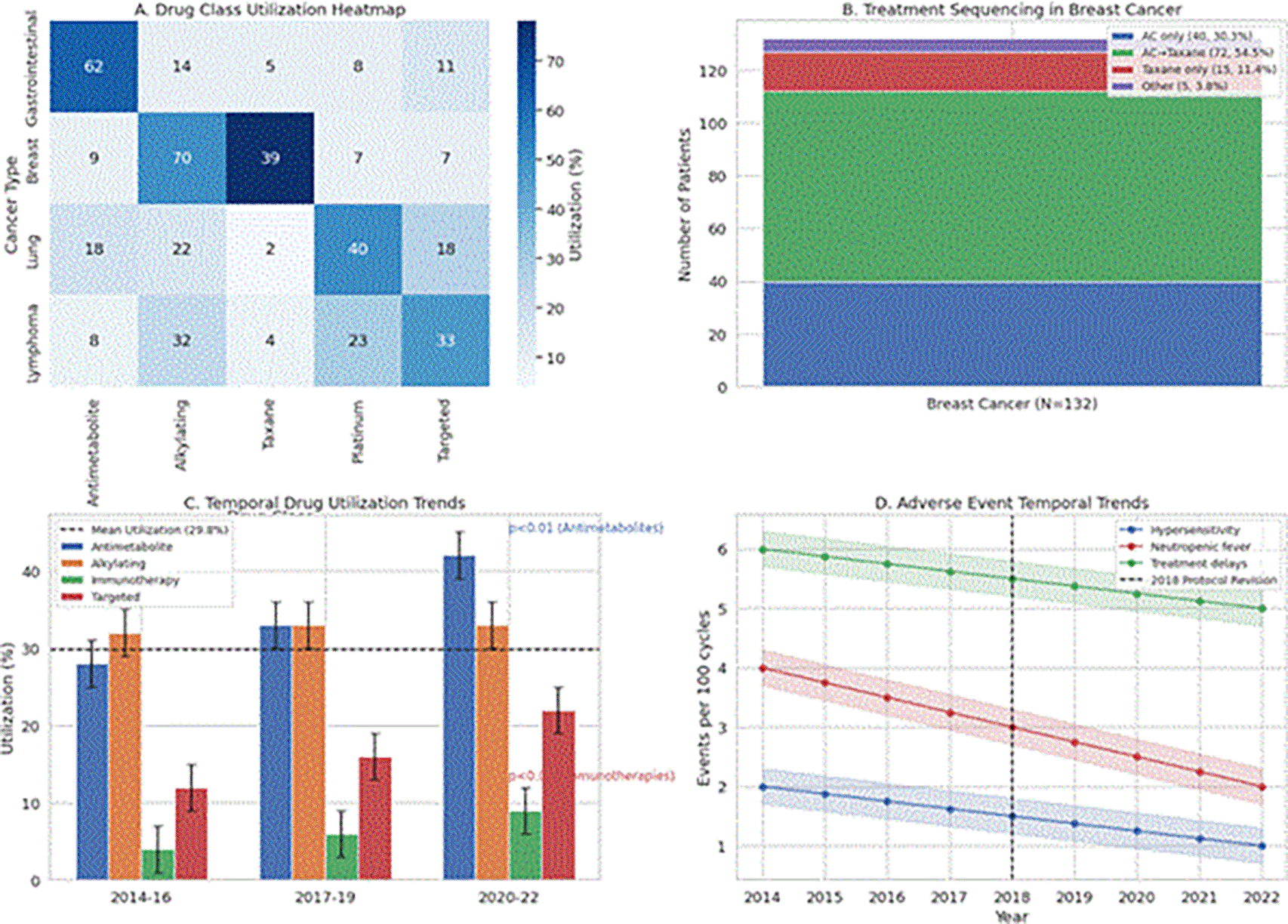
A. Drug Class Utilization Heatmap. Heatmap displaying the prescription frequency of major anticancer drug classes across four principal cancer types (gastrointestinal, breast, lung, and lymphoma) from 2014 to 2022. Color intensity reflects the percentage of patients within each cancer type receiving a given drug class, normalized to column totals. Absolute patient counts are annotated within each cell. Antimetabolite use predominated in gastrointestinal cancers, while alkylating agents were most frequent in breast cancer. B. Treatment Sequencing in Breast Cancer. Stacked bar plot illustrating first-line to second-line chemotherapy transitions among breast cancer patients (N=132). The AC regimen (doxorubicin/cyclophosphamide) was initiated in 72.7% of cases, with 54.5% progressing to taxane-based therapies (docetaxel or paclitaxel). Categories accounting for fewer than 5% of transitions are consolidated as ‘Other’. Bar segments are labeled with absolute patient numbers and percentages. C. Temporal Trends in Drug Class Utilization. Grouped bar chart showing annual utilization rates of major drug classes across three epochs (2014–2016, 2017–2019, 2020–2022). Error bars represent 95% confidence intervals calculated by the binomial exact method. Statistically significant trends are annotated (Mantel-Haenszel χ2 test: p<0.01 for antimetabolites, p<0.05 for immunotherapies). The dashed horizontal line indicates mean utilization across all periods (29.8%). D. Adverse Event Temporal Trends. Faceted line plots depicting rates of key treatment-related adverse events per 100 chemotherapy cycles, including (i) hypersensitivity reactions, (ii) neutropenic fever, and (iii) treatment delays. Shaded areas represent 95% confidence intervals. The dashed vertical line marks the 2018 protocol revision, which was associated with a reduction in neutropenia rates (Pearson’s r=–0.82, p=0.007). Abbreviations: AC, Adriamycin-cyclophosphamide; FOLFOX, folinic acid, fluorouracil, and oxaliplatin.
