Keywords
Apoptosis, BDNF, Neurodegeneration, SH-SY5Y
This article is included in the Cell & Molecular Biology gateway.
Apoptosis, BDNF, Neurodegeneration, SH-SY5Y
Neurological diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), are the leading cause of disability and the second leading cause of death worldwide. Over the past 30 years, the number of deaths and disabilities due to neurodegenerative diseases has continued to increase, particularly in poor and developing countries, and is expected to increase globally owing to population growth and aging.1 As hereditary and sporadic conditions, neurodegenerative diseases attack the brain and spinal cord cells, characterized by progressive degeneration, dysfunction, and death of neurons in the central nervous system.2 Neurodegeneration can be caused by several factors, including mitochondrial dysfunction, which increases oxidative stress, neuronal cell death (particularly in the limbic system and hippocampus), impairments of axonal transport caused by swelling of axons resulting in organelle accumulation, impairments of the ubiquitin–proteasome system, abnormal inflammatory cytokines, and excitotoxicity.3
It is known that therapy using human umbilical vein endothelial cells (HUVECs) has been used to cure various diseases, including neurodegenerative diseases. However, therapy with HUVECs still has several shortcomings, including its unknown long-term safety, varied treatment responses, ineffective delivery methods to donors, and lack of availability.4 Several research have proved that mesenchymal stem cells (MSCs) from bovine umbilical cord have similar content and express identical markers to the human umbilical cord stem cells.5 Conditioned medium (CM) is a culture medium from certain primary cells that contain secretory factors called secretomes.6 Thus, secretome products from bovine umbilical vein endothelial cells (BUVECs) can be used as an alternative.
Studies have been conducted on the use of CM for the treatment of various diseases, including fatty liver disease,7 vascular disorders,8 osteoarticular,9 neurology,10 organ failure,11 and diabetes.12 The CM of MSCs contains various antiapoptotic and proangiogenic growth factors, including exosomes, vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF)-1, angiogenin, interleukin (IL)-8, IL-6, stromal cell-derived factor (SDF)-1, and hepatocyte growth factor (HGF); these can trigger proliferation, and increase migration, differentiation, angiogenesis, and cell viability under oxidative stress.13
On the basis of these contents and properties, this study aimed to describe the ability of CM from BUVEC (BUVEC-CM) as an anti-inflammatory and antiapoptotic therapy that can prevent apoptosis of the neuron as well as stimulate neuroproliferation, which has been investigated using an in vitro model of trimethyltin (TMT)-induced neurodegeneration on the human neuroblastoma (SH-SY5Y cells).
Sterile cows’ umbilical cords were collected from local farms in Sleman Regency, Yogyakarta. The collection process was approved by the cows’ owners with informed consent. The umbilical cords were aseptically harvested from the cows that had just given birth, washed with DPBS (Capricorn, Ebsdorfergrund, Germany), stored in sample containers with ice to prevent sample damage, and subsequently taken to the laboratory for BUVEC isolation. Isolation and cultivation of BUVECs were performed following the methods described in our previous study.10 The sterile umbilical cord was washed with DPBS mixed with P/S. A collagenase solution was prepared by mixing collagenase powder (Gibco, Langenselbold, Germany) in HBSS (Capricorn, Ebsdorfergrund, Germany). The collagenase solution was poured into the umbilical vein lumen and subsequently incubated for 30 min. To obtain the cells, the umbilical vein lumen was flushed with DPBS; subsequently, the fluid obtained was centrifuged at 2,500 rpm for 5 min at 24°C. The pellets were then used for further culture in a T-75 flask (Greiner Bio-One, Frickenhausen, Germany) in 5 mL of complete medium containing DMEM (Gibco, Langenselbold, Germany), 1% P/S (Gibco, Langenselbold, Germany), and 0.5% amphotericin (Gibco, Langenselbold, Germany). BUVECs were incubated in a 5% CO2 incubator at 37°C. Cells that reached 80% confluence were then subcultured.
BUVEC-CM was harvested after two-time passaging and at the position of cell confluence at 80%. The BUVEC-CM volume for each harvest was approximately 15 mL. The BUVEC-CM was subsequently filtered in a sterile filter and stored in the refrigerator until used. In this study, the BUVEC-CM used was from the third and fourth passages.
In this study, an in vitro neurodegeneration model was constructed; the SHSY-5Y cells were induced with TMT and subsequently treated using BUVEC-CM. The cells were separated into seven groups: non-treated, positive control, commercial medicine as a comparison (TMT + donepezil), treatment A (TMT 10 μM + 25% BUVEC-CM [25% pure BUVEC-CM in 75% complete medium]), treatment B (TMT 10 μM + 50% BUVEC-CM [50% pure BUVEC-CM in 50% complete medium]), treatment C (TMT 10 μM + 75% BUVEC-CM [75% pure BUVEC-CM in 25% complete medium]), and treatment D (TMT 10 μM + 100% BUVEC-CM [pure BUVEC-CM without the addition of complete medium]). The cells were pre-treated with BUVEC-CM and donepezil for 1 h before being treated with TMT. The presentation of BUVEC-CM and TMT concentration is according to the protocols of our previous study.10
SH-SY5Y cells were purchased from the European Collection of Authenticated Cell Cultures and cultured with a complete medium containing DMEM (Gibco, Langenselbold, Germany), Ham’s F-12 nutrient mixture (Gibco, Langenselbold, Germany), 10% FBS (Gibco, Langenselbold, Germany), 1% Penicillin/Streptomycin (P/S) (Gibco, Langenselbold, Germany), and 0.5% amphotericin (Gibco, Langenselbold, Germany). SH-SY5Y cells were stored at −80°C and subsequently transferred to conical tubes for centrifugation at 3,000 rpm for 5 min. The collected pellet was transferred to a new flask and administered 5 mL of complete medium. The cells were incubated in a 5% CO2 incubator at 37°C until confluent and divided into the abovementioned seven groups.
SH-SY5Y cells that were 80% confluent in the T-25 flask were counted and subsequently subcultured onto a 96-well plate. The number of cells grown on the 96-well plate was determined after three-time optimization following the protocol described by Tarozzi et al.14 The optimization resulted in 3 × 104 cells monolayered with a density of 80% on a 96-well plate. Subsequently, 3 × 104 SH-SY5Y cells in 100-μL complete medium were seeded on a 96-well cell culture plate (Greiner Bio-One, Frickenhausen, Germany). The cells were incubated for 24 h and separated into the abovementioned seven groups; each treatment was replicated three times. Following the next 24-h incubation, 100 μL of 5-mg/mL MTT (Sigma, Munich, Germany) reagent was added to the cell and incubated for 2 h. Finally, 100-μL DMSO/well (Santa Cruz, TX, USA) was added. Results were read using an ELISA reader at 595 nm. The absorbance numbers obtained were then used to calculate the percentage of viability.
In the subsequent experiment, 3 × 104 SH-SY5Y cells in 100-μL complete medium were seeded on a 96-well cell culture plate (Greiner Bio-One, Frickenhausen, Germany). The cells were incubated for 24 h and separated into the abovementioned seven groups; each treatment was replicated three times. Following the next 24-h incubation, 100 μL of WST-8 reagent (Abbkine, (Wuhan, China) was added to the cell and incubated for 2 h; subsequently. Finaly, 100-μL DMSO/well (Santa Cruz, TX, USA) was added. The optical density was measured with an ELISA reader at 460 nm. The absorbance numbers obtained were then used to calculate the percentage of viability.
In this step, 5 × 104 SH-SY5Y cells in 500-μL complete medium were seeded on a 24-well cell culture plate (Greiner Bio-One, Frickenhausen, Germany). The cells were incubated for 24 h. After incubation, a 200-μL pipette tip (Vertex, PA, USA) was used to make scratches on the cell. The cells were then divided into the abovementioned seven groups. The cells were incubated for another 24 h and observed afterwards. The images were captured at 0 and 24 h. The scratch areas were calculated using ImageJ software (National Institute of Health, USA), and the percentage of the narrowing area was calculated using the following formula:
Next, 5 × 105 SH-SY5Y cells in 2,000-μL complete medium were seeded on a six-well cell culture plate (Greiner Bio-One, Frickenhausen, Germany). The cells were incubated for 24 h and separated into the abovementioned seven groups. Subsequently, the cells were incubated for another 24 h. The medium was changed using RIPA lysis buffer (Santa Cruz, TX, USA) to make cell lysates, which were then used as samples for ELISA. The ELISA test was performed using a sandwich human BDNF ELISA kit (ABclonal, Woburn, USA) according to the manufacturer’s protocol. The plate was read using an ELISA reader at 450 nm.
In this step, 15 × 103 SH-SY5Y cells in a 300-μL complete medium were seeded on an eight-well cell culture slide (Biologix, KS, USA). The cells were incubated for 24 h and separated into the abovementioned seven groups. After 24 h, the cells were washed with DPBS (Capricorn, Ebsdorfergrund, Germany), fixed with ice-cold ethanol for 15 min, and subsequently washed with DPBS three times. Then, in a dark room, 120 μL of 1-mg/mL Hoechst 33342 solution (Thermo Fisher Scientific, MA, USA) was added to each well. The wells were covered with aluminum foil and incubated for 10 min. Finally, the cells were washed with DPBS and observed under a confocal microscope with 10 and 20× magnifications.
