Keywords
RNA editing, ADAR, Parkinson’s Disease, nonsense-mediated decay, neurodegenerative disease, SLC11A2
This article is included in the Cell & Molecular Biology gateway.
Parkinson’s Disease (PD) is a complex disease with multiple phenotypes varying between individuals, as well as by age and sex. Males are diagnosed with PD at a much higher rate than females, and females experience later onset yet faster disease progression than males; the sexes also differ in PD by neuron composition, gene expression, and symptom progression. Because only a fraction of PD cases can be tied to genetic variants, it is likely that a complicated interaction between gene expression, hormones, the environment, and modifications to RNA transcripts plays a role in PD pathology and progression.
Here we explored changes in RNA editing through analysis of RNAseq between 243 healthy controls and PD patients aged 65 years or older enrolled in the Parkinson’s Progression Markers Initiative. Specifically, we analyzed editing through the actions of the adenosine deaminases acting on RNA (ADARs), which may cause nonsynonymous alterations to gene expression products including those that result in nonsense-mediated decay (NMD).
We observe differences in ADAR expression, number of putative ADAR edits, and the number of high/moderate impact edits between comparison groups and PD samples, which often show higher levels of ADAR expression and edits. PD males and females also differ in ADAR expression, number of edits, and the number of high and moderate impact edits with males exhibiting elevations compared to females in all three categories except in those edits associated with NMD, particularly in edits affecting SLC11A2, a gene coding for a transmembrane iron transporter. Likewise, differentially expressed genes between comparison groups were tied to NMD-related pathways
Our findings suggest that the dysregulation of ADAR editing may play a role in PD and that ADAR editing associated with NMD and genes functioning in NMD-related pathways may be integral to PD pathophysiology, particularly when comparing the sexes.
RNA editing, ADAR, Parkinson’s Disease, nonsense-mediated decay, neurodegenerative disease, SLC11A2
Parkinson’s Disease (PD) is a complex disease with multiple phenotypes that vary between individuals, as well as by age and sex (Lawton et al., 2018; Sandor et al., 2022). The characteristic symptoms of PD including resting tremor, bradykinesia, rigidity, and postural instability (Lill, 2016; Hughes et al., 1992; Gelb et al., 1999) are largely due to the loss of dopaminergic neurons in the substantia nigra pars compacta (Lill, 2016). A specific hallmark of PD is the accumulation of protein, particularly those composed of alpha-synuclein, called Lewy bodies, however, these aggregations are not common across all cases of PD (Lill, 2016; Gelb et al., 1999). Historically, PD subtypes were characterized by one variable such as age or tremor (Fereshtehnejad & Postuma, 2017) but have been expanded to include a wider variety of symptoms (Postuma & Berg, 2019) including subtypes that differ by sex (Sandor et al., 2022). Males are diagnosed with PD at a much higher rate than females (Gillies et al., 2014; Vaidya et al., 2021), and females experience later onset yet faster disease progression than males (Morgan et al., 2014; Vaidya et al., 2021). It has been suggested that estrogen promotes neuron survival, neurogenesis, synaptic functioning, and reduction of neuroinflammation through its interactions with estrogen receptors on neuron cell membranes (Azcoitia et al., 2011), and contributes to some of the discrepancies in onset and symptomology between the sexes (Wooten et al., 2004); however, the results of some studies have been inconsistent with this assertion (Russillo et al., 2022). Sex differences in PD progression have been well documented including alterations in motor features by body region and postural scores (Szewczyk-Krolikowski et al., 2014), increased drooling (Perez-Lloret et al., 2012; Cheon et al., 2008), speech problems (Perez-Lloret et al., 2012), swallowing in men (Cheon et al., 2008; Miller et al., 2009), postural instability in women (Baba et al., 2005; Solla et al., 2012), and more pronounced cognitive impairment (Nazem et al., 2009), daytime sleepiness (Martinez-Martin et al., 2012) in men, and recently, Sandor et al. observed a significant association between sex and anxiety and depression (2022).
The etiology of PD is multifactorial, including both genetic and environmental origins, and although only ~5-10% of PD diagnoses can be associated with genetic causes (Lill, 2016), it is likely that a complicated interaction between gene expression, hormones, and potentially other unknown factors contribute to a prognosis of PD (Mercer et al., 2023). One of the factors that influence the outcomes of gene expression is RNA editing. Unlike gene mutations, which result in permanent changes to the genome, RNA editing can be dynamically and differentially regulated and is controlled spatiotemporally in a nuanced manner Notaras et al., 2020 (Huntley et al., 2016; Piontkivska et al., 2019). Patterns of RNA editing by Adenosine Deaminases Acting on RNA (ADAR editing) vary among species in terms of editing levels, targets, and ADAR isoforms, with the majority of editing occurring in non-coding regions of the genome (Jaffrey & Wilkinson, 2018; Lee et al., 2021; Soreq et al., 2013). ADAR editing, which causes adenosine to inosine deamination, is the most common mechanism of post-transcriptional RNA editing in the nervous system and integral to neurodevelopment and response to infection/inflammation (Bass, 2002; Goldstein et al., 2017; Hundley & Bass, 2010; Lundin et al., 2020). Separate from gene expression, dysregulated RNA editing by ADARs can potentially cause changes in mRNA transcripts that result in alterations that can affect protein coding, cause nonsense mediated decay, or create changes in regulatory RNAs (Wu et al., 2023). Several studies have linked ADAR editing with neurological, neurodegenerative and psychiatric disorders such as epilepsy (Cheng et al., 2025; Grigorenko et al., 1998; Kortenbruck et al., 2001; Krestel et al., 2013; Vollmar et al., 2004), autism (Cheng et al., 2025; Eran et al., 2013; Tran et al., 2019), Amyotrophic Lateral Sclerosis (ALS) (Cheng et al., 2025; D’Erchia et al., 2017; Hideyama et al., 2012; Kawahara et al., 2004; Takuma et al. 1999), Huntington’s Disease (HD) (Annese et al., 2018), Alzheimer’s Disease (AD) (Akbarian et al., 1995; Annese et al., 2018; Cheng et al., 2025; Gaisler-Salomon et al., 2014; Khermesh et al., 2016), schizophrenia (Akbarian et al., 1995; Cheng et al., 2025; Di Narzo et al., 2014; Dracheva et al., 2003; Lyddon et al., 2013; Niswender et al., 2001; Sodhi et al., 2001; Weissmann et al., 2016), suicide (Di Narzo et al., 2014; Lyddon et al., 2013; Niswender et al., 2001; Weissmann et al., 2016), and depression (Gurevich et al., 2002; Iwamoto & Kato., 2003). The dysregulation of RNA editing may be an important factor in neurodegenerative disorders (Christofi & Zaravinos, 2019; Cheng et al., 2025), such as PD, and defining these patterns could elevate understanding of disease pathology, delineate changes to editing based on sex, and inform alternative therapies for treatment (Mercer et al., 2023). The substantia nigra pars compacta (SNpc) is a brain region integral to dopaminergic signalling and has been implicated in the pathophysiologic differences between males and females with PD (Bianco et al., 2023). A study of post-mortem brain samples illustrated that 2.6% of gene expression in the brain were differentially expressed based on sex, and additionally, that 95% of those genes were spliced differently dependent on sex (Trabzuni et al., 2013; Vaidya et al., 2021). The male SNpc contains a higher number of dopaminergic neurons, shows increased expression of PD-implicated genes such as SNCA and PINK1, and is associated with increased dopamine release when stimulated and increased vulnerability to drugs when compared to the female SNpc (Bianco et al., 2023); however, genes involving signal transduction and maturation of neurons are expressed at greater levels in females resulting in dopaminergic neurons that show greater resistance to degeneration (Cerri et al., 2019; Vegeto et al., 2020). Estrogen has been suspected to be neuroprotective as evidenced by the role of estradiol in dopamine processing observed in animal models (Cerri et al., 2019) and the potential inhibitory effects on the development of synuclein fibrils (Piscopo et al., 2021). Although PD pathology is most evident in the brain, crosstalk between the central nervous system (CNS) and peripheral organ systems provides clues to PD pathophysiology, including increasing evidence that circulating serum neurofilament light chains (Ygland Rödström et al., 2022), small non-coding RNAs (Kern et al., 2021), and circulating cytokines (Karpenko et al., 2018) found in blood vary between PD and control samples. In addition to variations in gene expression between PD males and females, a recent study based on a small number of PD skeletal muscle samples observed variations between the sexes in patterns of ADAR editing specifically when editing resulted in nonsense-mediated decay (Mercer et al., 2023).
