Keywords
16S rDNA, anti-cancer, apoptosis, breast cancer, gene expression, gut microbiome, Thai traditional formulary medicine, xenografted mouse models
This article is included in the Cell & Molecular Biology gateway.
This article is included in the Oncology gateway.
There is a limited range of treatment options for triple-negative breast cancer (TNBC), a cancer known for its aggressiveness; therefore, innovative alternative therapies are required. A link between TNBC progression and bacterial dysbiosis in the gut microenvironment has been demonstrated. Thai traditional formulary medicine (TTFM), comprising bioactive natural products and medicinal plants, exhibits anti-cancer properties. However, the effectiveness of TTFM against breast cancer (BC) has not been clarified. The present study aimed to identify the secondary metabolites of TTFM and investigate its effects on BC both in vitro and in vivo.
The metabolite profiles of TTFM extracts were investigated using LC-MS/MS analysis. The anti-cancer activities were examined using a cell viability assay. The effects on the apoptosis pathway and gene expression profiles were also investigated using an apoptosis assay and RNA sequencing analysis. Next, TTFM extracts were examined in 4T1 xenografted mouse models. The gut microbiota profiles and tumor gene expression were investigated by 16S rDNA sequencing and nanoString, respectively.
LC-MS/MS identified 302 compounds in the TTFM extract. In breast cancer cells, TTFM inhibited cell proliferation and induced apoptosis. Additionally, TTFM treatment partially restored the gut microbiota balance, increasing the abundance of Butyrivibrio hungatei and reducing Clostridium saccharolyticum. NanoString analysis showed that TTFM modulated immune responses by upregulating the Pias1 gene and downregulating pro-inflammatory cytokines (IL-1r2, IL-1β, IL-2) and cancer-related genes (Ccno, Nkd1).
This study highlights the anti-cancer potential of TTFM extracts, suggesting its future use in the development of novel therapeutic strategies for breast cancer.
16S rDNA, anti-cancer, apoptosis, breast cancer, gene expression, gut microbiome, Thai traditional formulary medicine, xenografted mouse models
Breast cancer (BC) is a major global health issue and a leading cause of morbidity among women worldwide, with incidence rising annually.1 According to a recent World Health Organization report, >20,000 new BC cases, accounting for 11.6% of all cancer cases, were reported in 2020.2 BC can be classified into several types, among which triple-negative BC (TNBC) tends to be more aggressive and progresses more rapidly than any other subtypes.3 TNBC treatment is challenging due to the lack of identified drug targets, with current treatments including surgery, radiation, chemotherapy, targeted therapy and immunotherapy.3 The effectiveness of these treatments is restricted by their adverse side effects, high cost and cancer recurrence. Thus, it is necessary to develop new complementary therapies.
Recent studies indicate that dysbiosis, an imbalance in microbial communities within the breast tissue and gut, is linked to the progression of breast cancer by promoting the growth of harmful microorganisms and reducing beneficial ones.4,5 Typically, the intestinal mucosal barriers are crucial to the host-microbe symbiosis. If these barriers are destroyed, microbes can invade the host circulatory system, provoking immune responses and producing proinflammatory that promote inflammation in the body or immunosuppressive microenvironments.5,6 Invading gut microbiota can upregulate Toll-like receptors (TLRs), particularly TLR4, thereby activating the NF-κB signaling pathway and increasing the release of pro-inflammatory cytokines (IL-6, IL-12, IL-17, IL-18, and TNF-α) that are essential for inflammation-related cancers. Dysbiosis disrupts the balance of the gut mucosa, weakening its barriers and allowing harmful components, such as lipopolysaccharides (LPS), to trigger TLR activation. This leads to chronic immune changes, including systemic inflammation and altered immune responses, which contribute to conditions such as inflammatory bowel disease, metabolic disorders, and tumor-promoting environments.4,5 Thus, maintaining gut microbiota equilibrium is considered a novel therapeutic strategy for BC.
Thai traditional formulary medicine (TTFM), a valuable legacy of Thai ancestral knowledge, has attracted considerable interest from Thai patients as an alternative therapy for digestive conditions and cancer7,8 due to its anti-fungal, anti-bacterial, anti-inflammatory and anti-cancer properties.9,10 The TTFM recipe utilized in the current investigation ( Table 1) was taken from the traditional Thai medicine, Atisaravak scripture.
| Scientific name | Common name | Part of used | Ratio (%) | Anti-cancer (Ref.) |
|---|---|---|---|---|
| Acacia catechu (L.f.) Willd | Catechu | Wood | 10 | Breast cancer11 |
| Aquilaria spp. | Agarwood | Stem bark and wood | 10 | Breast cancer12 |
| Dracaena loureiroi Gagnep | Dragon's blood | Wood | 10 | N/A |
| Myristica fragrans Houtt | Nutmeg tree | Seed | 10 | Colon cancer13 |
| Piper nigrum L | Black pepper | Fruit | 10 | Breast cancer14 |
| Quercus infectoria G.Olivier | Nutgall | Gall | 10 | Cervical cancer15 |
| Santalum album Linn | Sandalwood | Wood | 10 | Breast cancer16 |
| Styrax tonkinensis | Siam benzoin | Resin | 10 | Leukemic cancer17 |
| Uncaria gambir (Hunter) Roxb | Gambier | Leaf and wood | 10 | N/A |
| Zingiber officinale Roscoe | Ginger | Rhizome | 10 | Liver cancer18 |
Although studies on medicinal plants and traditional formularies of medicine on the gut microbiome are limited, recent studies suggest that bioactive secondary metabolites in these plants can restore the gut microbiome balance.19 For example, Wang et al20 revealed that ginger (Z. officinale) supplementation altered the gut microbiota composition, increasing the prevalence of Bifidobacterium genus and short-chain fatty acid (SCFA)-producing bacteria (Alloprevotella and Allobaculum), along with SCFA concentrations in mouse feces. Liu et al21 demonstrated that Traditional Chinese medicines, comprising Artemisia annua L., Gardenia jasminoides Ellis and Rheum Palmatum L., could modulate the gut microbiome composition. Moreover, diets high in micronutrients such as fiber, minerals, vitamins and phytochemicals have been associated with a lower risk of BC and other cancers.22,23 Together, these findings underscore the potential of dietary strategies and herbal supplements in promoting gut health and possibly reducing the risk of cancer. Additionally, micronutrient-rich diets are associated with a lower risk of breast cancer and other cancers, highlighting the potential of dietary strategies and herbal supplements in promoting gut health and reducing cancer risk. Plenty of phytochemicals derived from some herbs in the TTFM recipe have been reported to have the ability to fight various types of cancer, including BC in vitro.24,25 However, the effectiveness of TTFM against BC and its underlying mechanisms have not been thoroughly studied. The present study investigates the secondary metabolites from TTFM extracts and their effects on BC cells in vitro, including cytotoxicity and programmed cell death, as well as their impact on gut microbiota and tumor gene expression profiles in vivo.
The dried natural products and plant materials detailed in Table 1 were bought from a Thai traditional medicine shop (Chao-Krom-Poe Dispensary Pharmacy, Bangkok, Thailand). These materials, along with sulfur, were boiled in the supernatant derived from boiling 2.75 L of lac resin at 80-100°C for 30 min. The resulting mixture was then filtered through sterile voile fabric and centrifuged at 1,610 x g at 25°C for 20 min. The pellet was discarded, while the supernatants were lyophilized and kept at -20°C until use.
In accordance with previously described protocols, HPLC fractionation was employed prior to liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis. A total of 20 mg of dried TTFM extracts were incubated in 90% methanol at 25°C and shaken at 1,300 rpm for 20 min. Then, the mixture was centrifuged at 17,000 x g at 4°C for 10 min. Reversed-phase HPLC was performed using an Agilent 1200 HPLC device coupled with a 1260 Infinity II photodiode array detector (Agilent Technologies, CA, USA). The solvents for HPLC fractionation included ultrapure water (solvent A) and HPLC-grade acetonitrile (solvent B). The column used was graphitic porous carbon (Hypercarb; 100x2.1 mm; particle size, 3 μm; Thermo Fisher Scientific, MA, USA) maintained at 65±0.8°C. The mobile phase flow rate was 0.2 mL/min with specific gradient elution steps.26 Collected fractions were then analyzed by LC-MS/MS.