In a further experiment, 15 × 103 SH-SY5Y cells in 300-μL complete medium were seeded on eight-well cell culture slides (Biologix, KS, USA). The cells were incubated for 24 h and separated into the abovementioned seven groups. After 24 h, the cells were washed with DPBS (Capricorn, Ebsdorfergrund, Germany). AO/PI solution was prepared by mixing AO (Thermo Fisher Scientific, MA, USA) and PI (Thermo Fisher Scientific, MA, USA) solutions with a concentration of 50 g/mL each and a ratio of 1:1. Finally, 10-μL AO/PI solution was added to the cells, and the cells were observed under a confocal microscope with 10 and 20× magnifications.
Thereafter, 5 × 105 SH-SY5Y cells in 2,000 μL complete medium were seeded on six-well cell culture plates (Greiner Bio-One, Frickenhausen, Germany). The cells were incubated for 24 h and divided into seven groups as described above. Next, the cells were incubated for another 24 hours. The cells were then harvested and used for RNA extraction using the MiniPrep Plus Quick-RNA Kit (Zymo Research, CA, USA) as stated by the product’s protocol. After incubation, RNA lysis buffer was added to the cells and then centrifuged. The supernatant was added with absolute ethanol and then centrifuged. After that, the supernatant was discarded. For DNase I treatment, the column was washed with RNA wash buffer. Then, DNase I and digestion buffer were added to the column and centrifuged. Thereafter, the column was added with RNA preparation buffer, RNA wash buffer, and RNase-free water, and centrifuged. The concentration of eluted RNA was measured using a Nano-quant instrument and stored at −20 °C. The RNA isolation of the seven treatment groups had a ratio of 260:280 as follows: TMT: 2.12, TMT + donepezil: 2.11, TMT + 25% BUVEC-CM: 2.15, TMT + 50% BUVEC-CM: 2.14, TMT + 75% BUVEC-CM: 2.11, TMT + 100% BUVEC-CM: 2.14, untreated: 2.09. The RNA sample was then reverse-transcribed into cDNA to be tested by RT-qPCR.
Reverse transcriptase of the extracted RNA into cDNA was conducted using the SensiFAST cDNA Synthesis Kit (Bioline, TN, USA). RNA, TransAmp buffer, reverse transcriptase, and RNase-free water are mixed together. The thermal cycler program was set at 25°C for 10 min, 42°C for 15 min, 85°C for 5 min, and 4°C held. The cDNA concentration was then measured using a Nano-quant instrument and stored at -20 °C. For gene expression by quantitative RT-qPCR, cDNA was stored at a final concentration of 50 ng/μL.
The caspase-7, caspase-9, and GAPDH the primer was based on the publication references 15, 16 and we do additional experiment with the NCBI Blast to check the position of the forward and reverse primer. Meanwhile, for the human CD68 the primer was designed based on the NCBI accession number.
The volume mix per tube was as follows: 10-μL EvaGreen, 1-μL reverse primer, 1-μL forward primer, 7-μL nuclease-free water, and 1-μL cDNA. RT-PCR was performed using the SsoFast EvaGreen Kit (Bio-Rad, CA, USA) according to the manufacturer’s protocol. The primary sequences used are listed in Table 1 with GAPDH (Integrated DNA Technologies, IA, USA) as an internal reference. For thermal conditions, enzyme activation was performed at 95°C for 2 min; subsequently, 40 thermal cycles were started. Each cycle consisted of denaturation at 95°C for 10 s, annealing/extension at 58.5°C for 30 s, and melt curve at 65°C–95°C gradually, with 0.5°C increments every 5 s. Data were evaluated using the Bio-Rad CFX Manager software program and Microsoft Excel.
| Target gene | Primer sequence (5′–3′) |
|---|---|
| Caspase-7 | F: GCAGCGCCGAGACTTTTAG |
| R: GCTGCAGTTACCGTTCCCAC29 | |
| Caspase-9 | F: GAATGACGTGAAACACGACAG |
| R: TTAACGGCATCCCCCACTTAG29 | |
| CD68 | F: AAGGCCGTTACTCTCCTGCC |
| R: GTCCGGAGCTGGTGTGAACT | |
| GAPDH | F: GAGAAGGCTGGGGCTCATTT |
| R: AGTGATGGCATGGACTGTGG30 |
To analyze the relative expression of each gene, first, we calculated the ΔCt of the treatment group by subtracting the quantification cycle (Cq) of the target gene of the treatment group from the Cq of GAPDH. Then, we calculated the ΔCt of the non-treated group by subtracting the Cq of the target gene of the non-treated group from the Cq of GAPDH. Next, we calculated the ΔCt by subtracting the ΔCt of the treatment group from the ΔCt of the non-treated group. Subsequently, we calculated the normalized expression ratio using the following formula: 2(-ΔΔCt). If the normalized expression ratio was >1, then the fold change was increased as the value was that of the normalized expression ratio. If the normalized expression ratio was <1, then fold change was decreased as the value was calculated using the following formula: −1/(normalized expression ratio).17
In this experiment, 5 × 105 SH-SY5Y cells in 2,000-μL complete media were seeded on a six-well cell culture plate (Greiner Bio-One, Frickenhausen, Germany). The cells were incubated for 24 h and separated into the abovementioned seven groups. After 24 h, the cells were washed with serum free media, then given 1 mL of DCFH-DA (Abbkine, Wuhan, China) 10 μM. The cells were then incubated for 30 min in the dark. The cells were washed again with serum-free media, then given accutase to make cell suspension. Then, 1 mL of DPBS was added to the cell pellet. The cells were then analysed with flow cytometry with an excitation wavelength of 488 nm and an emission of 525 nm.
The results of our MTT assay (Figure 1) showed that the non-treated group had a 100% viability rate. Compared with the non-treated group, the positive control group experienced a decrease in viability percentage to 62.52% (p = 0.0004); the groups with BUVEC-CM at 25%, 50%, 75%, and 100% had viability percentages of 64.46% (p = 0.0006), 70.68% (p = 0.0020), 87.3% (p = 0.1453), and 95.835% (p = 0.9464), respectively; and the comparison group had a viability percentage of 99.88% (nonsignificant).
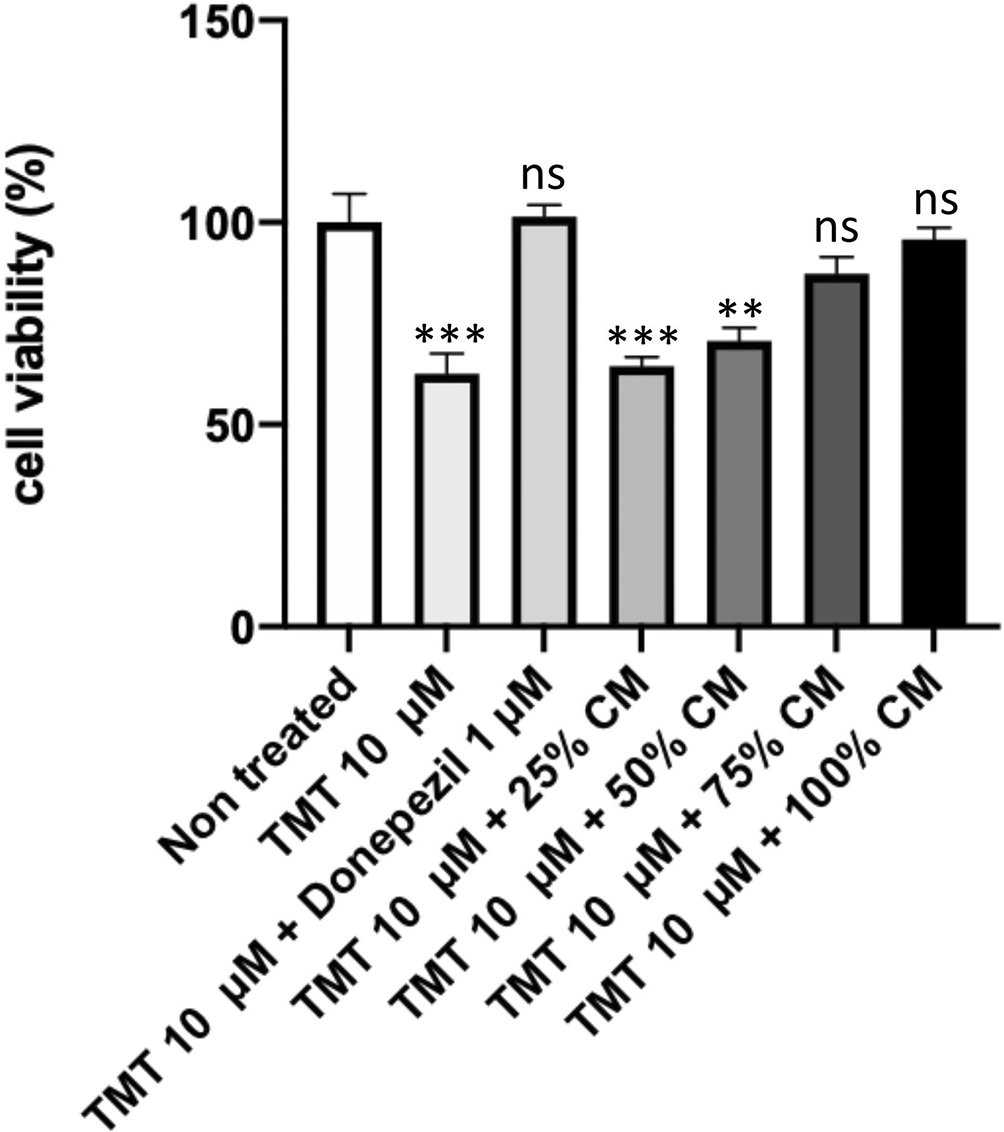
Before conducting the assay, the cells were cultured in several treatments: 10 μM TMT, 10 μM TMT + BUVEC-CM (25%, 50%, 75%, 100%), and 10 μM TMT + 1 μM Donepezil diluted in complete media. Untreated cells were used as negative control. Donepezil was used as a comparison drug for dementia. An increase of viability was observed in cells treated with graded concentration of BUVEC-CM. **p < 0.001, ns = nonsignificant, against non-treated groups.