The physiological progression and symptomology of PD differs between males and females (Abraham et al., 2019; Gillies et al., 2014; Jurado-Coronel et al., 2018; Russillo et al., 2022; Vaidya et al., 2021), and given the structural and biochemical differences that are observed between the sexes, particularly in the brain (Lawton et al., 2018; Sandor et al., 2022), it is conceivable that understanding these differences may be integral to understanding varying PD phenotypes.
While over 15 million RNA editing sites have been observed in humans (Mansi et al., 2021; Bazak et al., 2014), only a portion of them are found in protein coding regions and lead to non-synonymous re-coding (Pozdyshev et al., 2021). However, these substitutions are over-represented in transcripts known to function in neural tissues (Ramaswami & Li, 2016). In a recent study of BA9 brain samples from a small cohort of PD patients, differential A-to-I editing was observed in 160 sites, with half of those edits occurring in genes encoding glutamate ionotropic receptors. Overall, Pozdyshev et al. (2021) observed decreased editing in the PD brains as compared to controls. Alternatively, Mercer et al. (2023) observed elevated levels of high and moderate protein-coding impact ADAR edits in skeletal muscle tissue in genes with known associations to PD in a small males-only cohort. When examining brain tissue for editing in microRNA (miRNA), Lu et al., identified a number of miRNA editing sites that were edited at significantly different editing levels between PD and Healthy Controls (Lu et al., 2022). These findings suggest that changes in ADAR editing patterns may play an impactful role in the pathology of PD affecting various tissue types.
Nonsense-mediated decay (NMD) is an mRNA surveillance mechanism in eukaryotes which largely serves to eliminate nonsense mutations (Kurosaki et al., 2019; Lykke-Andersen & Jensen, 2015) that arise for a variety of reasons, including ADAR editing, and additionally function to regulate cellular processes such as stress responses (Nasif et al., 2018), and in the nervous system, moderates neuro-development, neurogenesis, neuronal differentiation (Lou et al., 2014; Goetz & Wilkinson, 2017), and synaptic plasticity (Magee & Grienberger, 2020; Notaras et al., 2020). NMD has been implicated in intellectual disabilities (Howe & Patani, 2023), neurodevelopmental disorders (Jaffrey & Wilkinson, 2018; Lee et al., 2021), ALS, frontotemporal dementia, and PD (Soreq et al., 2013; Ortega et al., 2020). A study of NMD events in the leukocytes of PD patients comparing samples taken before Deep Brain Stimulation (DBS) therapy and after DBS demonstrated that NMD events in-creased with disease progression suggesting that NMD is an important regulator of the transcriptome, but also that brain changes transcend into peripheral systems (Soreq et al., 2013). Soreq et al. also noted that NMD and alternative splicing events, along with a reduction in tremors associated with PD, decreased for a time following DBS (Soreq et al., 2013). NMD events are commonly associated with DNA repair genes and those that regulate alternative splicing, and thus, NMD events may provide a target for potential PD treatment (Soreq et al., 2013). The targeting of NMD and NMD-related factors has been suggested as a potential therapy for ALS and other neurodegenerative disorders (Jaffrey & Wilkinson, 2018).
Here we explored variations in RNA editing observed in whole-blood PD samples, specifically editing through the actions of the adenosine deaminases acting on RNA (ADARs) edits associated with nonsense-mediated decay and predicted changes in protein outcomes when comparing PD males and females to healthy controls.
The RNA-seq baseline data derived from blood samples of 179 PD and 64 healthy controls, aged 65 years or older was obtained from the PPMI (Marek et al., 2011) (https://www.ppmi-info.org/access-data-specimens/download-data ), downloaded on October 31, 2021, RRID:SCR 006431, with an average of 114 M (illion) reads (mean reads = 116M paired reads/sample). The PD group consisted of 114 males and 65 females, and the healthy control group consisted of 43 males and 21 females, all 65 years of age or older.
The Automated Isoform Diversity Detector (AIDD) pipeline (Plonski et al., 2020) was used to infer ADAR editing events as described by Mercer et al. (2024). Briefly, fastq files of individual patients were trimmed and aligned to the chosen human reference (GRCh37) using HISAT2 (Kim et al., 2015). Once aligned, the transcriptome was assembled using StringTie (Pertea et al., 2015), to estimate levels of individual gene expression as Transcripts Per Kilobase Million (TPM). Putative ADAR editing events here are those A-to-G or U(T)-to-C (referred subsequently as T-to-C) edits without Single Nucleotide Polymorphism Database annotation (dbSNP) (Sherry et al., 2001) associations that have been assigned as “Pass” (filters) or have met “basic snp filter” criteria as designated by GATK variant calling pipeline (McKenna et al., 2010) in AIDD (Plonski et al., 2020). SnpEff (Cingolani et al., 2012) was used to predict the effects and functional impacts of predicted edited variants, as described below. Variants identified are putative ADAR editing events described as such based on known ADAR editing sites, but for simplicity will be further described throughout this study as simply “editing events” or “edits”.
A list of 801 unique gene IDs with suspected associations to PD were downloaded from NCBI Gene (downloaded on November 4, 2023) and converted to Ensembl gene and transcript IDs, resulting in 901 gene and 7311 transcript IDs (Supplementary File 1) utilizing Ensembl Biomart (Kinsella et al., 2011). The NCBI PD Gene List (n = 901) is one of the multiple GeneRIF (Gene Relevance into Function) lists, which are created and frequently up-dated to reflect current research through three primary methods: extraction from published literature by the National Library of Medicine staff, summary reports from HuGE Navigator (Yu et al., 2008), and user submissions from a Gene record (Murphy et al., 2022). The NCBI Gene List can be found in Supplementary File 1. We used Reactome (Fabregat et al., 2017) to assess the pathways in which the NCBI PD genes were overrepresented filtering for FDR value < 0.05.
SnpEff (Cingolani et al., 2012) annotations were collated by sample and patient group. Edited NCBI PD genes found within PD and healthy control samples were compared between groups and analyzed for significant difference between groups (using Chi-square test). Only genes affected by high or moderate impact editing events occurring at a specific site in at least 25% of samples within a group were considered, similar to procedures followed by other studies (Choudhury et al., 2023; Ma et al., 2021; Mercer et al., 2023) in order to reduce stochastic editing events. Within comparison groups, using the limit of edited sites in at least 25% of samples, resulted in edits by coordinate occurring in at least 6 healthy control females, 11 healthy control males, 17 PD females, and 29 PD males. We focused on high and moderate impact events because those are the most likely changes to result in functional and/or structural consequences for relevant protein-coding sequences. High impact editing events include those A-to-G or T-to-C nucleotide changes that result in large duplications or deletions, deleted or duplicated exons, frameshifts, gene deletions, etc. Moderate impact editing events include those that result in exon deletion or duplication, codon deletion or insertion, nonsynonymous coding, etc. (Cingolani et al., 2012). Additionally, we compared the proportion of high and moderate impact editing events occurring in NCBI PD genes independently, between sample groups using Chi-square test.
DESeq2 (Love et al., 2014) R package version 3.18 (R4.3.2) with default parameters was used to identify differentially expressed genes, and results filtered for an adjusted p-value < 0.05 and log2 Fold Change less than -0.58 or greater than 0.58. The latter cut-off allowed us to consider genes that exhibited at least 50% change in their expression in either direction. Gene lists with greater than 2 members identified as being differentially expressed were analyzed via Reactome (Fabregat et al., 2017), or in the case of single genes, analyzed for function and potential connections to disease status. Gene lists were compared to the NCBI PD gene list (Supplementary File 1) to identify differentially expressed genes that may be more likely to contribute to PD physiology. When Reactome (Fabregat et al., 2017) was used for pathway analysis for gene lists other than the NCBI PD gene list, filters of FDR <0.05 and at least 10% of entities in pathways were used. ADAR isoform expression was also evaluated with DESeq2 R package version 3.18 (R4.3.2) with read counts of less than 10 disregarded.