A Dionex Ultimate 3000 HPLC coupled with an Orbitrap Q Exactive Focus mass spectrometer (Thermo Fisher Scientific, MA, USA) was employed for untargeted metabolomics studies.26 The Heated Electrospray Ionization source settings included a sheath gas flow rate of 30 arbitrary units, auxiliary gas flow of 10 L/min, spray voltage of 3 kV, capillary temperature of 350°C, S-lens RF level of 60 and auxiliary gas heater temperature of 300°C. Polar metabolites were separated using an Acclaim™ Polar Advantage II column (250x3 mm; particle size, 3 μm; Thermo Fisher Scientific, MA, USA). The mobile phases were as previously described. The LC gradient included: 0-5 min, isocratic elution of 1% B; 5-40 min, gradient elution of 1.5% B/min; 40-47 min, isocratic elution of 100% B (column wash); 47-65 min, isocratic elution of 1% B (reconditioning of the HPLC column).
Differential peak identification was performed using MS-Dial software version 4.90,27 with raw files converted to ABF format via an ABF file converter. In MS-Dial, converted files were processed with default parameters, using public MSP-formatted libraries for both positive and negative ionization modes. MS/MS analysis conditions included a minimum peak height of 1,000 amplitude, m/z search tolerance of 0.01 Da, data acquisition in centroid mode and peak alignment filtering before feature removal based on blank information.
The mouse 4T1 cell line (ATCC®; cat. no. CRL-2539™), representing stage IV human BC, was cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 1 mM sodium pyruvate, 10 mM HEPES, 4,500 mg/L glucose, 1,500 mg/L sodium bicarbonate, and 1% antibiotic-antimycotic, containing 10,000 units/mL of penicillin, 10,000 μg/mL of streptomycin, and 25 μg/mL of Gibco Amphotericin B (Gibco; Thermo Fisher Scientific, MA, USA). The MDA-MB-231 BC cell line (gift from Ketchart laboratory; Chulalongkorn University) was cultured in DMEM with 10% heat-inactivated FBS and 1% antibiotic-antimycotic (Gibco; Thermo Fisher Scientific, MA, USA). The normal breast cell line EpH4-Ev (ATCC®; cat. no. CRL-30639™) was used as a control and cultured in DMEM with 10% Calf Bovine Serum (ATCC, VA, USA), 1.2 μg/mL puromycin dihydrochloride (Merck, Darmstadt, Germany), and 1% antibiotic-antimycotic (Gibco; Thermo Fisher Scientific, MA, USA). All cells were maintained at 37°C under 5% CO2.
A cell viability assay using MTT was performed on 4T1, MDA-MB-231 and EpH4-Ev cells. Cells at a total density of 1x104 were seeded in 96-well plates and incubated for 24 h. TTFM extracts were then applied at a concentration range of 0-400 μg/mL. After 5 days of incubation, cells were treated with MTT (Abcam, Cambridge, UK) for 3 h, and the resulting formazan crystals were dissolved in DMSO (Merck, Darmstadt, Germany). Absorbance was measured at 570 nm, and IC50 values were calculated by nonlinear regression (curve fit) using GraphPad Prism (version 9.3.0; Dotmatics).
A total of 1 × 105 cells were seeded in 24 well-microplates and incubated for 24 h, followed by treatment with TTFM extracts for 72 h. The mock cells were treated with PBS, while the positive control cells were treated with the apoptosis inducer, 50 μg of dihydrochloride hydrate (Merck, Darmstadt, Germany). After incubation, cells were harvested, washed with cold PBS and stained with a FITC Annexin V Apoptosis detection kit with propidium iodide (BioLegend, London, UK) for 15 min in the dark. Fluorescent intensity was evaluated using a BD™ LSR II flow cytometer, and data were analyzed with BD FACSDiva™ (version 6.1.3; BD Biosciences, NJ, USA).
Total RNA was extracted from TTFM-treated and -untreated cells using the RNeasy mini kit (Qiagen, Hilden, Germany). The concentration and integrity of RNA were assessed with Qubit RNA assay kits (Invitrogen; Thermo Fisher Scientific, MA, USA), and quality was confirmed using a Bioanalyzer (Agilent Technologies, CA, USA). Library preparation and RNA-Seq were performed commercially by Vishuo Biomedical Pte., Ltd., adhering to standard procedures. A total of 1 gm of total RNA was used for poly(A) mRNA isolation with oligo (dT) beads, followed by mRNA fragmentation and first- and second-strand cDNA synthesis. Adaptors were ligated to the double-stranded cDNA using T-A ligation, followed by size selection. Libraries were validated and quantified before multiplexing and sequencing with a NovaSeq 6000 SP Reagent Kit (version 1.5; Illumina, CA, USA) for paired-end (2 × 150) sequencing.
The RNA-seq data were processed to analyze differentially expressed genes (DEGs).28 Trimmed reads were aligned to the mouse reference genome using HISAT2 v.2.1.0,29 and transcript prevalence was quantified with Cufflinks v.2.2.1.30 Differentially expressed genes were examined using DESeq2 v.1.24.031 with a significance cut-off of p < 0.05. Gene Ontology (GO) and biological pathways were identified using GOSeq v1.34.132 and the Kyoto Encyclopedia of Genes and Genomes (KEGG).33
A total of 24 six-week-old female BALB/c mice (~18-20 g) purchased from Nomura Siam International, Bangkok, Thailand, were held humanely in pathogen-free conditions (temperature, 21 ± 1°C; humidity, 50 ± 20%; 12 h light/dark cycle) and acclimated for 1 week before the experiment. The study procedures were approved by the Institutional Animal Care and Use Committee of Chulalongkorn University Laboratory Animal Center (approval no. 2173024). All animal experiments were conducted in accordance with ARRIVE guidelines (https://arriveguidelines.org/arrive-guidelines ). The sample size was calculated using the n4Studies program.34 For anesthesia, mice were anesthetized using a mixture of oxygen and isoflurane, at a flow rate of 0.5 L/min and 2-3% isoflurane vaporization. A total of 12 mice were injected with 3 × 105 4T1 cells into the left mammary fat pad via subcutaneous injection under aseptic techniques.
After a total of 2 weeks post-transplantation, mice were randomized into four groups (n = 6 per group): i) Untreated control (NC); ii) untreated CLDX (MD); iii) non-tumor treated with TTFM; and iv) CLDX treated with TTFM. Mice in the NC and MD groups were treated with a 0.9% sodium chloride solution (normal saline, A.N.B. Laboratories, Bangkok, Thailand) administered via oral gavage three times a week. For TTFM treatment groups, the animal equivalent dose (AED) was determined based on body surface area, using a Km ratio of 12.3, resulting in a TTFM dosage of 205 mg/kg for mice. TTFM, typically 16.67 mg/kg (1,000 mg/60 kg/day) for human cancer treatment, was administered in non-pharmaceutical grade form, dissolved in normal saline, sterilized and given via oral gavage thrice weekly. Treatments followed the protocol outlined in Figure 4A. Fecal samples were collected at weeks 0 and 3, stored in NAPseq™ (Bioentist, Bangkok, Thailand) at -80°C. At the end of the experiment, mice were euthanized via CO2 inhalation. Tumor tissues were collected, rinsed with PBS, stored in DNA/RNA Shield™ (Zymo Research, CA, USA), snap-frozen in liquid nitrogen and kept at -80°C until use.