The results of our CCK-8 assay (Figure 2) showed that the non-treatment group had a 100% viability rate. Compared with the non-treated group, the positive control group experienced a decrease in proliferation rate percentage to 26.455% (p < 0.0001); the groups with BUVEC-CM at 25%, 50%, 75%, and 100% had proliferation rate percentages of 32.81% (p < 0.0001), 37.715% (p < 0.0001), 49.695% (p < 0.0001), and 70.57% (p = 0.0013), respectively; and the comparison group had a proliferation rate percentage of 79.195% (p = 0.0092).

Before conducting the assay, the cells were cultured in several treatments: 10 μM TMT, 10 μM TMT + BUVEC-CM (25%, 50%, 75%, 100%), and 10 μM TMT + 1 μM Donepezil diluted in complete media. Untreated cells were used as negative control. Donepezil was used as a comparison drug for dementia. An increase of proliferation rate was observed in cells treated with graded concentration of BUVEC-CM. **p < 0.01, ****p < 0.0001 against non-treated groups.
The comparison results of the narrowing area percentage after 24 h (Figures 3, 4) showed that the non-treatment group had an increase of 43.91%. Compared with the non-treatment group, the positive control group had an increase of 27.97% (p = 0.0115); the groups with BUVEC-CM at 25%, 50%, 75%, and 100% had an increase of 43.31% (nonsignificant), 46.99% (nonsignificant), 47.57% (nonsignificant), and 68.51% (p = 0.0003), respectively; and the comparison group had an increase of 42.93% (nonsignificant).
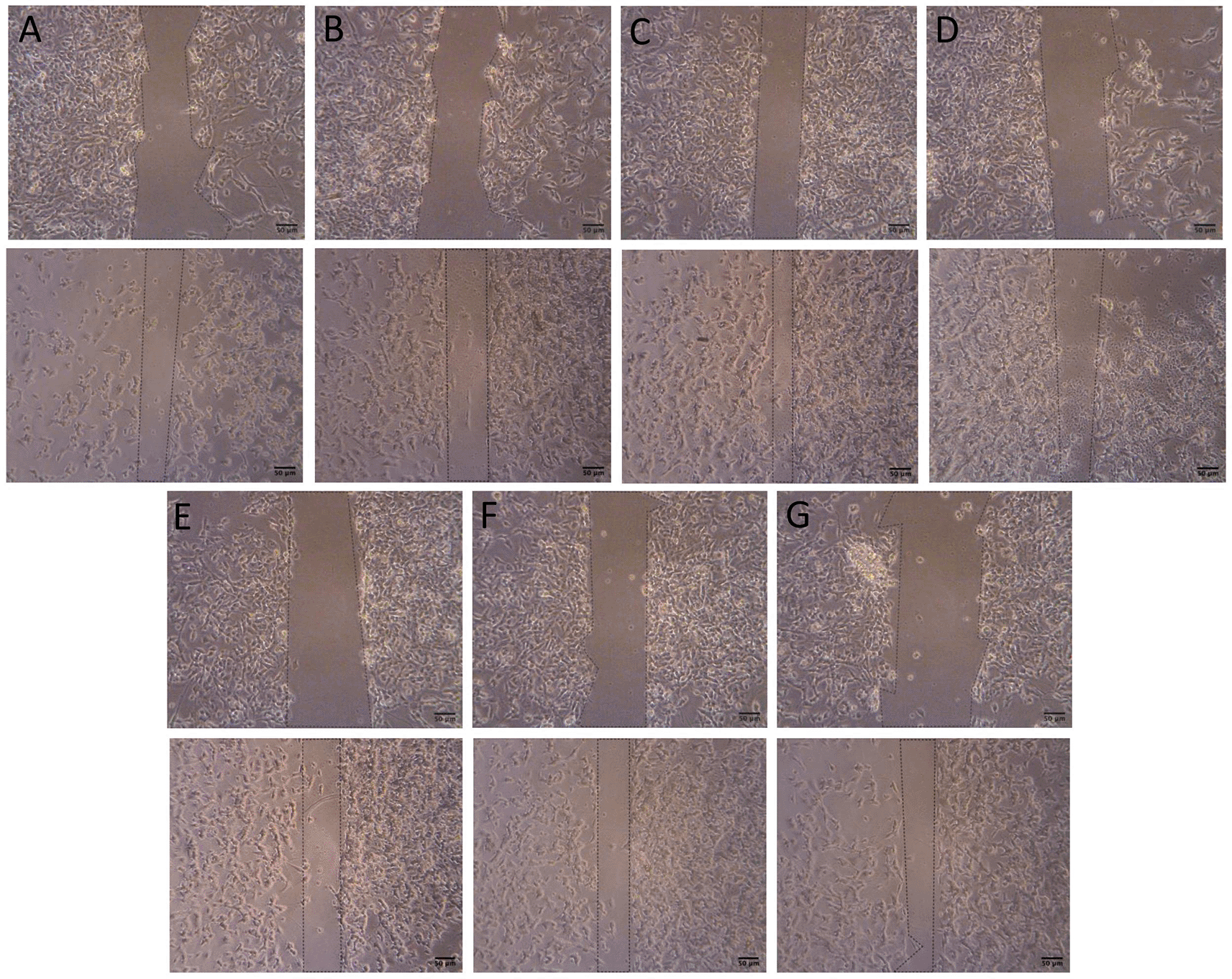
Before conducting the assay, the cells were cultured in several treatments diluted in complete media: (A) SH-SY5Y + TMT 10 μM (positive control group), (B) SH-SY5Y + TMT 10 μM + 25% BUVEC-CM), (C) SH-SY5Y + TMT 10 μM + 50% BUVEC-CM), (D) SH-SY5Y + TMT 10 μM + 75% BUVEC-CM, (E) SH-SY5Y + TMT 10 μM + 100% BUVEC-CM, (F) SH-SY5Y + TMT 10 μM + 1 μM Donepezil, (G) non-treated SH-SY5Y. Untreated cells were used as negative control. Donepezil was used as a comparison drug for dementia. The image on the top row was taken at 0 hour and the image on the bottom row was taken at the 24th hour. Narrowing areas were observed in cells treated with graded concentrations of BUVEC-CM. Scale bar: 50 μm.