The cohort received 1,021 anticancer drug administrations among 512 patients, yielding a mean of 1.99 ± 0.41 agents per treatment course. Single-agent therapy was employed in 314 cases (61.3%), while 198 patients (38.7%) received combination regimens ( Table 5).
This retrospective analysis of 512 cancer patients showed prominence of gastrointestinal and breast cancers, compromising 29.7% and 25.8% of cases. In addition, nearly half of cases (46.7%) presenting with metastatic disease at diagnosis. The observed therapeutic patterns-particularly the high utilization of doxorubicin (14.0%) and cyclophosphamide (13.3%), reflect a complex interplay of clinical and socioeconomic factors, including alignment with the regional cancer burden, formulary optimization for cost-effectiveness (86.7% WHO EML compliance), and limited accessibility to certain novel agents. These findings consistent with previous reports from Saudi Arabia’s Southern Region and highlighting the significant influence of resource availability on prescription behaviors, particularly in shaping regional formulary preferences based on cost-effectiveness and drug accessibility.12,15,20–23
The study showed high adherence to the WHO EML, with 86.7% of prescribed agents being EML-listed, and 92.4% compliant with the SFDA formulary. The generic prescribing rate reached 90.1%, substantially higher than previously reported rates in Saudi Arabia and other Middle Eastern countries,24,25 considerable geographic variation. A comparative review of national EMLs and the WHO EML revealed marked disparities, with the European region demonstrating the highest dissimilarity to WHO EML compared to South-East Asia region.26 The authors partially attributed this variation to the perception of the WHO Essential Medicines List as a resource primarily intended for high- and middle-income countries. However, their analysis revealed a lower adherence to the WHO EML among less affluent nations. Additional explanations included discrepancies in timing, with some national EMLs being updated prior to the release of the corresponding WHO EML editions.
Prescription pattern in Saudi Arabia is similarly affected by multiple socioeconomic and supply chain factors, including cost, availability, and institutional preferences.27 A prior study examining medication accessibility highlighted that public awareness and preferences for branded medications hinder the broader adoption of generic alternatives in the market.28 Supply chain limitations further exacerbate prescribing challenges, with periodic shortages of essential agents, such as BCG, aprepitant, and gemcitabine, reported in multiple Saudi healthcare centers, often resulting in treatment delays or regimen modifications.28,29 These shortages stem from global manufacturing issues, importation delays, and local distribution challenges Addressing these barriers through the integration and localization of pharmaceutical manufacturing could enhance accessibility and affordability, supporting a more consistent alignment with WHO EML.30–32 Moreover, regulatory policies and formulary updates guided by the Saudi FDA and alignment with the WHO Essential Medicines List ensure rational and standardized prescribing but also limit access to some novel agents, affecting utilization trends.33 For example, targeted therapies and immunotherapies have seen gradual uptake following regulatory approvals and guideline incorporation during the study period.34
Temporal analysis revealed a significant increase in the use of targeted therapies over the study period, rising from 27.9% to 41.6%, aligns with the global transition towards oral chemotherapeutic agents.35,36 In this study, immunotherapies exhibited the most dramatic relative growth, rising from 3.8% to 8.9%, compared to stable use of alkylating agents, maintaining around 32%. This trend was observed similarly in the United States, with alkylating agents reserved its role in the management of some hematological malignancies,37 compared to Europe that experienced a noticeable reduction in alkylating agents utilization with rise of immunotherapies.38
Supportive care practices in our cohort were characterized by the predominant use of metoclopramide-based antiemetics (76.1%), with dexamethasone co-prescribed in 90.1% of cases. This pattern is consistent with international guidelines for moderate-emetogenic chemotherapy and is likely influenced by local formulary constraints and clinician familiarity.39,40
Medications shortage affected around 8.4% of patients, most frequently involving BCG (0.8%) and gemcitabine (0.6%), leading to a median treatment delay of 7 days (interquartile range: 5–11 days). A prior analysis from the United States showed that 63% of cancer institutions experienced at least one medication shortage in monthly basis.41 Such shortages can result in treatment delays, regimen modifications, and potentially suboptimal clinical outcomes-a concern echoed in global oncology literature.42,43