Total DNA was extracted from mice fecal samples using the ZymoBIOMICS DNA Kit (Zymo Research, CA, USA). The full-length 16S rDNA (V1-V9 regions) of bacteria was amplified using in-house methods for DNA library preparation.35 PCR included 0.25 μM primer (each), 0.2 mM dNTPs, 0.2 U/μL Phusion™ Plus DNA Polymerase (Thermo Fisher Scientific, MA, USA) and 10 ng DNA template. The thermal amplification involved initial denaturation at 98°C for 30 sec, followed by 25 cycles (98°C for 10 sec, 60°C for 10 sec, 72°C for 45 sec) and a final extension at 72°C for 5 min. Subsequently, PCR was performed on each amplicon to attach multiplexing Nanopore barcodes using the PCR Barcoding Expansion 1-96 kit (cat. no. EXP-PBC096; Oxford Nanopore Technologies, Oxford, UK). The thermocyclers were set to: Initial denaturation at 98°C for 30 sec, five cycles of amplification (98°C for 10 sec, 60°C for 10 sec, 72°C for 60 sec), and a final extension at 72°C for 5 min. Following agarose gel electrophoresis and purification with the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany), DNA libraries were quantified using the Qubit™ DNA HS assay kit (Invitrogen; Thermo Fisher Scientific, MA, USA). Barcoded DNA was pooled, purified with 0.5x Agencourt AMPure XP beads (Beckman Coulter, CA, USA), and subjected to adaptor ligation before final library construction with the Sequencing Ligation Kit (cat. no. SQK-LSK112; Oxford Nanopore Technologies, Oxford, UK). The final adaptor-ligated DNA library was loaded onto flow cell R10.4 (cat. no. FLO-MIN112; Oxford Nanopore Technologies, Oxford, UK) and sequenced on MinION Mk1C (Oxford Nanopore Technologies, Oxford, UK).
All data were analyzed based on a previous study.35 Briefly, the raw FAST5 data of full-length 16S sequences from nanopore sequencing were basecalled using the super-accuracy mode of guppy basecaller (version 6.1.2; Oxford Nanopore Technologies, Oxford, UK). Sequence quality was checked with MinIONQC. Demultiplexing and adaptor trimming were performed using Porechop (version 0.2.4; https://github.com/rrwick/Porechop). The demultiplexed filtered reads were subsequently processed by NanoCLUST36 for clustering, polishing and taxonomic identification, using the Ribosomal Database Project. The data were analyzed with the QIIME2 pipeline (version 2021.2)37 and visualized using MicrobiomeAnalyst (https://www.microbiomeanalyst.ca).
Total RNA was isolated from tumor tissue using the Qiagen RNeasy Kit (Qiagen, Hilden, Germany). Gene expression profiling was performed with the nCounter® Mouse PanCancer Pathways Panel (nanoString Technologies, WA, USA), assessing 750 cancer-related genes. RNAs (100 ng) were hybridized and processed per the manufacturer’s protocols. mRNA counts were analyzed using nSolver and normalized against housekeeping genes, with data visualized as a heatmap. Functional enrichment analysis was conducted with g:Profiler software38 and volcano plots were generated using GraphPad Prism (version 9.3.0; Dotmatics).
Metabolite profiling of TTFM extracts was conducted using MS-Dial, integrating mass accuracy, isotope ratios and retention-time prediction. After HPLC purification, the LC-MS/MS analysis identified 302 compounds. Positive ion mode (ESI+) enabled ionization of a larger spectrum of chemicals, while negative ion mode (ESI−) minimized background noise, detecting 270 and 32 compounds, respectively (refer to extended data-Supplementary Table 1 and 2). Notably, several identified metabolites, such as 6-methoxydihydrosanguinarine, astaxanthin, costunolide, kaempferol, hinokitiol, berberine, 1-methylnicotinamide, benzyl isothiocyanate, dexamethasone, vincamine, spironolactone, (9Z)-9-octadecenoic acid, hexanedioic acid, N,N-dimethylglycine, d-limonene, pyridoxine, thymol, ginkgolide A and azelaic acid, are recognized for their anti-cancer properties across diverse cancer types ( Table 2).
| Compound name | Pathway | Anti-cancer | Ref. |
|---|---|---|---|
| (9Z)-9-Octadecenoic acid |
| Liver cancer | 41 |
| 1-methyl nicotinamide |
| Breast cancer | 57 |
| 6-Methoxydihydro sanguinarine |
| Breast cancer | 42 |
| Astaxanthin |
| Breast cancer | 43 |
| Azelaic acid |
| Myeloid leukemia | 50 |
| Benzyl Isothiocyanate |
| Breast cancer | 58 |
| Berberine |
| Breast cancer | 51 |
| Costunolide |
| Breast cancer | 44 |
| D-limonene |
| Colorectal cancer | 45 |
| Dexamethasone |
| Lung cancer | 52 |
| Ginkgolide A |
| Ovarian cancer | 53 |
| Hexanedioic acid |
| Liver cancer | 46 |
| Hinokitiol |
| Breast cancer | 59 |
| Kaempferol |
| Breast cancer | 54 |
| N,N-Dimethylglycine |
| Liver cancer | 47 |
| Pyridoxine |
| Colorectal cancer | 55 |
| Spironolactone |
| Lung cancer | 56 |
| Thymol |
| Colorectal cancer | 48 |
| Vincamine |
| Lung cancer | 49 |
The impact of TTFM extracts on BC cell viability was assessed using an MTT assay. The BC cell lines 4T1 and MDA-MB-231, along with normal breast cells (EpH4-Ev), were treated with varying concentrations of TTFM extracts for 5 days. A dose-response relationship was observed in the 4T1 and MDA-MB-231 cells, with increased extract concentrations associating with decreased cell viability ( Figure 1B and C). By contrast, EpH4-Ev cells demonstrated a high tolerance to TTFM extracts ( Figure 1A). The IC50 values for TTFM extracts against 4T1, MDA-MB-231 and EpH4-Ev cells were determined to be 14.13, 20.59 and 117.30 μg/mL, respectively ( Figure 1D).
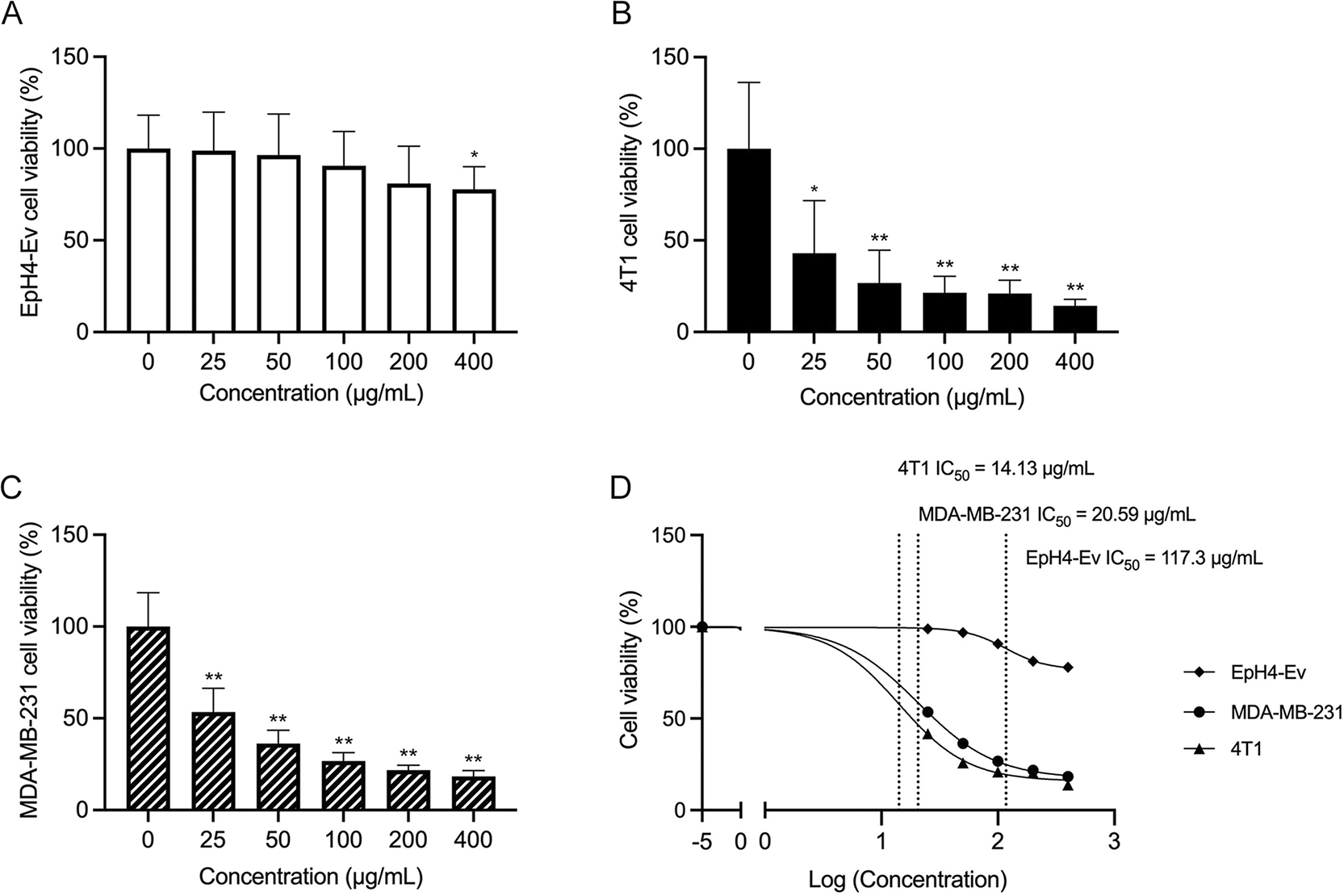
(A-C) Cell viability was assessed using the MTT assay and (D) the IC50 values for proliferation after 5 days of TTFM treatment are shown. Data are presented as mean ± standard deviation (n=3). * p < 0.01, ** p < 0.0001 (Unpaired t-test). n represents the number of independent experiments performed. TTFM, Thai traditional formulary medicine.