Chi-square test was used to compare the numbers of editing events across various categories, such as editing events with high and moderate impact, protein coding, and nonsense-mediated decay (NMD), using GraphPad Prism (version 10.1.2, 2023) when categorical data was being considered. For ADAR expression, non-parametric Kruskal-Wallis and Mann-Whitney tests were used to compare PD and healthy control subgroups by sex and as combined groups of PD patients and healthy patients, respectively. For comparing the total number of putative ADAR editing events per sample, as a proxy of ADAR editing levels, non-parametric Mann-Whitney test was used. For comparisons of ADAR isoform, unpaired t-test was used.
To clarify, this assessment of ADAR editing in PD is not meant to infer that putative edits are “causative” to PD, but rather that they may “contribute” to the pathophysiology of PD. To normalize editing estimates, we divided the total number of putative ADAR edits in a sample group and divided by the number of total reads (scaled by 100) similar to the methods of Breen et al., 2019. The highest mean edits per mapped read were observed in PD with 503.1 (SE ± 11.16) mean edits/read while 486.2 (SE ± 17.15) edits/read were observed in healthy controls when the sexes are combined. There was no significant difference in mean edits/read between the two groups (Mann-Whitney, p= 0.4436). When the data is parsed by sex, the highest mean edits/read were observed in PD males with 522.5 (SE ± 14.71) edits/read followed by 499.3 (SE ± 25.11) edits/read in healthy control females, 479.8 (SE ± 22.51) edits/read in healthy control males, and 469.0 (SE ± 15.98) mean edits/read in PD females. There was no significant difference between groups when separated by sex (Kruskal-Wallis, p = 0.1305).
When range (difference between the highest number of editing events observed in a sample group and the lowest number of editing events observed in the group) is considered for major comparison groups, the largest range is observed in PD (range = 163,348 events) followed by healthy controls (range = 116,762 editing events). When subgroups varying by sex are considered, the greatest range of ADAR editing events is observed in PD females (range = 163,348 editing events) have a higher range than PD males (range = 139,562 editing events); however, healthy control males (range = 112,347 editing events) show greater range than healthy control females (range = 100,143 editing events). There were no significant differences observed between sample groups when the number of putative ADAR edits are considered (Mann-Whitney, p > 0.05) (Supplementary Table 1).
A list of 801 unique genes with suspected associations to PD were downloaded from NCBI Gene (downloaded November 4, 2023) and converted to Ensembl gene and transcript IDs, resulting in 901 gene and 7311 transcript IDs utilizing Ensembl Biomart (Kinsella et al., 2011). Reactome (Fabregat et al., 2017) pathway analysis results were filtered for FDR < 0.05. NCBI PD genes were overrepresented in Reactome pathways Interleukin-4 and Interleukin-13 signalling (27%), Neutrophil degranulation (10.7%), Interleukin-10 signalling (31.4%), Post-translational protein phosphorylation (18.4%) and Platelet degranulation (17.0%). Results of Reactome (Fabregat et al., 2017) analysis of the NCBI PD gene list are found in Figure 1.
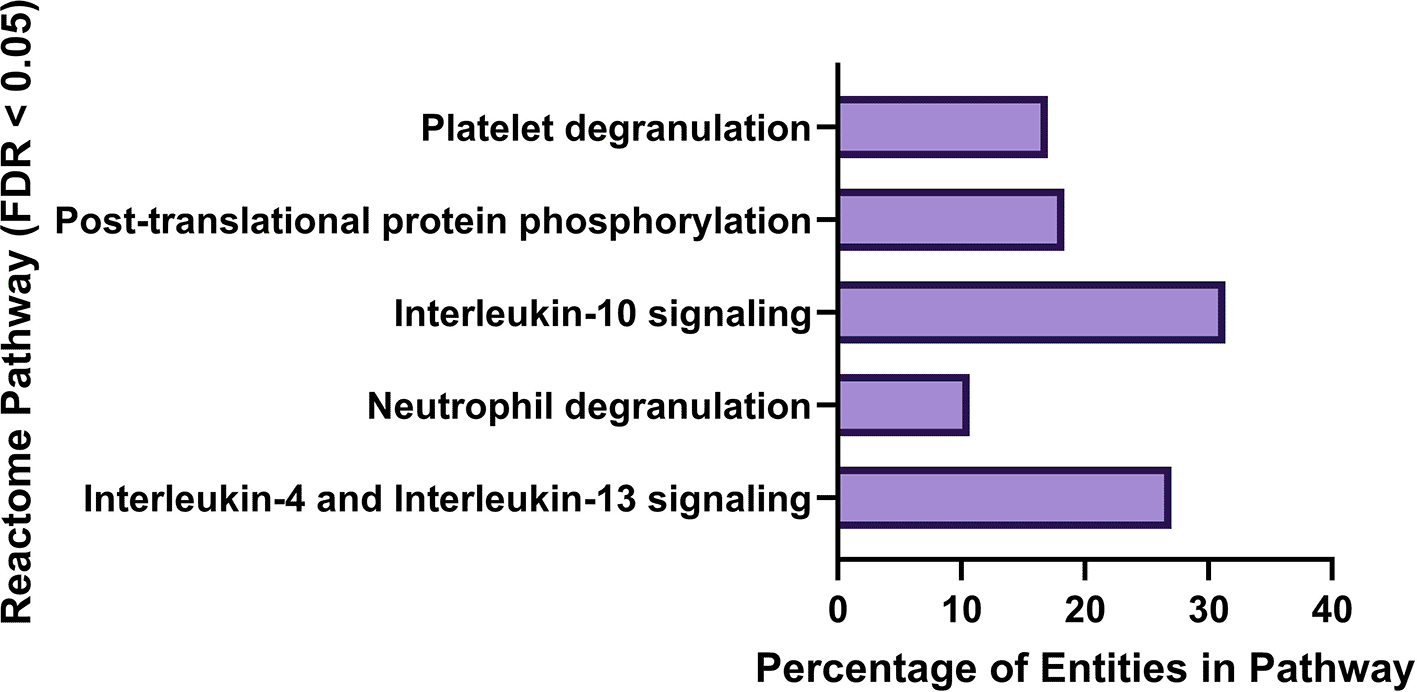
Interleukin-10 signaling pathway was enriched with 31.4% of Reactome (Fabregat, et al., 2017) entities represented in the NCBI PD gene list, Interleukin-4 and 13 signaling (27% of entities represented), Post-translational protein phosphorylation (18.4% of entities represented), Platelet degranulation (17% of entities represented), and Neutrophil degranulation (10.7% of entities represented).
SnpEff (Cingolani et al., 2012) annotations of putative ADAR editing events were filtered for genes in which high or moderate impact editing events occurred within genes from the NCBI PD Gene (Supplementary File 1) list and genome wide. Putative ADAR edits occurring in at least 25% of samples in a group were examined. Comparison groups can be found in Supplementary Table 2.
In addition to the number of high and moderate impact edits, differences in protein coding and nonsense-mediated decay biotypes with high or moderate impact were analyzed as these biotypes were consistently represented in sample groups. Significant differences were observed when comparing the number of high and moderate impact edits genome-wide between healthy control males and healthy control females (Chi-square, p = 0.0018, increased proportionately in healthy control males), and neared significance when comparing healthy control males to PD males (Chi-square, p = 0.075, increased proportionately in healthy control males). When comparing the number of high or moderate impact protein coding edits genome-wide, significance was achieved when comparing healthy control females and healthy control males (Chi-square, p = 0.0184, increased proportionately in healthy control males).
When only PD genes are considered, no significant difference is reached (Chi-square test) between comparison groups when all high or moderate impact edits, or when protein coding high or moderate impact edits are considered; however, significance is achieved when considering the number of high and moderate impact edits in PD genes resulting in NMD. Significance is reached when comparing NMD edits in PD genes between healthy controls and PD samples (Chi-square, p = 0.0106, increased proportionately in PD), healthy control females and PD females (Chi-square, p = 0.0106, increased proportionately in PD females), and PD females and PD males (Chi-square, p=<0.0001, increased proportionately in PD females). Statistical results can be found in Table 1 and Supplementary Tables 3-7. Comparisons of editing impacts can be found in Figure 2 (a,b) and Figure 3 (a,b).