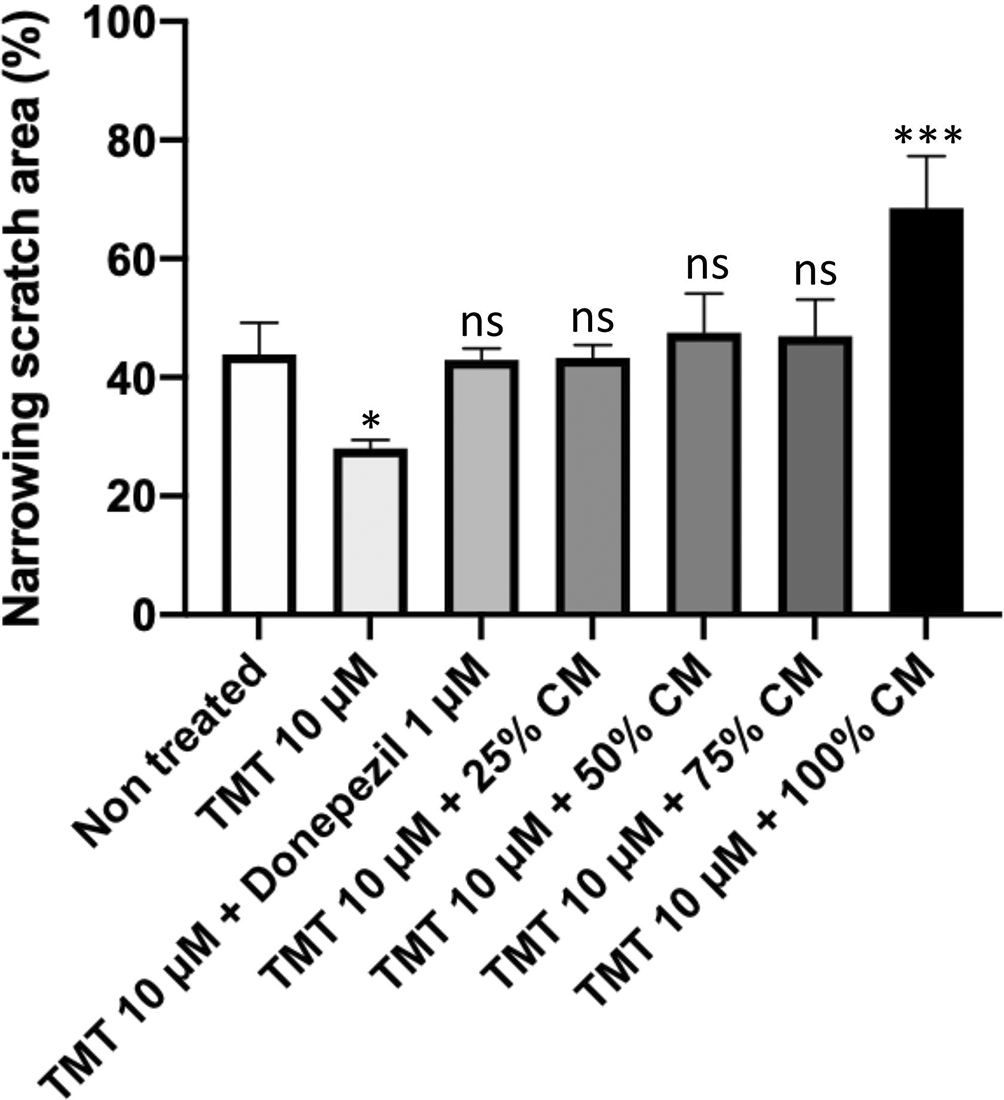
An increase of narrowing area was observed with cells treated with graded concentrations of BUVEC-CM. Data were presented as percentage of narrowing scratch area calculated with the formula mentioned in the method. A high percentage of narrowing area represents high migration of the cell. *p < 0.1, ***p < 0.001, ns = non-significant, against non-treated groups.
The results of the ELISA test (Figure 5) in the non-treated group showed a concentration value of 0.446 μg/mL. Compared with the non-treated group, the positive control had a decrease in concentration with a value of 0.208 μg/mL (nonsignificant). The groups with BUVEC-CM at 25%, 50%, 75%, and 100% had an increase in concentration with values of 0.569 μg/mL (nonsignificant), 0.622 μg/mL (nonsignificant), 0.66 μg/mL (nonsignificant), and 1.222 μg/mL (p = 0.0006), respectively. Meanwhile, the comparison group had a decrease in concentration with a value of 0.42 μg/mL (nonsignificant).
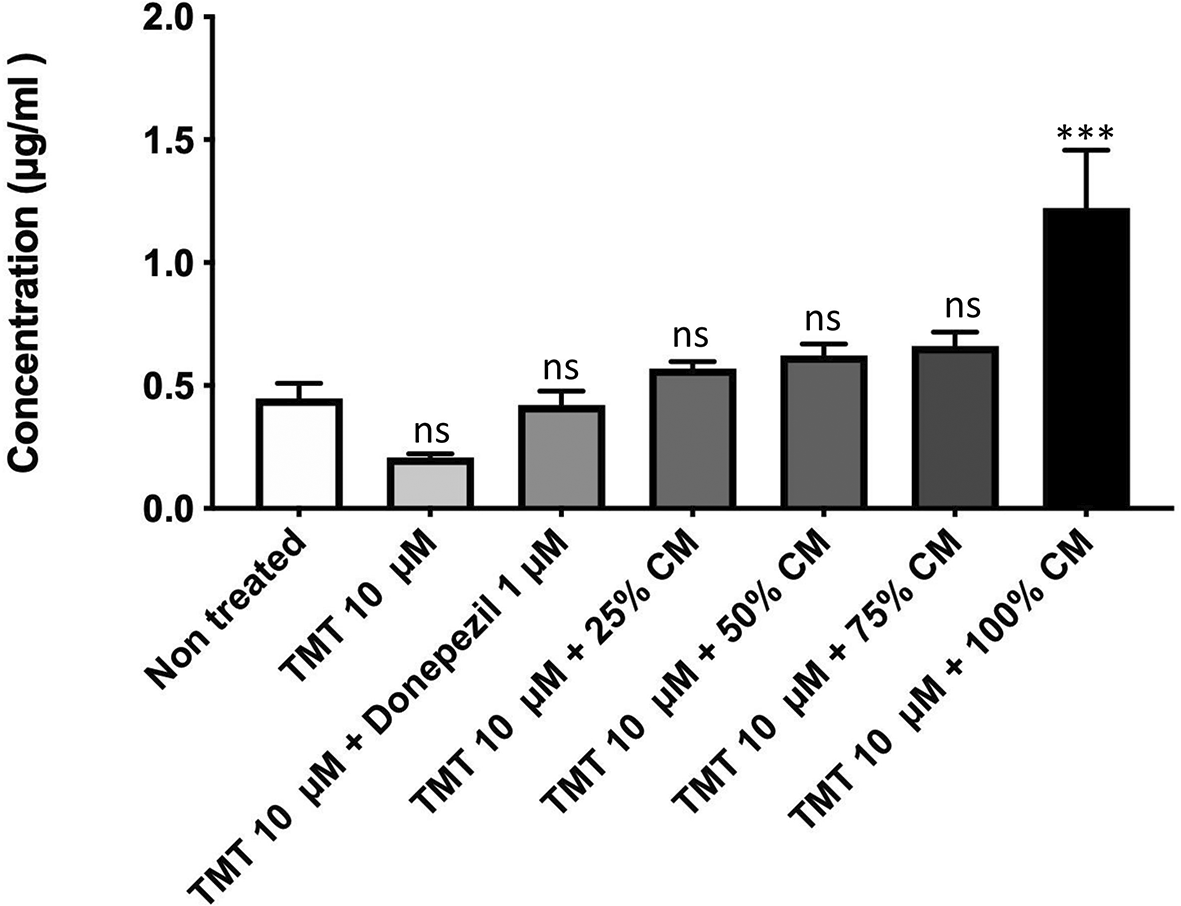
Before conducting the assay, the cells were cultured in several treatments: 10 μM TMT, 10 μM TMT + BUVEC-CM (25%, 50%, 75%, 100%), and 10 μM TMT + 1 μM Donepezil diluted in complete media. Untreated cells were used as negative control. Donepezil was used as a comparison drug for dementia. An increase of BDNF concentration was observed in cells treated with graded concentration of BUVEC-CM. ***p < 0.001, ns = non-significant, against non-treated groups.
The results for CD68 expression (Figure 6A) showed that the positive control group had a 3.7-fold increased CD68 expression compared with the non-treated group. The BUVEC-CM 25% and 50% groups had a 2.45- and 1.24-fold increased CD68 expression, respectively. Meanwhile, the BUVEC-CM 75%, 100%, and comparison groups had a 1.5-, 2-, and 1.49-fold decreased CD68 expression, respectively (the non-treated group vs. the positive control, BUVEC-CM 25%, 50%, 75%, 100%, and comparison groups: p = 0.0001, p = 0.0051, nonsignificant, p = 0.0002, p < 0.0001, and p = 0.0002, respectively).