Results showed that TTFM extracts induced apoptosis in BC cells. In the NC group, 84.4% of 4T1 cells were alive, with 7.3% in late apoptosis, 6.8% in early apoptosis and 1.5% dead ( Figure 2A and H). Addition of the apoptosis inducer significantly increased early and late apoptotic cells by 1.98- and 4.7-fold, respectively (p < 0.0001; Figure 2B and H). Low TTFM concentrations, 25 and 50 μg/mL, increased early apoptosis by 1.6- and 1.7-fold, respectively (p < 0.001; Figure 2C-D and H). Higher concentrations, 200 and 400 μg/mL, raised early apoptosis by 2.7- and 6.4-fold, respectively (p < 0.0001; Figure 2E and F). Late apoptosis increased by 1.5-fold at 400 μg/mL (p < 0.0001; Figure 2G and H). TTFM also induced apoptosis in MDA-MB-231 cells with increasing concentrations (Supplementary Figure 1).
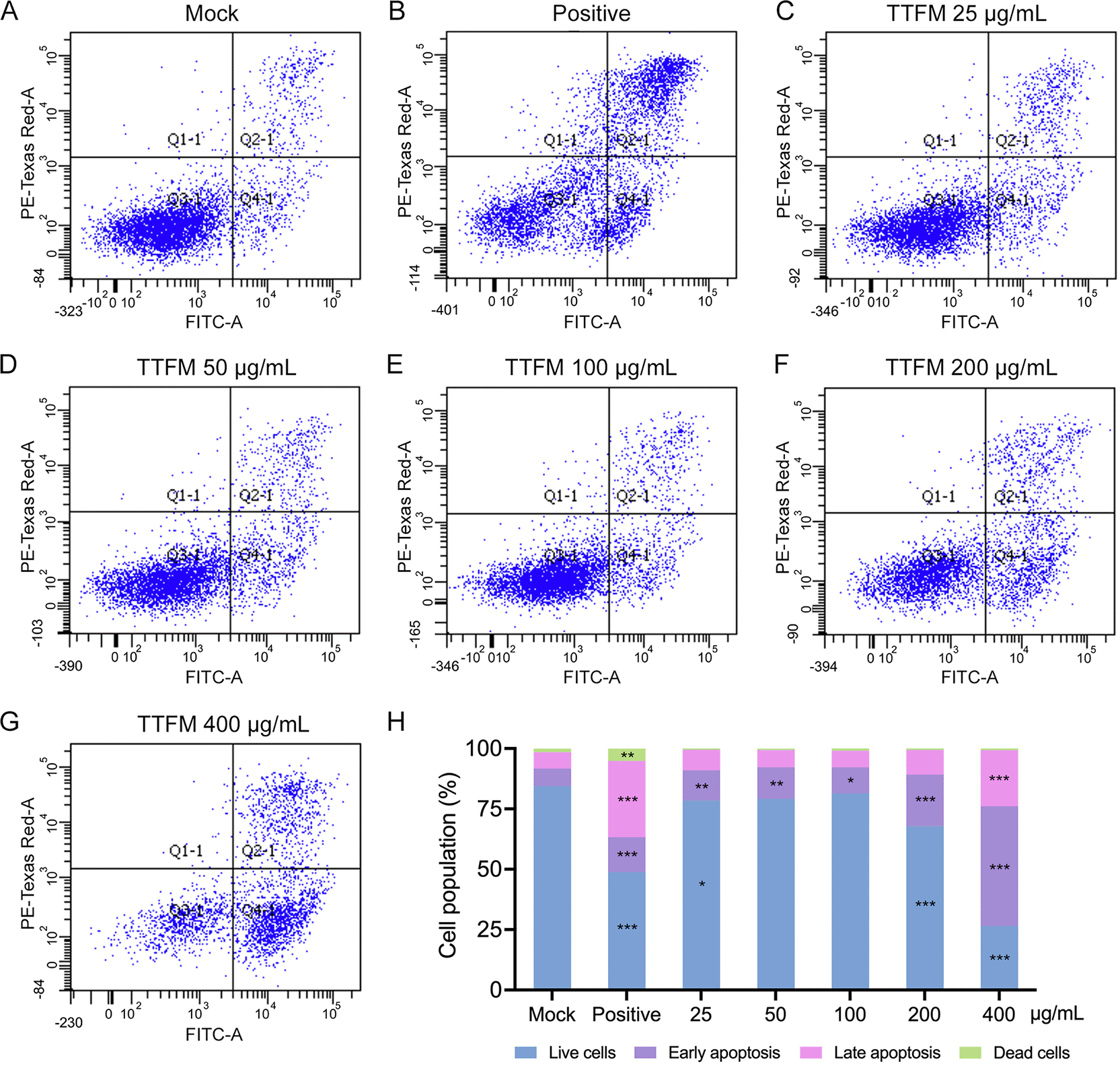
Apoptosis was analyzed after 72 hours of TTFM treatment at the following concentrations: (A) 0 μg/mL, (C) 25 μg/mL, (D) 50 μg/mL, (E) 100 μg/mL, (F) 200 μg/mL and (G) 400 μg/mL. (B) Positive control used 50 μg dihydrochloride hydrate. (H) Quantitative comparison of apoptosis rates. * p < 0.05, ** p < 0.001, *** p < 0.0001 indicate statistical significance differences when compared with mock (one-way ANOVA followed by Tukey’s post hoc test). TTFM, Thai traditional formulary medicine.
RNA-Seq analysis was conducted to elucidate the effects of TTFM on gene expression in BC cells. Cells were treated with TTFM extracts at a concentration of 25 μg/mL for 72 h, and total RNA was collected in triplicate. Sequencing identified 55,359 protein- and non-protein-coding genes in EpH4-Ev and 4T1 cells. TTFM treatment in EpH4-Ev cells resulted in the significant upregulation of 200 genes and downregulation of 66 genes, while in 4T1 cells, two genes were upregulated and eight genes were downregulated > 2-fold (|log2fold-change| > 1; p < 0.05) compared with the untreated control ( Figure 3A and B).