Significance is reached when comparing NMD edits in PD genes between Healthy Controls to PD samples (Chi-square, p = 0.0106, increased proportionately in PD), healthy control females and PD females (Chi-square, p = 0.0106, increased proportionately in PD females), PD females and PD males (Chi-square, p=<0.0001, increased proportionately in PD. NS indicates non-significance; NA indicates that pairwise comparison was not performed.
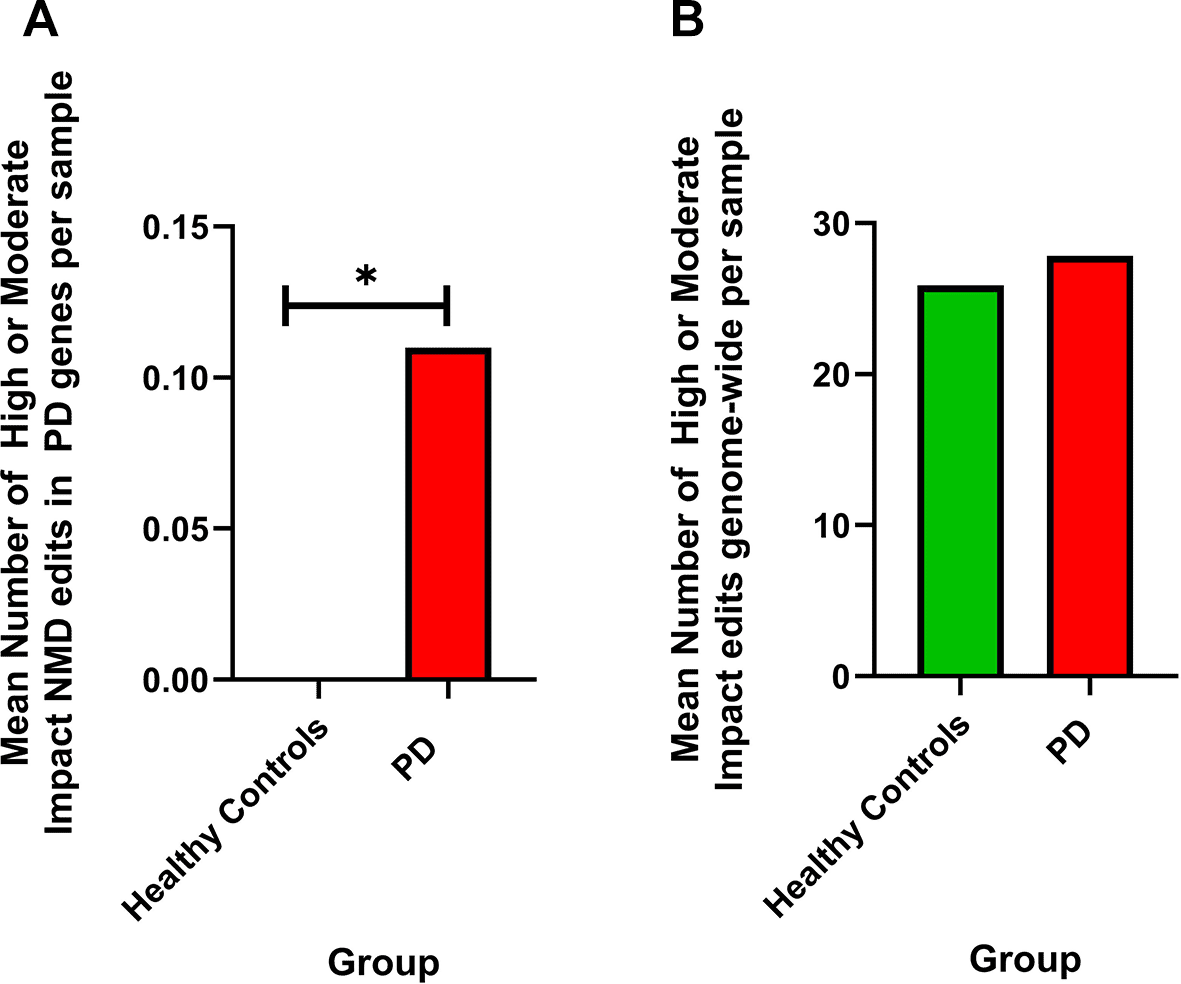
Shown is the mean number of high or moderate impact edits resulting in NMD in PD genes (healthy controls = 0, PD = 0.11, average edits per sample).
b) When comparing the number of high or moderate impact edits genome-wide resulting in NMD between sample groups, there is no significant difference (Chi-square, p > 0.05) between PD and healthy control samples. Shown is the mean number of high or moderate impact edits resulting in NMD in all major comparison groups (healthy controls = 25.9, PD = 27.84, average edits per sample.) *p=<0.05, **p=<0.01, ***p=<0.001
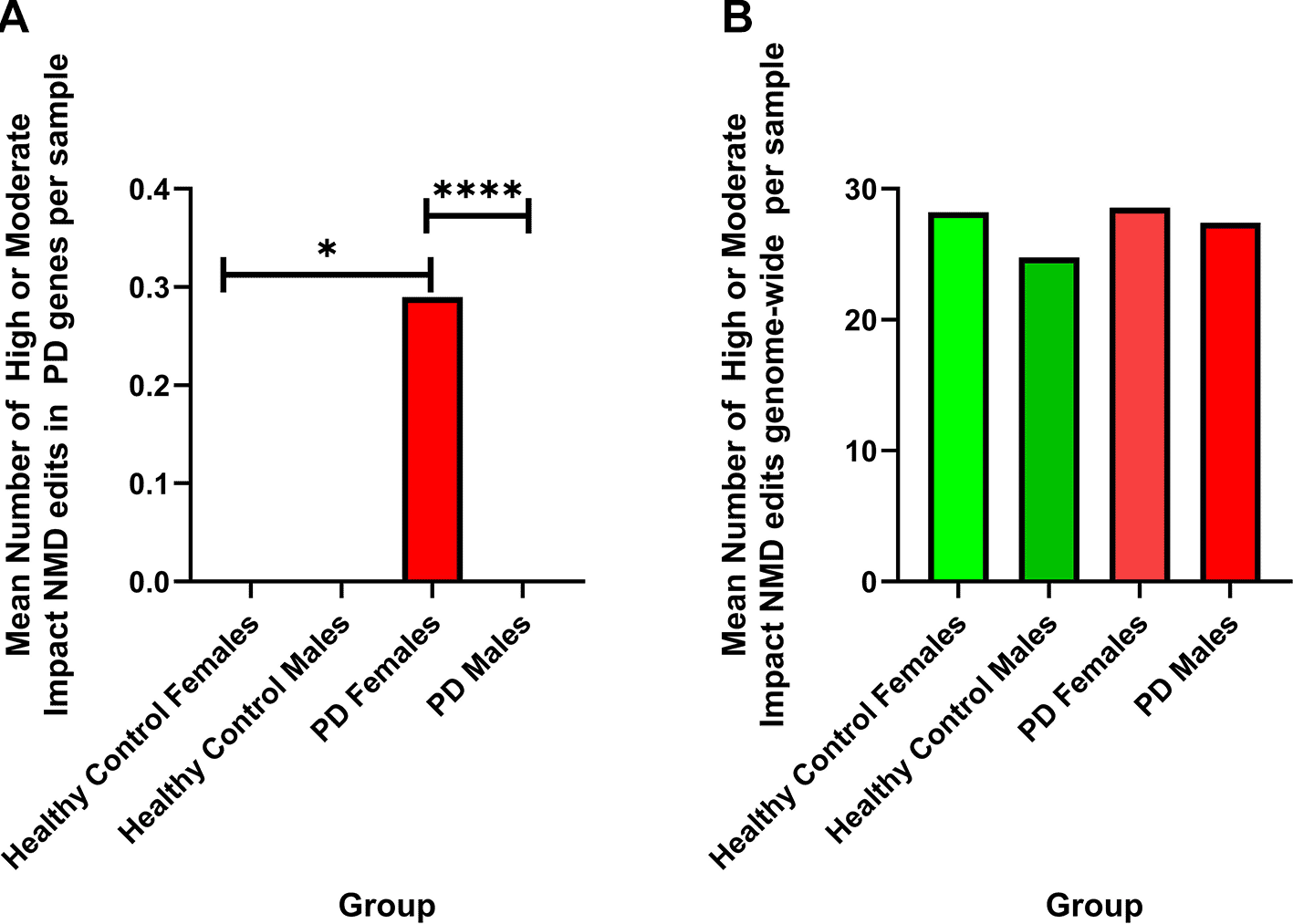
a) Significant differences are observed (Chi-square) when comparing the number of high or moderate impact edits in PD genes that results in NMD in healthy control females and PD females (Chi-square, p = 0.0106), and PD females to PD males (Chi-square, p = 0.0001). Shown are the mean number of high or moderate impact NMD edits in PD genes per sample (healthy control females = 0, healthy control males = 0, PD females = 0.30, PD males = 0).
b) There are no significant difference observed (Chi-square) when comparing the number of high or moderate impact edits resulting in NMD genome-wide between sample groups. Shown is the mean number of high or moderate impact NMD edits genome-wide by comparison group (healthy control females = 28.2, healthy control males = 24.77, PD females = 28.57, PD males = 27.42).