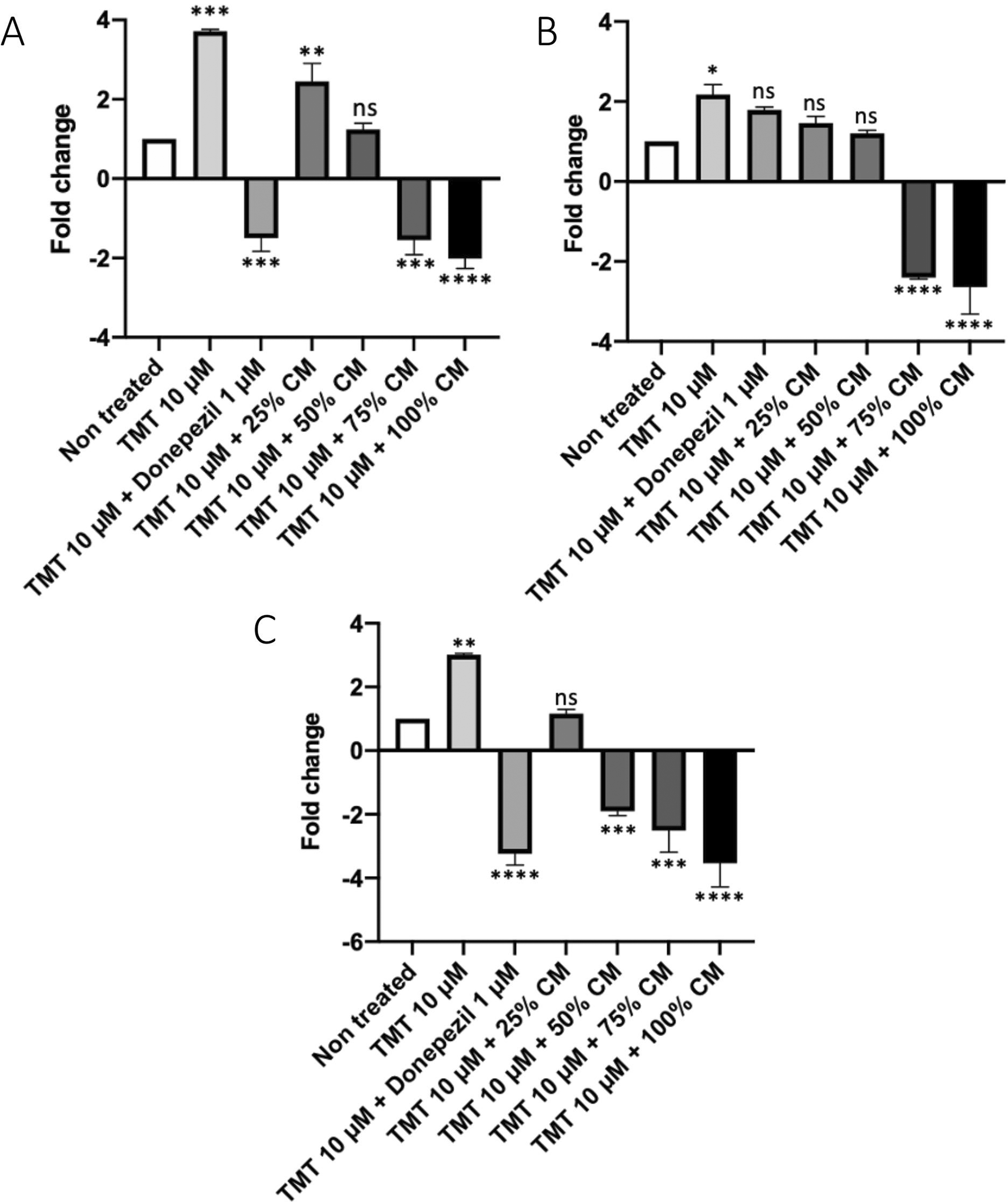
Before conducting the assay, the cells were cultured in several treatments: 10 μM TMT, 10 μM TMT + BUVEC-CM (25%, 50%, 75%, 100%), and 10 μM TMT + 1 μM Donepezil diluted in complete media. Untreated cells were used as negative control. Donepezil was used as a comparison drug for dementia. A decrease of CD68 expression was observed in cells treated with graded concentration of BUVEC-CM. Data were presented as normalized expression ratio proportional to CD68 expression. A high ratio represents high CD68 expression. *p < 0.1, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns = non-significant, against non-treated groups.
The results for caspase-9 expression (Figure 6B) showed that the positive control group had a 2.1-fold increased expression of caspase-9 compared with the non-treated group. The comparison group of treatment with the medication drug (donepezil) had a 1.8-fold increased caspase-9 expression. The groups with BUVEC-CM at 25% and 50% had a 1.5- and 1.2-fold increased caspase-9 expression, respectively. The groups with BUVEC-CM at 75% and 100% had a 2.4- and 2.5-fold decreased caspase-9 expression, respectively. Compared with the positive control group, the BUVEC-CM 25%, 50%, 75%, and 100% groups were significantly different (p = 0.0067, p = 0.0013, p < 0.0001, and p < 0.0001, respectively). Meanwhile, the comparison group was insignificantly different.
The results for caspase-7 expression (Figure 6C) showed that the positive control group had a three-fold increased caspase-7 expression compared with the non-treated group. The group with BUVEC-CM at 25% had a 1.1-fold increased caspase-7 expression. The groups with BUVEC-CM at 50%, 75%, and 100% had a 1.9-, 2.1-, and 2.3-fold decreased caspase-7 expression, respectively. The comparison group had a 1.8-fold decreased caspase-7 expression. Compared with the positive control group, the 25%, 50%, 75%, and 100% BUVEC-CM treatment groups were significantly different (p = 0.0003, p < 0.0001, p < 0.0001, and p < 0.0001, respectively). Furthermore, the comparison group was significantly different (p < 0.0001).
From the Hoechst 33342 staining results (Figure 7), it was observed that the cells in the non-treated group had a nucleus with a round shape and evenly stained bluish color. Meanwhile, the positive control group was dominated by cells with a fragmented nucleus (+++). In the groups with graded concentrations of BUVEC-CM, the higher the concentration provided, the fewer cells underwent apoptosis (BUVEC-CM 25% = ++, BUVEC-CM 50% = ++, and BUVEC-CM 75% = +). In the BUVEC-CM 100% and comparison groups, it was observed that the cell morphology was dominated by cells with a round nucleus (+).
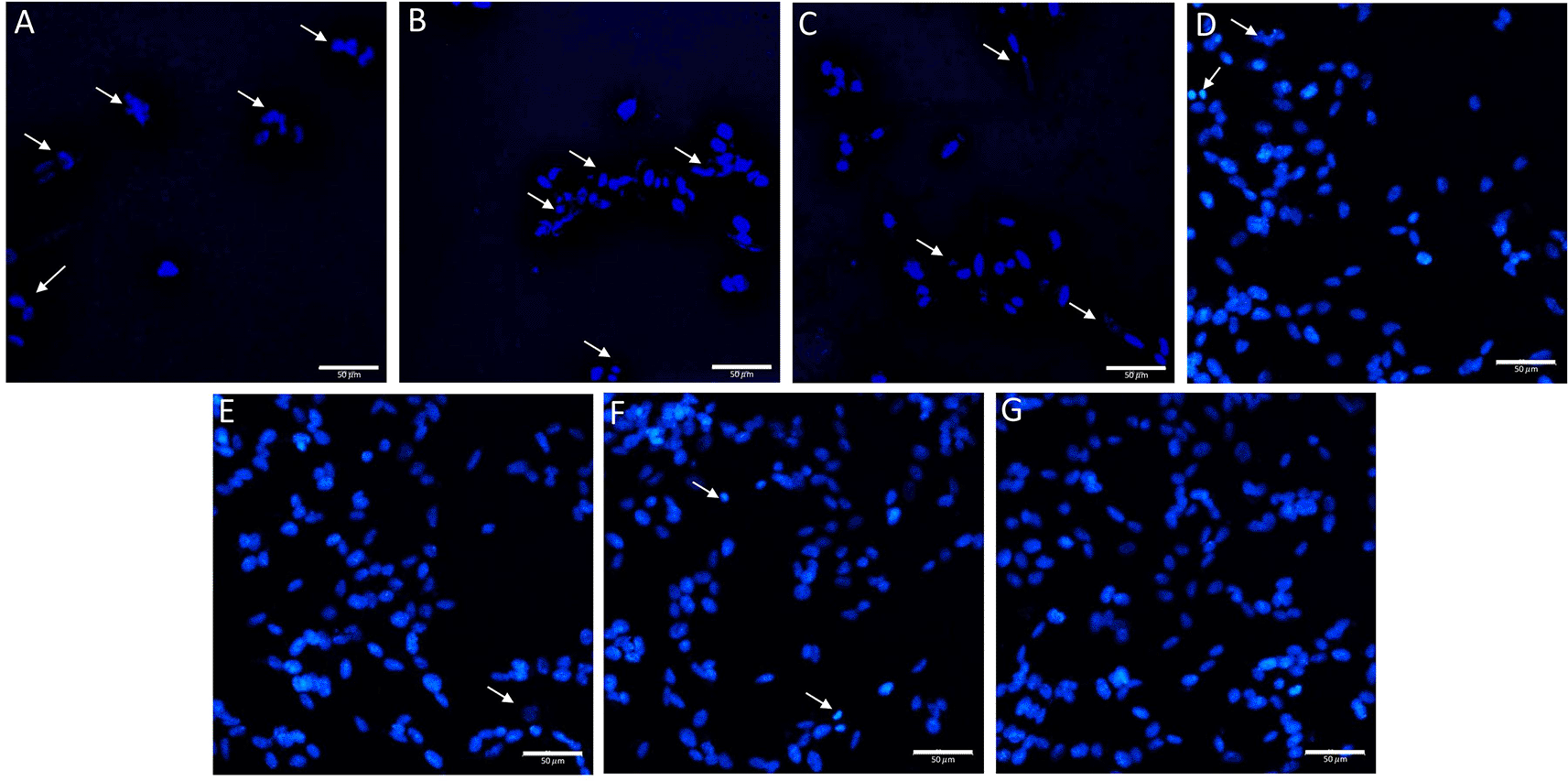
Before the staining, the cells were cultured in several treatments diluted in complete media: (A) SH-SY5Y + TMT 10 μM (positive control group), (B) SH-SY5Y + TMT 10 μM + 25% BUVEC-CM), (C) SH-SY5Y + TMT 10 μM + 50% BUVEC-CM), (D) SH-SY5Y + TMT 10 μM + 75% BUVEC-CM, (E) SH-SY5Y + TMT 10 μM + 100% BUVEC-CM, (F) SH-SY5Y + TMT 10 μM + 1 μM Donepezil, (G) non-treated SH-SY5Y. Untreated cells were used as a negative control. Donepezil was used as a comparison drug for dementia. Arrow signs indicate cells undergoing apoptosis characterized by nuclear fragmentation due to DNA condensation. Apoptotic cells were observed less in cells treated with graded concentrations of BUVEC-CM. The data is measured as semi quantitative as +++ (strong), ++ (moderate), + (weak). Scale bar: 50 μm.