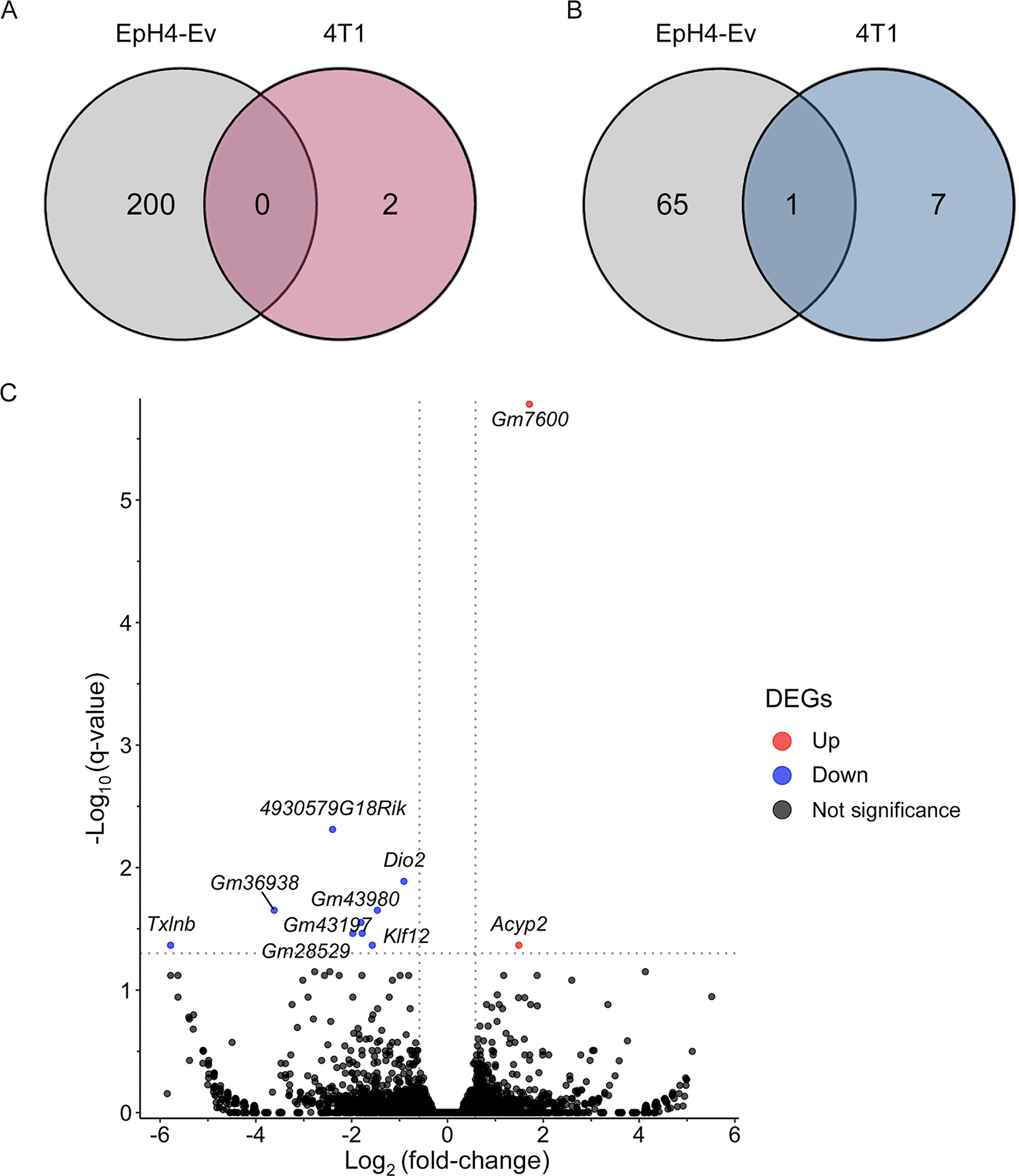
(A) Venn diagram of upregulated genes. (B) Venn diagram of downregulated genes. (C) The volcano plot illustrates the significantly upregulated and downregulated genes in 4T1 cells, generated using DESeq2 with a significance cut-off of p < 0.05. * p < 0.05, ** p < 0.01, *** p < 0.001, *** p < 0.0001 indicate statistical significance differences when compared with the 4T1-treated or untreated groups. TTFM, Thai traditional formulary medicine.
These findings indicate that TTFM regulates five known genes in 4T1 BC cells ( Figure 3C; refer to extended data - Supplementary Table 3). TTFM treatment increased the expression of Acyp2, while decreasing the expression of Klf12, Dtnbos, 4930579G18Rik and Txlnb. The significantly DEGs impacted by TTFM were categorized into four major biological processes based on KEGG pathway enrichment analysis, primarily metabolism. Notably, TTFM treatment significantly reduced the expression of Klf12 ( Figure 3C; refer to extended data Supplementary Table 3). On the other hand, the top 5 upregulated genes following TTFM treatment were Dclk1, Slc1a3, Cadm2, Anks1b, and Tmod2, while Slc15a3, 7SK, Mir221, Gbp10, and Slfn4 were downregulated in EpH4-EV cells (refer to extended data Supplementary Table 3).
After 8 weeks of treatment, TTFM had no significant effect on the body weight of normal mice compared with the NC group ( Figure 4B). After 3 weeks of treatment, xenografted mice in the TTFM group had higher body weight than those in the MD group (p = 0.0051; Figure 4C; refer to extended data Supplementary Table 4). TTFM treatment appeared to inhibit tumor growth in 4T1 xenograft mice ( Figure 4D and G; refer to extended data Supplementary Table 4). The tumor weight and volume in the TTFM group were lower than those in the MD group, but the differences were not statistically significant. At the end of the experiment, the tumor volume in the TTFM group was 800.98±195.74 mm3, compared with 1086.77±199.03 mm3 in the MD group ( Figure 4D, E and G). The tumor weight in the TTFM group was 0.95±0.2 g, while in the MD group it was 1.32±0.17 g ( Figure 4F).
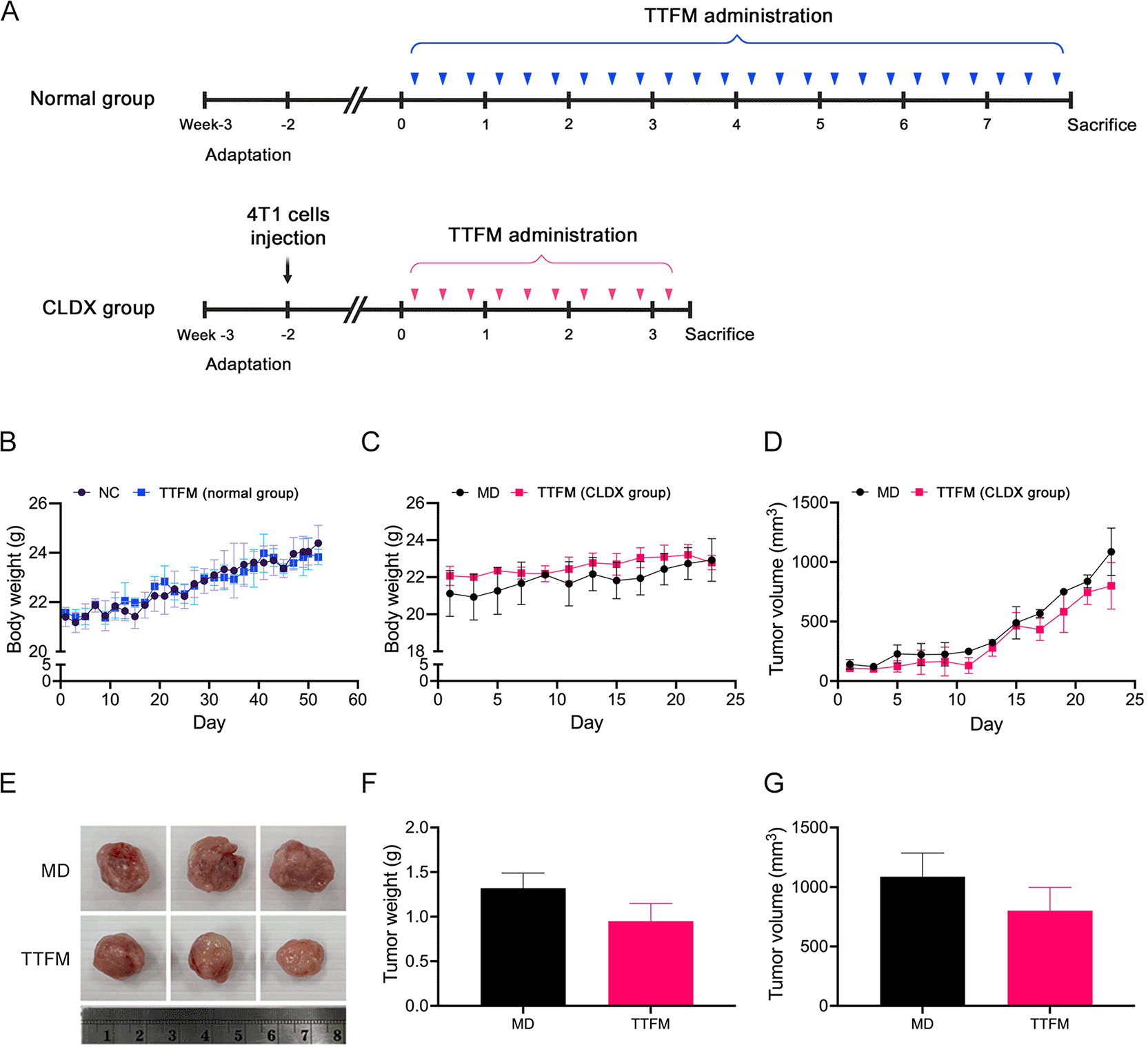
(A) Experimental design. (B) Body weight of normal group. (C) Body weight of CLDX group. (D) Tumor volume. (E) Tumor size, (F) weight and (G) volume at week 3. An unpaired t-test was used for statistical analysis. NC, normal control; MD, model; TTFM, Thai traditional formulary medicine; CLDX, cell line-derived xenograft.