There were no high or moderate impact edits resulting in nonsense-mediated decay found in PD males or healthy control males or females however, one specific edit is highlighted as a major difference when comparing editing in PD females to all other groups. All the high or moderate impact edits resulting in NMD observed in PD females were associated with one site on Chr 12:51399999 and associated with the gene SLC11A2 (ENSG00000110911). This T/C edit affecting transcript ENST00000549625 was observed in 19 of the 65 PD females used in this analysis, representing 29.2% of PD female samples as compared to 26 of 114 PD males (22.8%), 4 of 21 healthy control females (19%), and 7 of 43 healthy control male samples (16.2%).
Differential gene expression analysis with DESeq2 did not identify ADAR genes as differentially expressed as per our filtering criteria (log2 Fold Change < -0.58 or > 0.58, padj=<0.05) when the entire transcriptomes of male and female healthy controls, and PD patients were considered. However, because there is a nuanced non-linear relationship between the levels of ADAR editing and expression of individual ADAR genes (Nishikura, 2016), we wanted to explore whether any differences in normalized ADAR genes expression can be identified among subject groups. ADAR expression levels as transcripts per million (TPM) were compared between groups revealing marked differences between groups; however, no significance was achieved (Kruskal-Wallis test or Mann Whitney test, p > 0.05). The highest mean expression of ADAR (ADAR1), which includes the interferon inducible isoform ADARp150 (MacMicking, 2012), was seen in PD male samples with 49.88 mean TPM (±2.604) followed by PD females (mean TPM = 48.26 ± 3.570). The lowest expression of ADAR was demonstrated in healthy control females (mean TPM = 43.25 ± 5.080) and healthy control males (mean TPM = 44.70 ± 3.559). When males and females are combined, the PD groups show the highest expression of ADAR (mean TPM = 49.77 ± 2.614) followed by healthy controls (mean TPM = 44.22 ± 2.893). The expression of ADARB1 (ADAR2) was similar between all groups; however, when males and females are combined, ADAR2 mean expression is highest in PD (3.105 TPM ± 0.1235) followed by healthy controls (2.969 ± .2077). While ADAR 3 is generally only expressed in the brain, there were low levels of expression in blood samples with the highest expression observed in PD (0.8422 TPM ± 0.09130) as compared to 0.7171 TPM (±0.1677) in healthy controls. When separated by sex, the highest mean expression was observed in healthy control males (0.9029 ± 0.2404) and the lowest was observed in healthy control females (0.3366 ± 0.1034). Results of ADAR1, 2, and 3 TPM-based expression analyses can be seen in Figure 4 (a-f ).
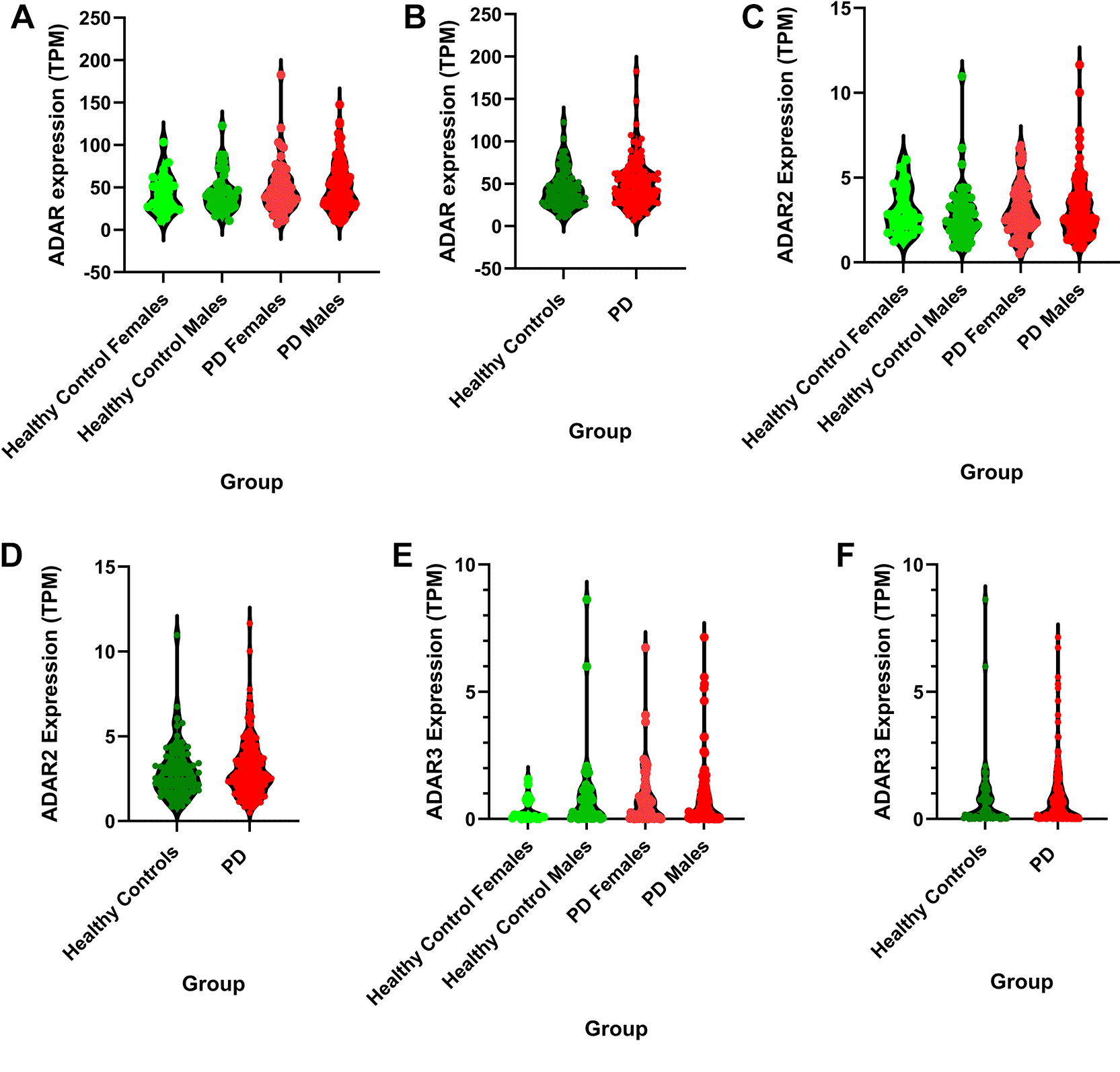
a) The highest mean expression of ADAR was observed in PD males and the lowest expression was observed in healthy control females with no significance observed between groups (Kruskal-Wallis, p = 0.6723). The highest range of ADAR expression between samples within a sample group was observed in PD females (range = 175.8 TPM) and the lowest range of ADAR expression was observed in healthy control females (range = 92.63 TPM).
b) When male and female samples are combined, the highest expression of ADAR was observed in PD samples with the lowest mean expression observed in healthy control samples with no significance observed between groups (Mann Whitney, p = 0.1764). The highest range of ADAR expression was observed in PD (range = 175.8 TPM) followed by healthy controls (range = 112 TPM).
c) The highest mean expression of ADAR2 was observed in healthy females with no significance observed between groups (Kruskal-Wallis, p = 0.6377).
d) When male and female samples are combined, the highest expression of ADAR2 was observed in PD samples with the lowest expression observed in healthy control samples with no significance observed between groups (Mann Whitney, p = 0.4750).
e) The highest mean expression of ADAR3 was observed in healthy males and the lowest expression was observed in healthy males with no significance observed between groups (Kruskal-Wallis, p = 0.2609).
f ) When male and female samples are combined, the highest expression of ADAR3 was observed in PD samples with the lowest expression observed in healthy control samples with no significance observed between groups (Mann Whitney, p = 0.4125).