From the AO/PI staining results (Figure 8), in the non-treated group, green cells were noted with protrusion of the cytoplasm. In the positive control group, red cells were more prevalent (++++). In the groups of cells administered with BUVEC-CM with graded concentrations, the higher the BUVEC-CM concentration, the fewer red cells were observed as apoptotic (BUVEC-CM 25% = +++, BUVEC-CM 50% = ++, and BUVEC-CM 75% = +). At a 100% BUVEC-CM concentration, red cells were no longer visible. In the comparison group, no red cells were noted. The group of cells with 100% BUVEC-CM treatment and the comparison group showed cell morphologies similar to the cells without treatment.
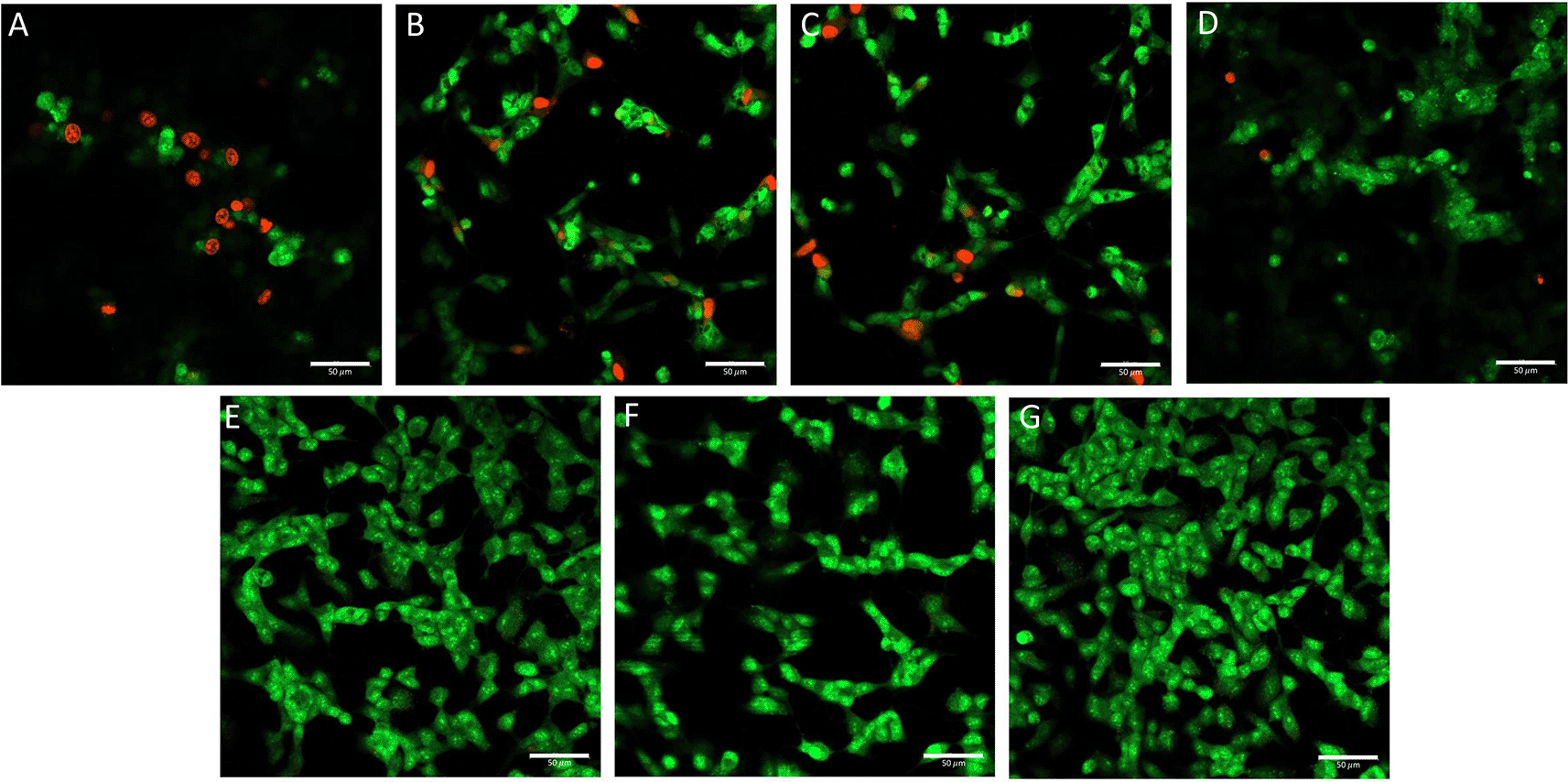
Before the staining, the cells were cultured in several treatments diluted in complete media: (A) SH-SY5Y + TMT 10 μM (positive control group), (B) SH-SY5Y + TMT 10 μM + 25% BUVEC-CM), (C) SH-SY5Y + TMT 10 μM + 50% BUVEC-CM), (D) SH-SY5Y + TMT 10 μM + 75% BUVEC-CM, (E) SH-SY5Y + TMT 10 μM + 100% BUVEC-CM, (F) SH-SY5Y + TMT 10 μM + 1 μM Donepezil, (G) non-treated SH-SY5Y. Untreated cells were used as negative control. Donepezil was used as a comparison drug for dementia. Red colour indicates cells undergoing apoptosis and green colour indicates viable cells. Green coloured cells were observed dominant in cells treated with graded concentration of BUVEC-CM. The data is measured as semi quantitative as +++ (strong), ++ (moderate), + (weak). Scale bar: 50 μm.
The results of the DCFDA staining (Figures 9, 10) in the non-treated group showed the lowest intensity percentage of 11.1%. Meanwhile, the positive control (TMT) reached the highest intensity percentage with a value of 41.93% (p < 0.0001). The groups in the presence of graded concentration of BUVEC-CM at 25%, 50%, 75%, and 100% had a gradually decreased intensity value of 33.36% (p < 0.0001), (30.96% (p < 0.0001), 30.90% (p < 0.0001), and 28,56% (p < 0.0001), respectively. Meanwhile, the comparison group had an intensity percentage at 30.46% (p < 0.0001).