Fecal samples from experimental mice were subjected to full-length 16S rDNA sequencing to investigate the impact of TTFM on gut microbiota diversity and composition. Data quality was validated by rarefaction analysis, confirming adequate sequencing depth (Supplementary Figure 2). α-diversity metrics such as Chao1 and Shannon indices were used to assess community diversity. A total of 2 weeks post-cancer cell transplantation, the CLDX group exhibited significantly higher Chao1 and Shannon indices compared with the normal group ( Figure 5A). However, Bray-Curtis dissimilarity-based PCoA plot analysis showed no significant group-level differences ( Figure 5B).
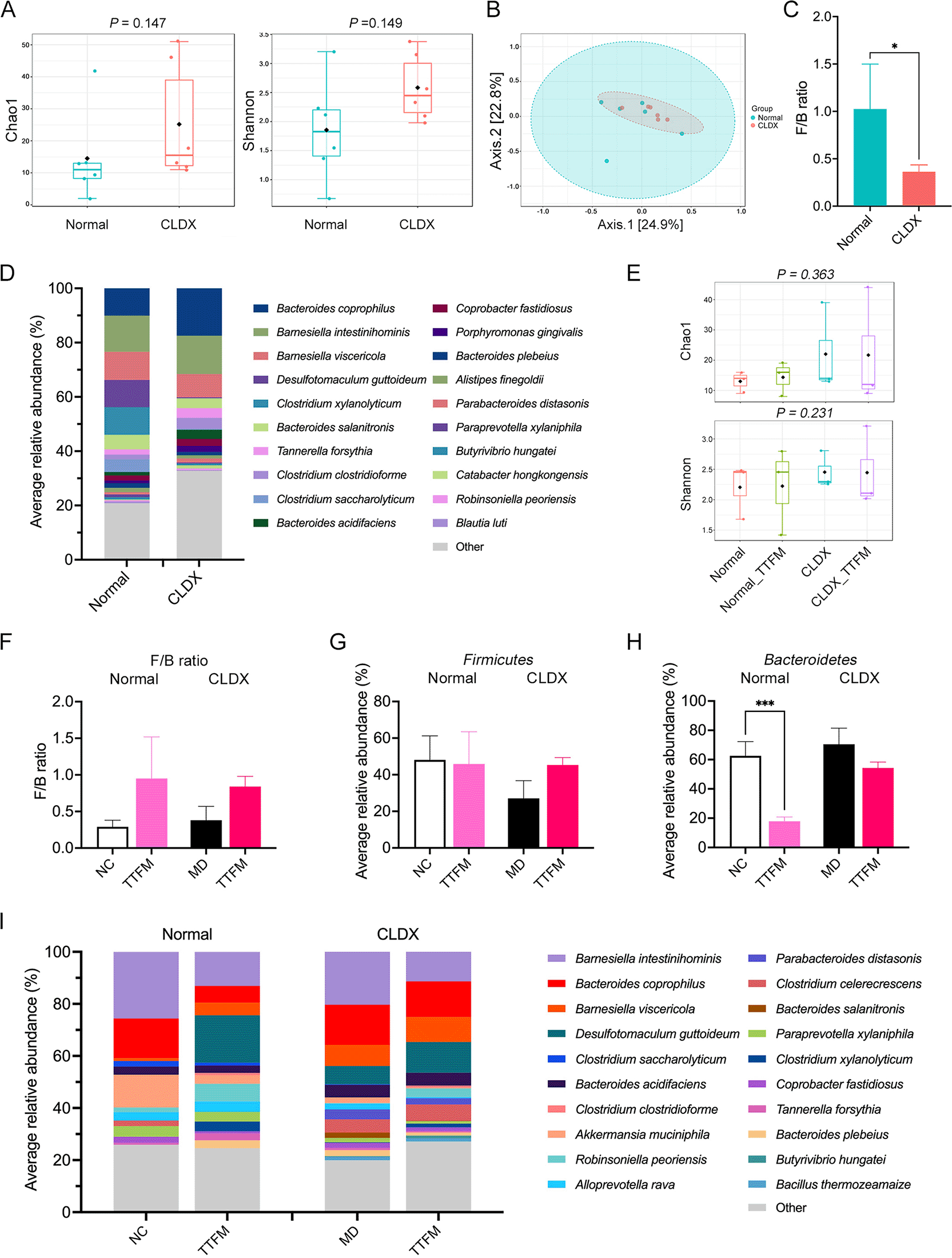
Fecal samples of normal and CLDX mice collected at week 0: (A) α-diversity analysis (Chao1 and Shannon indexes); (B) β-diversity analysis (Permutational multivariate ANOVA, PERMANOVA); (C) F/B ratio (Mann-Whitney U test); (D) Average relative bacterial abundance at species level (Top 20). Effects of TTFM at week 3: (E) α-diversity analysis (Chao1 and Shannon indexes); (F) F/B ratio (Mann-Whitney U test); (G) Average relative abundance of F at phylum level. (H) Average relative abundance of B at phylum level. (I) Average relative bacterial abundance at species level (Top 20). TTFM, Thai traditional formulary medicine; CLDX, cell line-derived xenograft; F, Firmicutes; B, Bacteroidetes.
At the phylum level, Bacteroidetes (B) and Firmicutes (F) dominated the bacterial composition, with a notable increase in B and decrease in F abundance in the CLDX group compared with the normal group (p = 0.0472; Supplementary Figure 2). The F/B ratio was significantly lower in the CLDX group (p = 0.0351) than in the normal group ( Figure 5C). Species-level analysis ( Figure 5D) indicated that tumor development altered the gut microbiome, with increased abundance of species such as Bacteroides coprophilus (p = 0.0197), Barnesiella intestinihominis, Tannerella forsythia and others, while some species like Barnesiella viscericola, Clostridium xylanolyticum and others decreased, though not significantly between groups.
Following TTFM treatment, α-diversity indices (Chao1 and Shannon) did not significantly change across groups ( Figure 5E), but Bray-Curtis dissimilarity-based PCoA analysis revealed distinct clustering (Supplementary Figure 3). At the phylum level (Supplementary Figure 3), B and F remained dominant. The F/B ratio in TTFM-treated CLDX mice was 2.21-fold higher than in untreated CLDX mice ( Figure 5F), indicating increased F and decreased B prevalence ( Figure 5G-H). At the species level ( Figure 5I), notable changes included increased Butyrivibrio hungatei and decreased Clostridium saccharolyticum abundance in TTFM-treated CLDX mice.
NanoString gene expression analysis was employed to assess the impact of TTFM on tumor-related gene expression in 4T1 xenografted mice. Following sacrifice, tumor tissues were isolated for total RNA extraction and subsequent analysis of 750 tumor-related genes. Expression of DEGs was visualized using volcano plots (log2fold-change > 1; p < 0.05) compared with untreated controls ( Figure 6A; refer to extended data Supplementary Table 5 and 6). TTFM treatment notably upregulated Pias1 expression ( Figure 6A and B), while the top five downregulated genes included Ccno, IL-1r2, IL-1β, IL-2 and Nkd1 ( Figure 6A and B). Functional pathway analysis using gProfiler revealed enriched biological processes such as regulation of protein metabolic processes, regulation of cell death, IL-1 signaling pathway, positive regulation of protein catabolic processes and positive regulation of the p38MAPK cascade ( Figure 6C and refer to extended data Supplementary Table 7).