Differential expression of ADAR isoforms, p110 and p150, were also compared between groups showing no significant difference in expression (unpaired t-test, p > 0.05). ADAR p150, induced by IFN, is primarily located in the cytoplasm while p110 is constitutively expressed and localizes in the nucleus (Li et al., 2022b). When differential expression of ADAR p110 (ENST00000368471) was compared between healthy controls and PD, there was no significant difference in expression with p values of 0.066 (unpaired t-test). Likewise, no significant differences in expression were observed for ADAR p150 (ENST00000368474) for comparisons between the major groups (healthy controls vs PD; p = 0.48). Results are shown in Figure 5 (a,b).
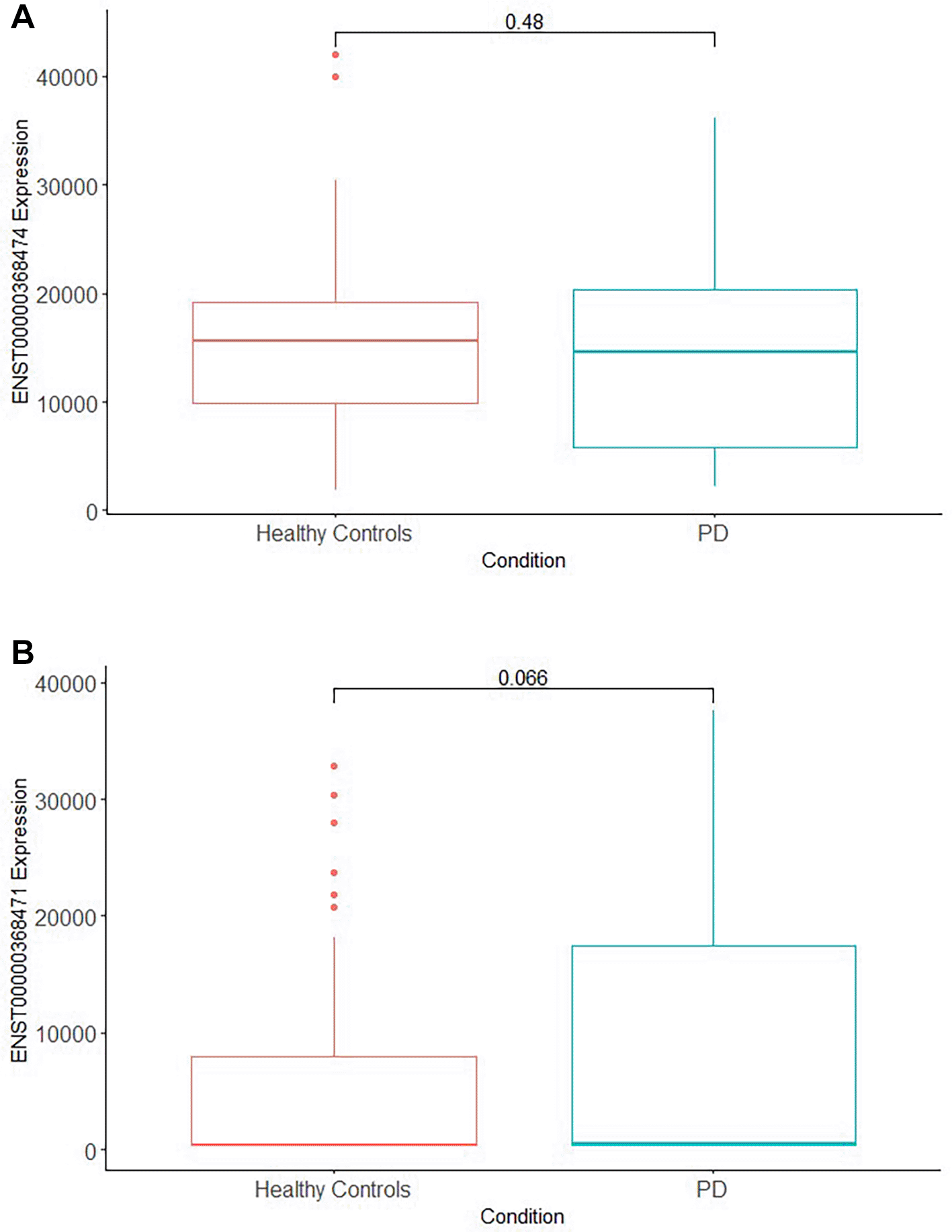
a) Interferon-inducible ADAR isoform p150 (ENST00000368474) is not expressed with significant difference between PD and healthy controls (unpaired t-test, p = 0.48).
b) ADARp110 (ENST00000368471) expression (right) is not significantly different between the two groups (unpaired t-test, p = 0.066).
Five genes were differentially expressed when comparing PD and healthy controls. ENSG00000243695 (RP11-290L7.3), a TGF Beta-Inducible Nuclear Protein 1 Pseudogene (log2 Fold Change = -1.31, padj = 0.03), ENSG00000265037 (MIR4707) (log2 Fold Change = -2.3, padj = 1.39 × 10−8) a microRNA involved in post-transcriptional gene regulation, ENSG00000108825 (PTGES3L-AARSD1) (log2 Fold Change = -2.4, padj = 1.04 × 10-7), a protein coding gene affiliated with Distal Spinal Muscular Atrophy (O’Leary et al., 2016), and ENSG00000200983 (SNORA1) (log2 Fold Change = -1.7, padj = 6.25 × 10−5), a small nucleolar RNA, were downregulated in controls. No Reactome pathways were identified as over-represented when analyzing these four genes. Only 1 gene from the NCBI PD gene list was differentially expressed in this analysis with ENSG00000258761 (RP11-24J19.1), an RNA gene affiliated with lncRNA with its transcript antisense to SLC03A1 which enables sodium-independent transmembrane transporter activity, was observed to be upregulated (log2 Fold Change = 1.1, padj = 0.04) in healthy controls.
Six hundred and nine genes were observed to be differentially expressed between PD males and PD females with 55 genes upregulated (including 1 NCBI PD gene) and 554 genes downregulated (including 6 NCBI PD genes) in PD females. One Reactome over-representation pathway met the filtering criteria (FDR < 0.05 and ≥10% entities in pathway) when considering the upregulated 55 genes, namely, HDMs demethylate histones. Of those 55 genes, Xist (ENSG00000229807), is the lone differentially expressed gene from the NCBI PD gene list (log2 Fold Change = 8.90, padj = 1.97 × 10-72). Xist, coding for a lncRNA, is important for X inactivation in mammalian females with higher serum levels observed in both males and female PD patients and those levels associated with PD stage (Zhang et al., 2022). Xist has been suggested to play a role in the male bias in some disease processes, including neurodegenerative disorders like PD (Li et al., 2022a).
Reactome overrepresentations analysis of the 554 downregulated genes revealed no pathways that met the filtering criteria (FDR < 0.05, ≥10% entities in pathway). Six downregulated genes were included on the NCBI PD gene list. When the 6 PD genes are considered for pathway over-representation, Dopamine Receptors and Chylomicron clearance are highlighted with FDR <0.05 and ≥10% entities in pathway. As a sex-determining gene located on the Y chromosome, as expected, SRY gene is observed as downregulated (log2 Fold Change = -4.94, padj = 3.31 × 10−25) in females. Noteworthy, dysregulation of the SRY gene has been suggested to play a role in PD pathology with a recent study of rat brains and human neuroblastoma cell lines demonstrating persistent upregulation of SRY and that SRY inhibition may be a strategy for mitigating male PD progression (Lee et al., 2019).