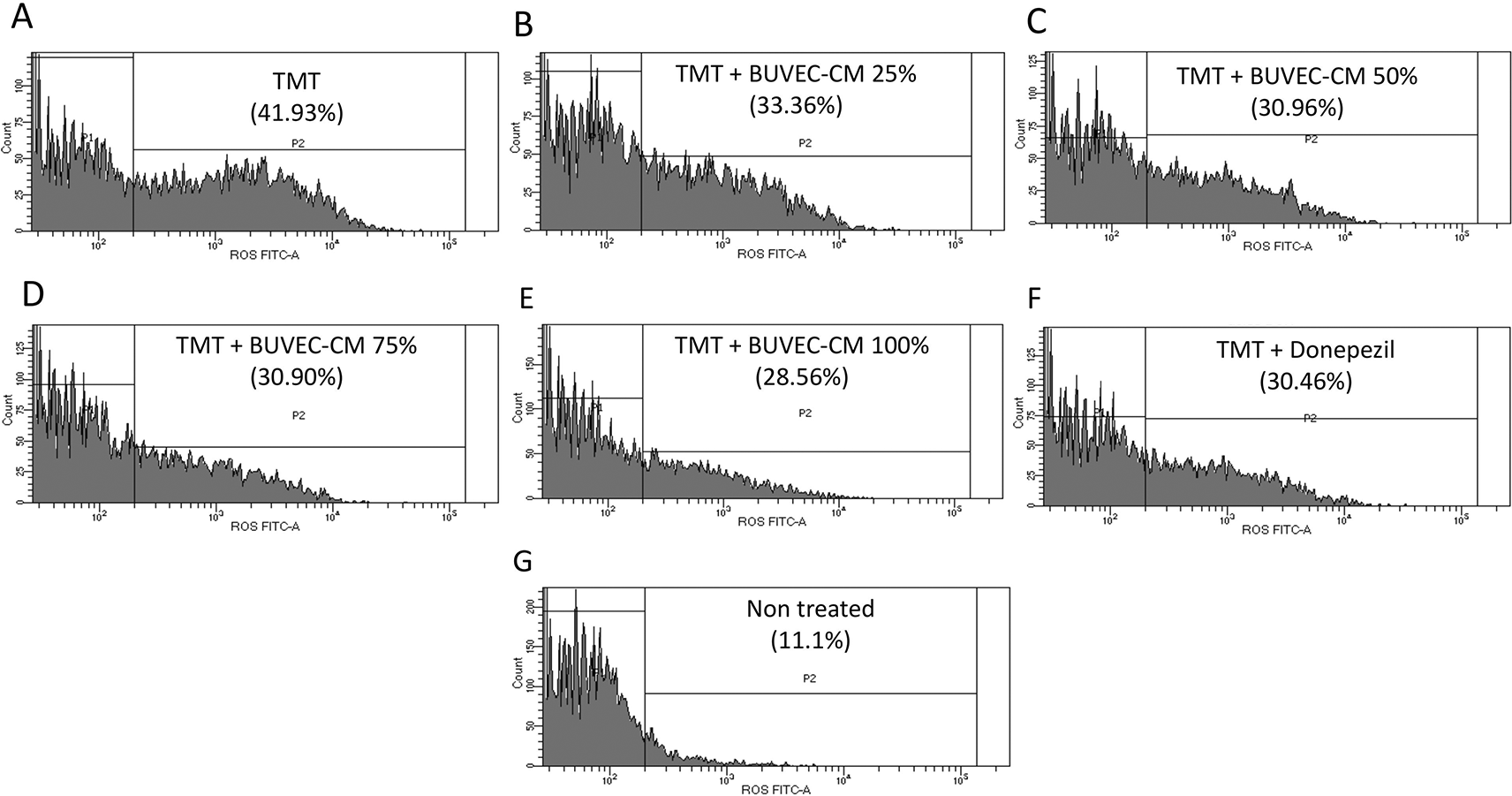
The SH-SY5Y cells were stained with DCFH-DA. The cells were cultured in several treatments diluted in complete media: (A) SH-SY5Y + TMT 10 μM (positive control group), (B) SH-SY5Y + TMT 10 μM + 25% BUVEC-CM), (C) SH-SY5Y + TMT 10 μM + 50% BUVEC-CM), (D) SH-SY5Y + TMT 10 μM + 75% BUVEC-CM, (E) SH-SY5Y + TMT 10 μM + 100% BUVEC-CM, (F) SH-SY5Y + TMT 10 μM + 1 μM Donepezil, (G) non-treated SH-SY5Y.
In the development of neurodegeneration, mainly in AD and PD, microglial activation parallels to the upregulation of inflammatory factors in the affected brain areas. In AD, oxidation can induce neuronal axon demyelination and microglial overactivation, which are the main sources of ROS. In this study, BUVEC-CM with graded concentrations of 25%, 50%, 75%, and 100% preserved viability and morphological integrity, trigger cells’ proliferation, protect cells from apoptosis, increase BDNF concentrations, and decrease caspase-7, -9, and CD68 expression in TMT-induced neurodegeneration (AD) on the human neuroblastoma (SH-SY5Y) cells.
Morphological integrity can be observed from the results of MTT and CCK-8 assays, wherein among the seven treatment groups, the positive control group had the lowest viability. From these results, it can be concluded that the treatment of SH-SY5Y cells with TMT had a cytotoxic effect and decreased cell viability. In the CCK-8 assay, the decreasing cell viability is caused by cell adhesion inhibition, as evidenced by the low percentage. Meanwhile, the 100% BUVEC-CM group had the highest viability value and approached the non-treated group, which had a 100% viability rate, indicating that all cells in this group were alive. This means that BUVEC-CM at 100% can maintain cell viability from TMT treatment. Moreover, the CCK-8 results were consistent with those of a study by Larasati et al.,10 who reported that TMT administration can reduce SH-SY5Y cell viability in the CCK-8 assay and that BUVEC-CM administration in graded concentrations can increase the viability of these cells.
The ability of BUVEC-CM to trigger cell proliferation can be observed from the scratch-wound assay result, wherein among the seven treatment groups, the TMT-treated group had the least increased narrowing area, whereas the group with BUVEC-CM at 100% had the most increased narrowing area. Therefore, BUVEC-CM can enhance wound healing by stimulating cell migration. One of the goals of neuronal proliferation is to promote axon growth and stimulate the formation of new connections. Several anti-inflammatory cytokines can act as modulators of plasticity and neuronal growth, which are critical for neuron proliferation. Anti-inflammatory factors, including IL-4, IL-10, IL-13, LIF, and TGF-b, play a role in the regulation of cell survival, proliferation, and migration; cytokines, IL-4, LIF, and TGF-b, have even more effects on neuronal regeneration.18 The results from our scratch-wound assay showed that BUVEC-CM can also trigger cell proliferation. Chang et al.19 stated that VEGF, HGF, bFGF, LIF, IGF-1, PGE2, SDF-1, MCP-1, IL-2, and PDGF are the main secretome components in MSCs that can induce proliferation. These components are similar to those of BUVEC-CM described in previous studies.5
The ability of BUVEC-CM to increase the BDNF concentration can be observed from the results of the ELISA test. From the test results, BUVEC-CM administration in graded concentrations could be observed to increase the BDNF concentration, with the BUVEC-CM 100% group having the highest OD number. This was observed since BUVEC-CM contains several growth factors, including VEGF, FGF, TGF, TNF, BDNF, and many amino acids.5 Chang et al.19 reported that BDNF, NGF, and GDNF are the main secretome components of MSC that are involved in inducing neuroprotection. It is known that BDNF plays a significant role in maintaining plasticity, neuronal maturation, and development of their connections. Several studies have also shown that therapy with BDNF has the potential to prevent depression, neurodegeneration, and brain cancer.20 A study by Aboutaleb et al.13 using CM from amniotic mesenchymal stem cells (AMSC-SM) as therapy for focal cerebral ischemia in vivo showed that AMSC-CM exerts a neuroprotective effect by activating the extracellular signal-regulated protein kinase (ERK)1/2-BDNF signaling pathway, triggering neurogenesis and angiogenesis, and suppressing apoptosis. BDNF exerts a neuroprotective effect by regenerating damaged neurons. BDNF expression after focal cerebral ischemia can be activated via several cell-signaling pathways, such as the ERK1/2 and PI3K/Akt pathways. ERK1/2, which belongs to the mitogen-activated protein kinase (MAPKs) family, modulates neuronal survival, and apoptotic cell death. The activation of this complex causes cytoplasmic and membrane protein phosphorylation. ERK1/2 translocation from the cytosol to the nucleus also causes phosphorylation of a different transcription factor, the cAMP response element-binding protein (CREB) protein, which is a pro-survival protein and a major regulator of the BDNF, Bcl-XL, and Bcl-2 genes. BDNF is generally decreased in cases of neurodegenerative diseases, including AD, and PD. Decreased BDNF level in AD is associated with amyloid β accumulation, τ phosphorylation, neuroinflammation, and neuronal apoptosis. In AD, increasing BDNF expression through therapy is believed to reduce behavioral disorders, neuronal abnormalities, and synaptic degeneration, as well as prevent neuronal loss.21 A review by Zhang et al.22 on the use of the secretome from neural stem cells also produced similar results, wherein the secretome from neural stem cells has been shown to contain growth factors, including NGF, GDNF, VEGF, and BDNF, which act as neuroprotectants; trigger neuronal regeneration and angiogenesis; and regulate immunity.
To detect changes in the expression levels of apoptosis and inflammation-related genes, gene expression was measured using qRT-PCR. From the results, it could be noted that the administration of TMT to SH-SY5Y cells caused increases in caspase-9, -7, and CD68 expression. TMT administration caused increases in caspase-7, -9, and CD68 expression in the positive control group. This was because of TMT-induced neuronal damage. Neurotoxic action by TMT can occur through various mechanisms, including glutamate excitotoxicity, intracellular calcium excess, impaired neurotransmission, oxidative stress, and hippocampal sparring activation. The TMT action is triggered by various molecular and cellular processes, including the activation of various kinases, transcription factors, stress proteins, and early response genes, thereby leading to cytotoxic responses. Furthermore, TMT-induced neurodegeneration is associated with astrocyte and microglia activation, along with proinflammatory cytokine upregulation.23
Caspase activation is the final stage of the apoptotic process. Caspase-9 is activated when a cell death stimulus induces Bcl-2 family proteins, such as Bax, or Bak, to oligomerize, and cause mitochondrial outer membrane permeability. This change in permeability makes cytochrome c and another proapoptotic factors released, which move from the mitochondrial intermembrane space to the cytosol. Caspase-9 itself is activated following the release of cytochrome c. Caspase-9 subsequently activates effector caspases (caspase-3 and -7), which develop cellular substrates to facilitate apoptosis. Caspase-7 is believed to have no significant role in intrinsic cell death; however, it is responsible for the production of reactive oxygen species (ROS) and detachment from the extracellular matrix.24,25 This is consistent with the results of this study, wherein cells in the positive control group underwent apoptosis and mostly failed to adhere to the flask surface. From the results of qRT-PCR assays, we noted that BUVEC-CM administration with graded concentrations can decrease the relative expression of caspase-9 and -7, indicating that BUVEC-CM can prevent apoptosis in neurons through the mitochondrial pathway. Similar results were also observed in a study by Larasati et al.,10 wherein BUVEC-CM administration to TMT-induced PC12 and SH-SY5Y cells could decrease caspase-9 and -3 expression. According to Chang et al.,19 VEGF, G-CSF, bFGF, IGF-1, HGF, IL-2, IL-6, IL-9, and STC-1 are the main secretome components in MSCs that act as antiapoptotic agents.