Our findings have several implications for practice and policy. The high rates of EML and generic prescribing provide a model for other institutions seeking to optimize resource utilization without sacrificing quality. Addressing supply chain weaknesses should be a priority for health authorities to ensure uninterrupted access to essential medicines. Establishing a comprehensive regional oncology registry would facilitate ongoing surveillance of prescribing patterns, supply chain issues, and patient outcomes. Such a registry could enable benchmarking across centers, support quality improvement initiatives, and provide a robust platform for future prospective studies linking drug utilization to clinical endpoints.
While this study provides comprehensive data on anticancer drug utilization, several limitations warrant consideration. First, the single-center design, based solely on data from King Khaled Hospital, may limit the generalizability of findings across the diverse healthcare settings in Saudi Arabia. Multicenter studies are needed to validate these results and enhance their applicability nationwide. Second, the absence of survival and long-term outcome data-attributable to inconsistent follow-up documentation-precludes assessment of the clinical impact of prescribing patterns on patient survival. Addressing this gap requires prospective studies with systematic outcome tracking. Third, although a significant increase in antimetabolite use was observed, unmeasured confounding factors, such as changes in reimbursement policies or drug availability, may have independently influenced prescribing behaviors apart from guideline updates. Finally, the reported adverse event rate of 18.9% likely underestimates the true toxicity burden, given the retrospective reliance on clinician documentation rather than prospective, systematic adverse event monitoring. These limitations underscore the need for further research employing multicenter, prospective designs with comprehensive clinical and outcome data to validate and expand upon the current findings.
This study demonstrates optimal alignment with the WHO EML and national formulary guidelines in cancer therapy. Prescribing practices were evidence-based and cost-effective, with generic utilization and regimen selection exceeding regional and international benchmarks. These findings reinforce the applicability of the WHO model in guiding rational oncology prescribing at the regional level. However, observed supply chain vulnerabilities highlight ongoing challenges that require targeted policy and operational interventions. Establishing robust, regionally adapted strategies to ensure continuity of essential cancer treatments remains a priority for optimizing patients outcomes.
On March 13, 2022, the Ethics Research Committees of King Khalid Hospital approved this study (ID: 2022-11 E), which was carried out in compliance with the Helsinki Declaration. Due to the anonymous retrospective nature of the study, written informed consent from the included patients was not required.
Figshare: Anticancer Drug Utilization and Prescription Practices at a Saudi Cancer Center: A Retrospective Study (2014–2022). figshare. Dataset. https://doi.org/10.6084/m9.figshare.25387114.v4.44
Supplementary Figure 1. Utilization patterns of anticancer agents (N = 1,021 prescriptions) has been deposited in the approved repository Figshare and is available as extended data associated with this article with DOI: https://doi.org/10.6084/m9.figshare.25387114.v4.44
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: AI in Healthcare
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Given my familiarity with similar studies conducted in this field, I am well acquainted with the data collection methods and analyses employed in this study. Additionally, my ongoing research in cancer treatments provides valuable insights, enabling me to effectively contribute to reviewing the oncological aspects of this study.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: GI cancer, GU cancer, clinical trials
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Given my familiarity with similar studies conducted in this field, I am well acquainted with the data collection methods and analyses employed in this study. Additionally, my ongoing research in cancer treatments provides valuable insights, enabling me to effectively contribute to reviewing the oncological aspects of this study.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 24 Jul 25 |
read | read | |
|
Version 1 31 May 24 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)