NRXN2, encoding cell adhesion molecules and receptors in vertebrate nervous systems, is also downregulated (log2 Fold Change = -0.93, padj = 0.008) in PD females. In a recent study in cell cultures, overexpression of NRXN2 led to elevated levels of proteins associated with AD, ALS, and PD (Cuttler et al., 2022). MMP16, also a downregulated gene identified in this comparison (log2 Fold Change = -1.95, padj = 0.02), is a matrix metalloproteinase tasked with the breakdown of the extracellular matrix during embryonic development and tissue remodeling but has also been related to inflammation and metastasis (Kapoor et al., 2016; Wang & Khalil, 2018). Additionally, a variant in MMP16, rs60298754, was deemed significant in a GWAS study of variants related to PD (Nalls et al., 2014). GRIN2C, also downregulated (log2 Fold Change = -0.78, padj = 0.005), encodes a subunit of N-methyl-D-aspartate (NMDA) receptor, a subtype of glutamate receptor in the CNS, and is related to memory, synaptic development and learning (O’Leary et al., 2016). Pozdyshev et al. (2021), in a recent PPMI brain sample study, identified differentially edited sites of which over half were ionotropic glutamate receptors. Other subunits of NMDA receptors such as GRIN1 and GRIN2D have been observed with elevated expression in PD patients compared to controls in a small PD differential expression study (Liu et al., 2016). Zhang et al. (2016) suggested that hyperactivity of NMDA receptors can lead to excitotoxicity and may play a role in neurodegenerative disorders like AD and PD. DRD4, is a subtype of the dopamine G-protein coupled receptor and is used as a target for medications used to treat schizophrenia and Parkinson’s Disease (O’Leary et al., 2016) and was observed as differentially expressed in this study (log2 Fold Change = -0.97, padj = 0.002). Additionally, variants in DRD4 have been identified as a risk factor for PD diagnosis in a sample of patients from Eastern India (Dipanwita et al., 2022) and further, DRD4 has been connected to the development of neuropsychiatric disorders such as attention deficit disorder and schizophrenia (Ptáček et al., 2011). APOE, or Apolipoprotein E, functions to transport lipids in the plasma associating with chylomicrons to assist with their clearance from the serum (Sehayek & Eisenberg, 1991) and is observed to be differentially expressed in this analysis (log2 Fold Change = -1.2, padj=0.04). APOE ε4 allele is a risk factor for sporadic Alzheimer’s (van der Kant et al., 2020) with recent data suggesting that APOE ε4 plays a role in alpha-synuclein pathology (Davis et al., 2020; Zhao et al., 2020) and current evidence inferring that the variant is associated with cognitive decline in PD (Gomperts et al., 2013; Monsell et al., 2014).
RNA editing is a phenomenon that adds a multidimensional dynamic regulatory aspect to gene expression, and specifically, ADAR editing is a nuanced spatiotemporally regulated (Huntley et al., 2016; Piontkivska et al., 2019) process that varies by individual, developmental stage, and possibly other unknown factors (Nishikura, 2016; Tsivion-Visbord et al., 2020). Because ADAR editing is non-linear relative to the expression of ADARs, we cannot expect that patterns of ADAR editing will closely imitate patterns of ADAR expression; however, we do observe that ADAR expression and the numbers of editing events putatively attributed to ADAR follow similar patterns, although without significance, and that editing may be dysregulated in diseased samples.
Although no statistical significance (Mann Whitney, p > 0.05) was observed between groups when considering the highest mean number of ADAR editing events per sample, the general trend shows a higher proportion of edits in PD samples compared to healthy controls and a higher proportion of edits in PD males compared to PD females mirroring our findings in a previous small cohort study (Mercer et al., 2023); however, the trend of higher editing proportions in males is reversed when comparing healthy control males and females.
While the findings that PD samples contain the highest number of putative editing events is perhaps not surprising, given the relationship between ADARp150 isoform, its IFN induced expression, and the inflammatory aspect of PD physiology, there was no significant difference observed between comparison groups when ADAR expression was considered, although ADAR1 expression was highest in PD samples when sexes are considered separately, and when combined (Mann Whitney, p > 0.05). ADAR2 and 3 expression was also highest in PD samples depending on whether sexes are combined. Other studies have observed decreased editing frequency (Wu et al., 2023) and significant differences in ADAR expression (Li et al., 2024) in PPMI PD blood; however, these studies did not compare PD males to PD females in their analyses and included samples regardless of age, whereas we chose samples from individuals 65 years or older.
SnpEff analysis of putative functional consequences of ADAR editing outcomes reveals significant differences when comparing the number of high and moderate impact NMD edits genome-wide, and in PD genes when comparing healthy controls to PD with the higher proportion of NMD edits observed in PD. When male and female healthy controls are compared within a group, significant differences in the number of high and moderate impact edits genome-wide and high and moderate impact protein coding edits are observed with a greater proportion of those edits occurring in healthy males in both cases. In PD, significance is observed when comparing the number of high and moderate impact NMD edits in PD genes although NMD edits are elevated in PD females and there are no NMD edits that met our criteria in PD genes in PD males. High and moderate impact edits in PD genes associated with NMD were significantly elevated in PD females compared to healthy control females. Where significant differences were observed in within-group comparisons, male samples showed a greater proportion of the edits than females except in NMD edits in PD genes where PD females had more edits compared to PD males suggesting that the manner in which transcripts are edited may differ between the sexes.
Interestingly, the high and moderate NMD edits in at least 25% of PD females were all associated with gene SLC11A2, marking a further distinction between editing patterns in PD females compared to other subgroups. SLC11A2, also known as DMT1 (Clingman & Ryder, 2013), is a transmembrane transporter involved in iron uptake, has been observed to be required for hemoglobin production during erythrocyte development (Gunshin et al., 2005) and imperative to iron influx (Liu et al., 2025). The dysregulation of iron homeostasis has been linked to PD in some studies (Jiang et al., 2017; Matak et al., 2016; Salazar et al., 2008; Santiago & Potashkin, 2017), with Lee et al. (2010) observing an increase in iron in the dopaminergic neurons of PD patients. Abnormal accumulation of iron was also reported in HD (Dexter et al., 1991), with altered iron metabolism already present during early stages of HD (Agrawal et al., 2018; Rouault, 2013; Van Bergen et al., 2016), and microstructural disruptions preceding the disease onset by at least two decades (Johnson et al., 2021). In addition to PD, SLC11A2 has been implicated in other neurodegenerative diseases, with Robertson et al. (2024) observing sex differences in knockdown mice in a murine Alzheimer model, and Blasco et al. (2011) observing associations between a polymorphism in SLC11A2 and disease duration in ALS patients. While the precise role of iron transport in neurodegeneration remains unclear, studies have associated irregularities associated with iron transport to ferroptosis, which has recently been identified as a form of cell death distinct from the other well-known processes such as apoptosis and autophagy (Dixon et al., 2012). Briefly, it is believed that polyunsaturated fatty acids associated with lipid membranes are transformed to lipid peroxides by ROS accumulation which in turn, when in the presence of divalent iron, causes the further accumulation of lipid related ROS species resulting in ferroptosis (Mi et al., 2019). A ferroptosis regulator, Gpx4, has been implicated in AD (Joshi et al., 2015), PD (Cardoso et al., 2017; Shahid et al., 2025; Bellinger et al., 2011), and HD (Sun et al., 2022). Additionally, Ferrostatin-1 is an antioxidant that traps peroxyl radicals, and thus serves as a potent inhibitor of ferroptosis while rescuing nerve cells as a neuroprotectant against Huntington’s disease and preventing neurotoxicity from excess glutamate in cell models (Skouta et al., 2014). As SLC11A2 expression and iron levels increase in the brain with age (Lee et al., 2023) and elevated iron levels in the brain have been associated with PD (Martin-Bastida et al., 2021), AD (Ward et al., 2022), and HD (Muller & Leavitt, 2014; Berg & Youdim, 2006; Bartzokis et al., 2007), it is possible that changes in iron transport associated with NMD-related ADAR editing plays a role in PD physiology.