The ability of BUVEC-CM to inhibit apoptosis can also be observed from the results of Hoechst 33342 and AO/PI staining. From the Hoechst 33342 staining results, apoptotic cells are characterized by a fragmented nucleus due to DNA condensation, which can be noted to predominate in the positive control group. These characteristics are consistent with the results of a study by Crowley et al.26 However, viable cells are indicated by having a round nucleus, which can be observed to predominate in the BUVEC-CM 100% and comparison groups. From the AO/PI staining results, viable cells can be indicated by green cells with protrusion of the cytoplasm, which can be noted to dominate in the non-treated and BUVEC-CM 100% groups. Meanwhile, dead cells, which undergo apoptosis, can be observed as red cells, which can be observed to dominate in the positive control group. Consequently, it can be concluded that BUVEC-CM can act as an antiapoptotic therapy.
To ensure the capability of BUVEC-CM in reducing neuroinflammation, measurement of CD68 expression was performed. From the results, it could be observed that BUVEC-CM administration at graded concentrations could reduce the expression of CD68 as a biomarker of inflammation in TMT-induced SH-SY5Y cells. CD68 is highly expressed in macrophages, microglia, osteoclasts, and myeloid dendritic cells.27 CD68 can enter the endosome from the cell surface following inflammatory stimulation. Inflammatory processes and microglial activation comprise the pathophysiology underlying several diseases of the central nervous system. Under physiological conditions, microglia, and astrocytes play a role in maintaining neuronal plasticity and synaptic homeostasis and producing trophic factors. Under pathological conditions, activated microglia, astrocytes, and macrophages migrate through the damaged blood–brain barrier and contribute to neuroinflammation. They lose their homeostatic function, decrease neurotrophic factor secretion, and produce large amounts of proinflammatory cytokines and chemokines, which can cause not only the clearance of pathogens and toxins but also neuronal dysfunction and damage. Moreover, microglial activation can damage the synaptic structure.28 M1 activation in the microglia can cause cytotoxicity, inflammation, and expression of NADPH oxidase, which can form superoxide, and ROS. The microglia can secrete proinflammatory cytokines, including IL-1β and IL-6, TNF-α, IFN-γ, chemokines (CCL-2, CX3CL1, and CXCL10), glutamate, and nitric oxide as transmitters of inflammation.29
Nevertheless, several studies are needed to provide stronger evidence of the potential of BUVEC-CM to maintain viability and enhance neuron migration. Here, the studies that we conducted remain in vitro experiments. Therefore, in vivo studies are also needed as the basis for preclinical trials.
In this study, inflammation that occurred in the positive control group due to TMT administration was evidenced by increased CD68 expression, and apoptosis via mitochondria was evidenced by AO/PI and Hoechst staining and increased expression of caspase-7 and -9 genes. In the groups given BUVEC-CM with graded concentrations, the results showed that BUVEC-CM could increase the level of BDNF, and in the same groups, the expression of CD68, caspase-7 and caspase-9 was reduced. Taken together, our results demonstrate that graded concentrations of BUVEC-CM can prevent apoptosis, inflammation, and protect the structure of neurons in a BDNF-dependent mechanism (Figure 11).
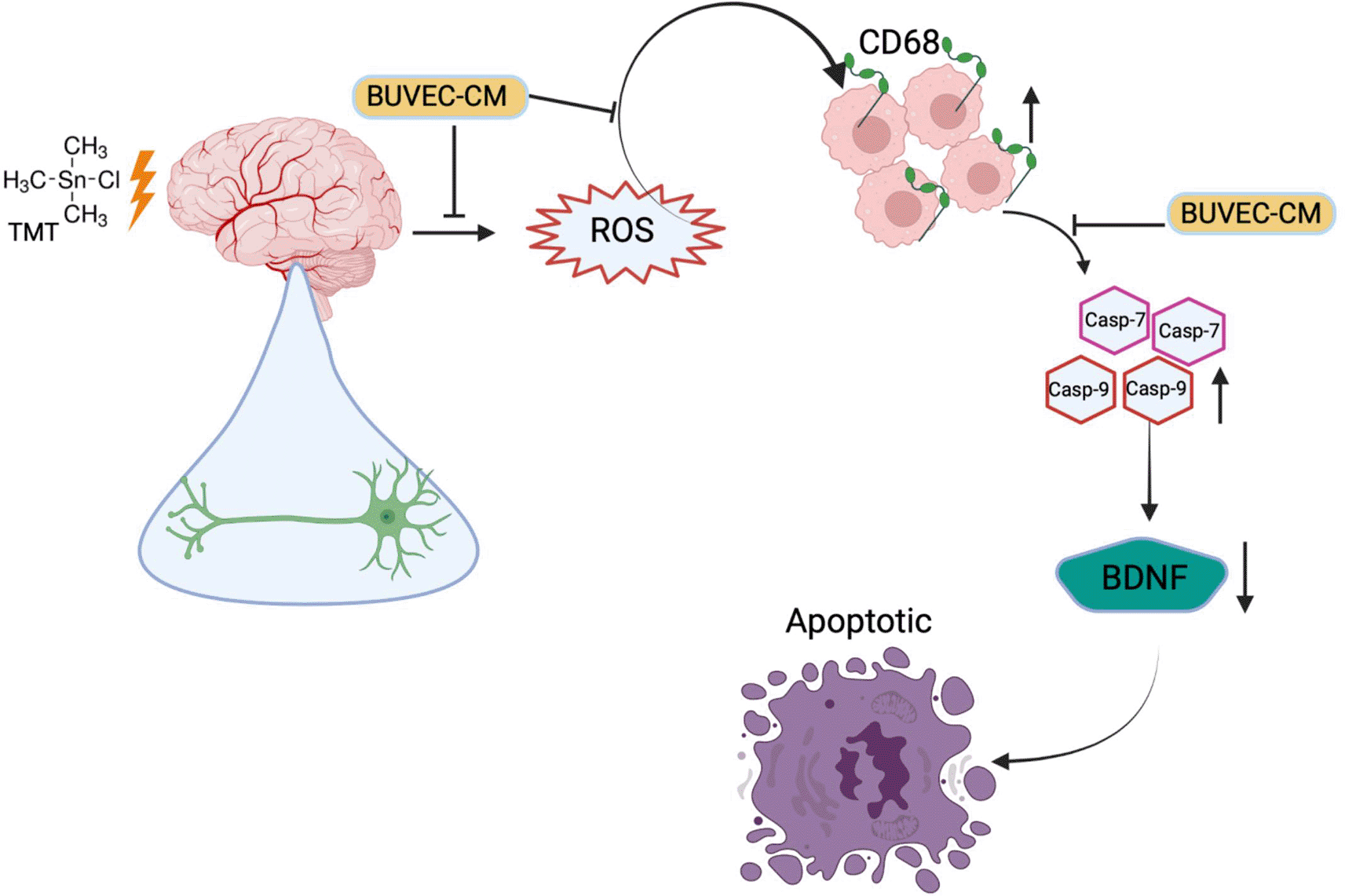
This shows the potential effect of BUVEC-CM as a neuroprotectant agent that inhibits inflammation and apoptosis in neurodegenerative diseases.
The experimental procedures were approved by the Ethical Committee of the Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia (approval number: 020/EC-FKH/Int./2022). Informed consent from the cow’s owner to acquire the umbilical cord from the animals was also obtained.
Figshare: Raw Data for The Bovine Umbilical Vein Endothelial Cells Conditioned Medium Prevents TMT-Induced Neurotoxicity Mediated by the Upregulation of Brain-Derived Neurotropic Factors on the Human Neuroblastoma SH-SY5Y Cells, https://doi.org/10.6084/m9.figshare.22153859.v1. 30
This project contains the following underlying data:
- Raw MTT Assay.csv
- Raw MTT Assay.xlsx
- Raw CCK assay.csv
- Raw CCK Assay.xlsx
- Raw scratch wound assay.csv
- Raw scratch wound assay.xlsx
- Raw ELISA BDNF.csv
- Raw ELISA BDNF.xlsx
- Raw CD68.csv
- Raw Caspase-9.csv
- Raw Caspase-7.csvRaw microscopy images for all assays
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like to thank Universitas Gadjah Mada for Thesis Recognition Grant in the year of 2022 for funding this research. The authors also thank the Department of Pharmacology and Therapy, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, the Integrated Research Laboratory (LRT), Universitas Gadjah Mada, and the Integrated Laboratory for Research and Testing (LPPT), Universitas Gadjah Mada for the laboratory facilities.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)