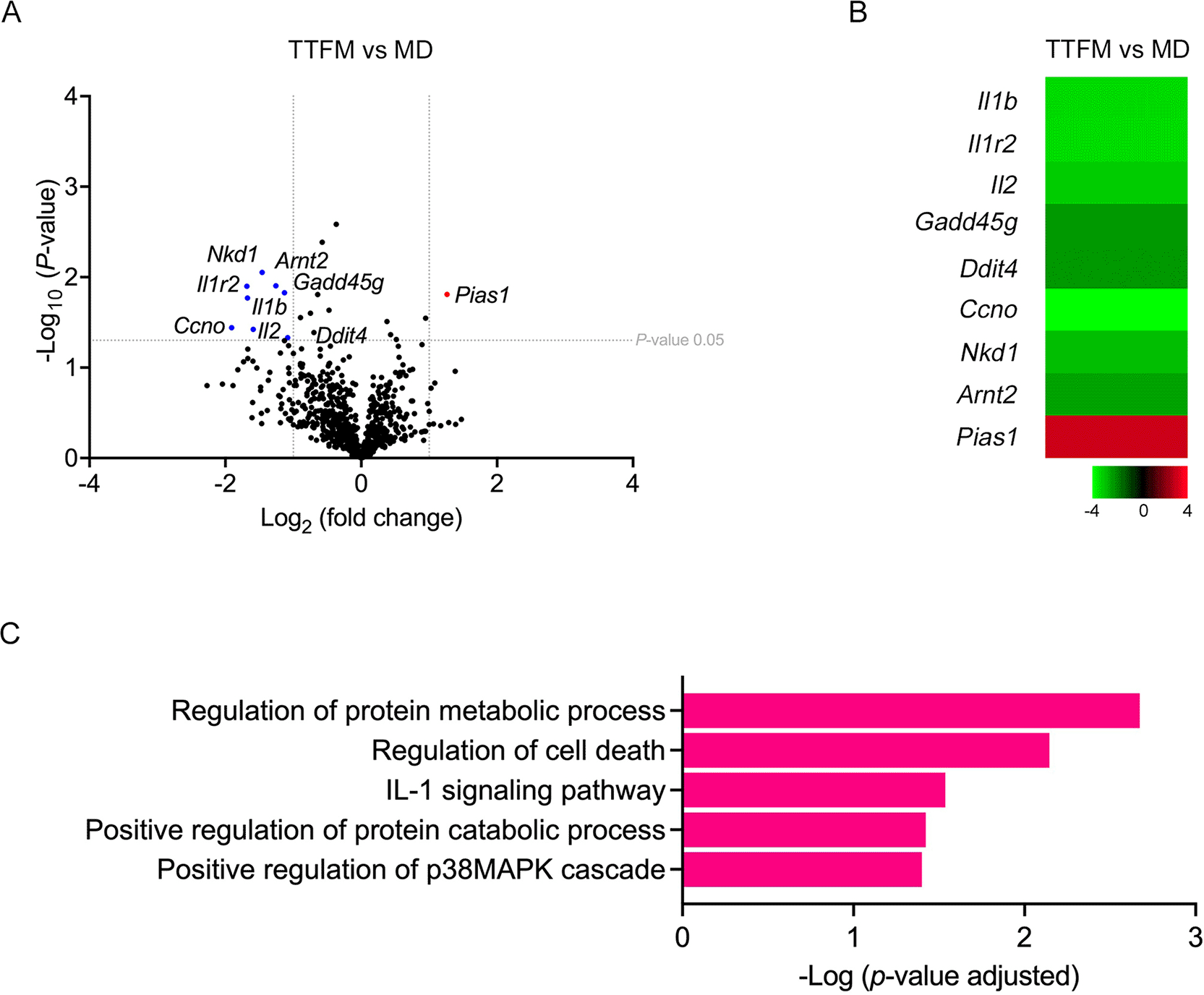
(A) Volcano plot of DEGs following TTFM treatment (log2fold-change > 1; p < 0.05). (B) Heatmap of significantly downregulated (green) and upregulated (red) genes. (C) Functional enrichment analysis was conducted with g:Profiler software. DEGs, differentially expressed genes; TTFM, Thai traditional formulary medicine.
Several studies26,39,40 have identified medicinal plants as a superior source of drugs, particularly for their bioactive compounds that exhibit anti-inflammatory, antitumor and anti-cancer properties with reduced cell toxicity and adverse reactions.39 These compounds are considered potential alternative cancer treatments due to their inhibitory effects on various cancers and their ability to induce apoptosis while having minimal impact on normal cells, suggesting their safety for long-term use.10 The present study specifically examined the impact of TTFM extracts on BC both in vitro and in vivo. The MTT assay results revealed that TTFM extracts possess anti-BC activity, demonstrating a differential effect on cancer cells compared with that on normal cells, with a tendency to induce greater cytotoxicity in BC cells. The TTFM extract demonstrated inhibitory effects on the growth of 4T1 and MDA-MB-231 breast cancer cell lines. However, for the apoptosis assay, an incubation period of 3 days was used instead of the standard 5 days. This shorter incubation period may have resulted in reduced responsiveness of MDA-MB-231 and 4T1 cells to the TTFM extract, particularly in comparison to 4T1 cells. Moreover, differences in species origin may result in variations in apoptosis regulation due to species-specific differences in cellular signaling, gene expression, and the regulation of apoptotic pathways.
LC-MS/MS analysis identified several secondary metabolites in the TTFM extracts (refer to extended data Supplementary Table 1 and 2), some of which have been reported to exhibit anti-cancer activity ( Table 2). Notable compounds such as (9Z)-9-octadecenoic acid,41 6-methoxydihydrosanguinarine,42 astaxanthin,43 costunolide,44 D-limonene,45 hexanedioic acid,46 N,N-dimethylglycine,47 thymol48 and vincamine49 have demonstrated the capacity to induce apoptosis in cancer cells, thereby inhibiting their proliferation and growth. This prompted the exploration of the effect of TTFM extract on the apoptosis process.
In the current study, the apoptosis assay results aligned with previous findings that metabolites from medicinal plants can promote apoptosis in 4T1 and other BC cells, potentially reducing their viability.25,42,43,46 Compounds such as azelaic acid,50 berberine,51 dexamethasone,52 ginkgolide A,53 kaempferol,54 pyridoxine55 and spironolactone,56 identified in the TTFM extracts, were reported to inhibit cancer cell proliferation and migration through various pathways. Additionally, metabolites like 1-methylnicotinamide,57 benzyl isothiocyanate58 and hinokitiol59 demonstrated antitumor effects in the xenografted mouse models. Previous studies indicated that mixed metabolites and crude plant extracts exhibited synergistic effects, enhancing their anti-cancer properties.7,43 The present study hypothesized that the anti-BC activity of TTFM extracts significantly relies on the synergistic effects of the various metabolites. Furthermore, previous study reported that some natural products revealed the potential signaling pathways involved in apoptosis induction. For example, berberine could induced apoptosis through the activation of caspase-9 and CytoC, effectively inhibiting the proliferation of TNBC cells both in vitro and in vivo.60 Kaempferol may cause MDA-MB-231 cells to undergo apoptosis by upregulating the levels of p-ATM and cleaved caspase-9/3.61 Nonetheless, additional research is required to explore the specific genes influenced by TTFM extract.
Changes in the expression of certain transcripts have been linked to promoting cancer cell growth, with previous study62 indicating that Klf12 plays a role in the development and progression of cancer. Klf12 enhances the proliferation of BC cells by suppressing p21 transcription in a p53-dependent and -independent manner.62 Klf12 may promote cell death in response to therapeutic treatments. A prior study found that the percentage of early apoptotic cells significantly decreased in KLF12-overexpressing MCF-7 cells when treated with CDDP. Additionally, knocking down KLF12 expression in ZR-75-30 cells resulted in an increased number of cells in the G0/G1 and G2/M phases.62 Following TTFM treatment, a decrease in Klf12 expression was observed, suggesting that TTFM may induce cytotoxicity and cell death in BC cells.