In our previous analysis of ADAR editing in the skeletal muscle of PD patients, a significant difference in the number of high or moderate impact edits resulting in NMD was observed (Chi-square, p = 0.0001) when comparing healthy age-matched males (n = 9) to a small subset of PD male patients (n = 4) who participated in exercise training, although these results were obtained from a small sample (Mercer et al., 2023). In our current analysis, we also observe significant differences in NMD-associated edits between groups although exercise training was not a factor in this study. This editing pattern suggests differences in the outcomes of ADAR editing particularly associated with NMD when comparing healthy controls to diseased states and between males and females. NMD is an important process in detecting deleterious nonsense sequences in mRNA transcripts and eliminating them from the cell with their regulation serving an important role in neurodevelopment, neural differentiation, and neural maturation (Lee et al., 2021). RNA misprocessing, which includes dysregulation of NMD and alternative splicing, plays an important role in other neurodegenerative diseases, including ALS and frontotemporal dementia (Abramzon et al., 2020; Cook & Petrucelli, 2019; Howe & Patani, 2023; Lee et al., 2021), and HD (Ayyildiz et al., 2023; Lin et al., 2016; Nguyen et al., 2025). The role of ADAR editing in NMD that may contribute to PD pathophysiology should be further explored with an in-depth look at the specific genes affected, particularly SLC11A2; however, that remains out of the scope of this study.
Certainly, the genes identified as differentially expressed between PD and healthy controls, particularly those suggested to play a role in PD pathology, warrant further analysis for editing patterns. The comparison of expression in PD genes between PD males and PD females, particularly concerning transcripts of DRD4, GRIN2C, MMP16, NRXN2, and APOE, should be further scrutinized for altered editing patterns. Several of the Reactome (Fabregat et al., 2017) pathway analyses of differentially expressed genes highlighted overrepresented pathways that relate to NMD, which parallels our findings of ADAR editing associated with NMD disproportionately between comparison groups, altogether suggesting that the role of NMD and NMD-related pathways in PD pathology should be further investigated.
PPMI data availability (Marek et al., 2011) has stimulated numerous studies of PD etiology, including imaging (Cousineau et al., 2017; Lee et al., 2021; Solana-Lavalle & Rosas-Romero, 2021; Yang et al., 2022), cerebrospinal fluid (CSF) (Yang et al., 2022; Huh et al., 2021; Mollenhauer et al., 2019; Stewart et al., 2019), dopamine transporter single photon emission tomography (DAT SPECT) (Kim et al., 2018; Tagare et al., 2017), motor symptoms (Aleksovski et al., 2018; Kanagaraj et al., 2021; Vavougios et al., 2018), and cognitive impairment (Caspell-Garcia et al., 2017; Jones et al., 2018); however, few studies have included ADAR editing in their analyses of PD.
Li et al. (2024) observed 17 A-to-G editing sites in NCOR1, BST1, and KANSL1 genes with potential risk associations for PD in a study of 380 PD and 178 healthy controls samples from PPMI. Additionally, alterations in ADAR editing at 57 sites correlated with the progression of cognitive symptoms in PD patient (Li et al., 2024). In another study of PPMI whole blood transcriptomes, 72 ADAR editing events were observed that affected miRNA binding, 8 of which may alter the expression of many other gene (Wu et al., 2023). Editing within seed regions of miRNA transcripts can potentially change editing targets and cause further dysregulation of gene expression (Wu et al., 2023). ADAR editing has also been associated with AD and PD in a study of blood and brain samples from both AD and PPMI PD patients identifying 198 loci in protein coding regions, of which 35 are potentially related to neurodegeneration (Wu et al., 2023). Our study of PPMI whole blood transcriptomes also notes differences in ADAR editing when comparing healthy controls to diseased samples when focusing on the downstream consequences of editing events and further distinguishes between editing patterns between PD males and PD females. Additionally, in our focus on editing impacts, we note that edits associated with NMD may be of particular interest in PD etiology, especially concerning the sex differences associated with PD phenotypes (Lawton et al., 2018; Lawton et al., 2015; Sandor et al., 2022).
The imbalance of male and female samples in our comparison groups is a limitation of this study as the observed trends may be influenced more heavily by the number of male samples when the sexes are combined. In addition, inferences of the downstream consequences of ADAR edits as analyzed by SnpEff may be underestimated. Lastly, we did not attempt to separate our PD samples by suspected “axis” or PD phenotype (Lawton et al. 2018; Sandor et al., 2022), which could potentially illuminate differences in ADAR editing between the groups.
All data was de-identified by Parkinson’s Progression Markers Initiative prior to download and therefore affords no risk of confidentiality to subjects. This study was reviewed by the Institutional Review Board of Kent State University and the University of Mount Union and qualified under exemption 4.
Extended Data can be found in the Zenodo repository: Variations in ADAR editing of nonsense-mediated decay targets in PD males and females. https://doi.org/10.5281/zenodo.16781183 (Mercer et al., 2025)
This project contains the following extended data:
Supplementary File 1: 901 National Centre for Bioinformatics Information (NCBI) Parkinson’s (PD) genes and 7311 transcripts
Supplementary Table 1: The total number of A-to-G and T-to-C edits with no dbSNP annotation (Sherry et al., 2001) were compared between groups with no significance observed (Mann-Whitney, p = > 0.05).
Supplementary Table 2: Comparisons made between sample groups. When editing patterns are compared between healthy controls and Parkinson’s (PD) samples, the effects of PD are compared. When sex is indicated, the effects of either PD are being analysed between males and females.
Supplementary Table 3: High or Moderate Impact Edits Genome-wide.
Supplementary Table 4: High or Moderate Impact Edits in Parkinson’s (PD) genes.
Supplementary Table 5: High or Moderate Impact Protein Coding Edits Genome-Wide.
Supplementary Table 6: High or Moderate Impact Edits associated with nonsense-mediated decay (NMD) genome-wide.
Supplementary Table 7: High or Moderate Impact Protein Coding Edits in Parkinson’s (PD) genes.
Data is available under the terms of the Creative Commons Attribution 4.0 International.
Original RNA-seq data is freely available upon request from the Parkinson’s Progression Markers Initiative (PPMI), https://www.ppmi-info.org/access-data-specimens/download-data (Marek et al., 2011). A pre-print of a previous version of this manuscript can be found at Doi: https://doi.org/10.1101/2024.05.17.594716 (Mercer et al., 2024).
Zenodo repository: Variations in ADAR editing of nonsense-mediated decay targets in PD males and females. https://doi.org/10.5281/zenodo.16781183 (Mercer et al., 2025)
This project contains the following underlying data:
Figure 1. The NCBI PD gene list (downloaded November 4, 2023) was overrepresented in 5 Reactome pathways (FDR < 0.05).
Figure 2. Variations in high and moderate impact NMD edits in PD genes and genome-wide by group.
Figure 3. Variations in high and moderate impact NMD edits in PD genes and genome-wide by sex.
Figure 4. ADAR expression varied between comparison groups with no significance (Kruskal-Wallis, p=>0.05, Mann Whitney, p=>0.05).
Figure 5. ADAR isoform expression is differentially expressed without significance (unpaired t-test, p=>0.05) when comparing PD samples to healthy controls.
Data is available under the terms of the Creative Commons Attribution 4.0 International.
We would like to thank the participants, and the researchers involved in the Parkinson’s Progression Markers Initiative (PPMI) for sharing their data to enable this study. PPMI – a public-private partnership – is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including 4D Pharma, Abbvie, AcureX, Allergan, Amathus Therapeutics, Aligning Science Across Parkinson’s, AskBio, Avid Radiopharmaceuticals, BIAL, BioArctic, Biogen, Biohaven, BioLegend, BlueRock Therapeutics, Bristol-Myers Squibb, Calico Labs, Capsida Biotherapeutics, Celgene, Cerevel Therapeutics, Coave Therapeutics, DaCapo Brainscience, Denali, Edmond J. Safra Foundation, Eli Lilly, Gain Therapeutics, GE HealthCare, Genentech, GSK, Golub Capital, Handl Therapeutics, Insitro, Jazz Pharmaceuticals, Johnson & Johnson Innovative Medicine, Lundbeck, Merck, Meso Scale Discovery, Mission Therapeutics, Neurocrine Biosciences, Neuron23, Neuropore, Pfizer, Piramal, Prevail Therapeutics, Roche, Sanofi, Servier, Sun Pharma Advanced Research Company, Takeda, Teva, UCB, Vanqua Bio, Verily, Voyager Therapeutics, the Weston Family Foundation and Yumanity Therapeutics.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)