However, significant changes in gene expression could disrupt normal cellular homeostasis or trigger off-target effects, which, in the long term, might contribute to the onset of other diseases, particularly if pathways related to cell proliferation, apoptosis, or immune regulation are involved. Further investigation into the specific genes and pathways affected is necessary to assess the long-term safety and potential risks associated with TTFM treatment in the future.
Recent studies have linked BC progression to bacterial dysbiosis in the gut microenvironment.4,63 The gut mucosa typically maintains a symbiotic relationship between hosts and microbes, but an imbalance in microorganism composition can disrupt these barriers, inducing tumor immune responses and creating proinflammatory or immunosuppressive microenvironments.63 The current study compared gut bacterial profiles between normal and CLDX mouse models, finding higher α-diversity in the CLDX group but no difference in β-diversity. Additionally, the CLDX group showed an increased abundance of B and a decreased abundance of F, resulting in a lower F/B ratio compared with normal mice. These findings are consistent with another study that showed that a lower F/B ratio is associated with poor prognosis in HER2+ BC and TNBC subtypes.64
The gut microbiome in mice was disturbed by tumor development and progression. Previous studies65 reported that Porphyromonas gingivalis, an anaerobic Gram- bacterium, promoted cancer proliferation through interaction with epithelial cells via TLRs and the IL-6-STAT3 axis, and also by activating the MAPK/ERK signaling pathway in colorectal cancer cells.65 The 4T1 CLDX group showed an enhanced abundance of Porphyromonas gingivalis and other Gram- bacteria such as Bacteroides coprophilus, Barnesiella intestinihominis, Tannerella forsythia, Coprobacter fastidiosus, Porphyromonas gingivalis and Parabacteroides distasonis. The lipopolysaccharide in the outer membrane of these bacteria increased BC cell invasiveness and metastasis by triggering inflammatory and oncogenic signaling pathways.66
The current study investigated the anti-cancer potential of TTFM in a murine 4T1 BC xenograft model. Oral administration of TTFM slightly reduced tumor volume and weight compared with the MD and other treatment groups. Previous study22 reported that consuming a diet rich in micronutrients, including fiber, minerals, vitamins and phytochemical compounds, has also been linked to cancer prevention and a lower risk of BC.22 Although the α-diversity analysis showed no significant changes, the gut microbiota composition was slightly modulated following the oral administration of TTFM in the CLDX mice. The F/B ratio is widely accepted to have an essential influence on maintaining normal intestinal homeostasis. Compared with the MD group, the abundance of the phylum B was decreased, while F was increased in the TTFM groups. In the CLDX model, TTFM treatment decreased B, which is associated with fasting glucose levels, a risk factor for BC.64 Treatment with TTFM enhanced the abundance of F, which produced SCFAs from dietary fiber. SCFAs are one of the primary metabolites produced by gut bacteria in the large intestine via anaerobic fermentation of resistant starch and indigestible dietary fiber.67 Among these, butyrate, a metabolite produced by F,68 plays a key role in anti-cancer activity.69 Butyrate is essential for suppressing inflammation by inhibiting the transcription factor NF-kB in intestinal epithelial cells and macrophages. This mechanism helps maintain intestinal barrier integrity and prevents inflammation triggered by microbial translocation.70 In the present study, the abundance of F increased following TTFM treatment, with an improvement in the butyrate-producing bacteria Butyrivibrio hungatei in CLDX mice. An increase in Butyrivibrio may elevate butyrate levels, which in turn stimulates the release of the anti-inflammatory cytokine IL-10 from regulatory T cells (Tregs). It also supports gut health by promoting the secretion of IgA from plasma cells, thereby inhibiting the growth of harmful bacteria.71 Consequently, the F/B ratio was enhanced after TTFM treatment.
Following TTFM treatment, the abundance of Clostridium saccharolyticum, which controls intestinal polyamine levels, was reduced. Polyamines are known to enhance DNA double-strand break repair and silence tumor-suppressor genes in cancer.72 These findings suggest that TTFM treatment may inhibit tumor occurrence by modulating the gut microbiome composition. Additionally, TTFM treatment did not cause toxicity, as indicated by the stable body weight of normal mice during prolonged treatment.
Although the treatment slightly affected the gut microbiome, the treatment differed in the expression of tumor genes, according to gene expression analysis. It has been demonstrated that changes in specific cancer-related transcripts can promote tumor growth. To further investigate the effects of TTFM on tumor gene expression, a nanoString gene expression analysis was conducted on murine 4T1-BC xenograft models. Following treatment with TTFM, the top upregulation was a protein inhibitor of activated STAT 1 (Pias1), which involves the regulation of the JAK-STAT signaling pathway and is typically downregulated in BC. Pias1 influences immune response control by modulating various cytokine-associated genes and inhibiting genes induced by interferons.73 In the current study, Pias1 was upregulated in the CLDX mouse models treated with TTFM extracts.
On the other hand, the top downregulated genes after TTFM treatment were proinflammatory cytokines. IL-2, which is upregulated in cancer and associated with breast tumor growth, was downregulated post-TTFM treatment.74 As a result, IL-2 was downregulated after TTFM treatment. Additionally, IL-1β and IL-1r2, which are involved in the MAPK pathway and promote tumor invasion through proinflammatory signaling, were also downregulated. These findings align with previous studies40,75 showing that piperine, a compound in black pepper, can reduce proinflammatory cytokines such as TNF-α and IL-1β by inhibiting the migration of dendritic cells and T cells.75 Furthermore, piperine can suppress IL-1β-induced IL-6 expression and inhibit the activation of p38 MAPK and STAT3 in cancer cells.40
A previous study reported that Ddit4 was expressed at high levels in TNBC cells and tissue. It plays a role in acetylation in the promoter region and promotes cell proliferation.76 However, it was downregulated after TTFM treatment. Moreover, the expression of Ccno and Nkd1, which involves the DNA damage repair and Wnt pathway, respectively, were downregulated after TTFM treatment as well. However, there are some limitations in this study, including the lack of HE staining, immunohistochemistry, and Western blotting validation.
The current study demonstrated that TTFM water extracts exhibit anti-BC activity by inhibiting BC cell proliferation through the induction of apoptosis. TTFM may also alter gut microbiota profiles in CLDX mouse models and reduce tumor formation by downregulating proinflammatory and cancer-related genes. TTFM extracts are suggested as a potential for further development of anti-BC therapeutics. However, further research is required to elucidate the precise regulatory mechanisms of TTFM on genes and proteins, with validation needed through human clinical trials. Translating results from animal models to humans remains challenging due to physiological differences. Additionally, the current study is limited by its small sample size of mice, moderate effect sizes, the short-term nature of the investigation, and a lack of deeper exploration into the interactions between gut microbiota and changes in gene expression.
The experimental procedures used in the present study were performed according to the Institutional Animal Care and Use Committee of Chulalongkorn University Laboratory Animal Center (approval no. 2173024).
NCBI GenBank: RNA-sequencing datasets. Accession number PRJNA830310; https://www.ncbi.nlm.nih.gov/bioproject/?term=(PRJNA830310) (Khamwut et al., 2023). NCBI GenBank: DNA-sequencing datasets. Accession number PRJNA1037291; https://dataview.ncbi.nlm.nih.gov/object/PRJNA1037291?reviewer=43onopv82ss6j2eb1asuvv2ft4 and PRJNA1043837; https://dataview.ncbi.nlm.nih.gov/object/PRJNA1043837?reviewer=os4i00poicubqj1l0o1udtbdlq (Khamwut et al., 2024). Gene Expression Omnibus: NanoString gene expression data. Accession number GSE282235; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE282235 (Khamwut et al., 2024).
Figshare: Supplementary materials. https://doi.org/10.6084/m9.figshare.2926258177
This project contains the following extended data:
Supplementary materials.docx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: ARRIVE Guidelines. https://doi.org/10.6084/m9.figshare.29966497.78
ARRIVE Guidelines.pdf
Data are available under the terms of the Creative Commons Zero Public Domain Dedication (CC0).
The authors would like to thank Dr. Wannarasmi Ketchart, Faculty of Medicine, Chulalongkorn University, for providing the MDA-MB-231 cells (ATCC®; cat. no. HTB-26).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microbiome–cancer interactionsNatural product pharmacologyMolecular biology and gene expression analysis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 06 Oct 25 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)