Revised
Amendments from Version 1
In the revised version, we have made corrections and clarifications based on the reviewer’s comments. The introduction has been shortened and made more focused. The methods section has been clarified, especially with respect to how LRTI was diagnosed and how antibiotic sensitivity testing was done. The results tables were cleaned for consistency and corrected for minor numerical formatting issues. Some sentences were rewritten for better readability, and repetition was removed. In the discussion, we have more clearly explained the significance of the resistance patterns seen and the possible impact on treatment delays. No new data has been added or removed, and the analysis remains unchanged. Overall, the revisions improve clarity, reduce redundancy, and ensure that the findings are presented in a straightforward and accurate manner
In the revised version, we have made corrections and clarifications based on the reviewer’s comments. The introduction has been shortened and made more focused. The methods section has been clarified, especially with respect to how LRTI was diagnosed and how antibiotic sensitivity testing was done. The results tables were cleaned for consistency and corrected for minor numerical formatting issues. Some sentences were rewritten for better readability, and repetition was removed. In the discussion, we have more clearly explained the significance of the resistance patterns seen and the possible impact on treatment delays. No new data has been added or removed, and the analysis remains unchanged. Overall, the revisions improve clarity, reduce redundancy, and ensure that the findings are presented in a straightforward and accurate manner
See the authors' detailed response to the review by Jiankang Zhao
Introduction
Head and Neck Cancers (HNCs) cause a significant burden on global health, with India contributing substantially. In 2022, an estimated 2.4 lakh new cases of head and neck cancer were diagnosed in India.1 The high incidence of HNCs in India is attributed to the use of tobacco, betel nut chewing, alcohol, and environmental factors such as exposure to pollution.2,3
The management of head and neck cancer is often a multimodal treatment that includes surgery, radiotherapy, chemotherapy, immunotherapy, or a combination of the three.4 Of these, definitive chemoradiation therapy has been the standard of care for the treatment of locally advanced and some early stage head and neck cancers.5 One of the major complications of this treatment is lower respiratory tract infections (LRTIs), which are common in immunocompromised populations.6 The immunosuppressive action of chemotherapy and mucosal defense disruption with radiotherapy increase the vulnerability of patients to pulmonary infection.7 Dysphagia can also lead to aspiration, thus increasing the risk of respiratory complications including pneumonia.8 LRTIs in patients undergoing chemoradiation for head and neck cancer are a major cause of morbidity, leading to prolonged hospital stays, increased healthcare costs, and delays in the completion of treatment.6
Studies have reported an incidence of infection of 12–19% in patients undergoing chemoradiotherapy for HNCs.6,9 In a large cohort of 5674 head and neck cancer patients, 238 bloodstream infections were reported, with increasing age, advanced stage, and poor performance status as key risk factors, while S. aureus was the predominant early pathogen and BSIs significantly contributed to early non-cancer mortality.10
Infections during chemoradiation in patients with head and neck cancer contribute significantly to increased morbidity, financial toxicity, and treatment interruptions, which ultimately compromise oncologic outcomes. Treatment-related infections, most notably pneumonia, have been shown to prolong hospital stays and increase healthcare expenditures by as much as US$17,000 per episode, thereby amplifying the overall financial burden on patients.11 Moreover, these adverse events intensify out-of-pocket expenses and potential loss of income, which are associated with a poorer quality of life and reduced treatment compliance.12 In addition, infectious complications often necessitate unplanned treatment gaps. Studies indicate that interruptions in radiotherapy exceeding five days can reduce locoregional control rates and diminish overall survival.13
Although infections are a major cause of morbidity and mortality, the effects of these infections are further aggravated by the increasing trend in antimicrobial resistance (AMR). The increase in antimicrobial resistance (AMR) has added to the problems of infection control in patients with cancer. Hospitalized cancer patients have 1.5–2 times higher antimicrobial resistance rates than non-cancer patients do.14,15 Widespread and inappropriate use of antibiotics in both inpatient and outpatient settings has resulted in an increase in the number of drug-resistant organisms. As a result, infections that were once manageable with routine antibiotics became harder to treat, leading to longer hospital stays and the need for costly and higher-end antibiotics.16
The aim of this study was to evaluate an essential but poorly researched aspect of head and neck cancer (HNC) treatment, lower respiratory tract infections (LRTIs), in patients undergoing chemoradiation. While it is commonly thought that such patients are at an increased risk of infection due to factors such as immunosuppression, disruption of the mucosal barrier, and aspiration, there is a significant knowledge gap about the microbial epidemiology, predictors of risk of infection, and the emerging resistance patterns of the responsible pathogens. Understanding these factors is essential to maximize infection prevention strategies, guide empirical antibiotic therapy, and enhance clinical outcomes. By systematically analyzing the microbiological profile and resistance trends, this study aimed to provide valuable insights that can help clinicians make informed decisions, reduce treatment delays, and ultimately enhance the quality of care for HNC patients.
Methods
Study design and population
This study aimed to evaluate the spectrum of antimicrobial resistance and antibiotic sensitivity patterns in patients with head and neck cancer undergoing definitive radiation therapy, with or without chemotherapy, who developed lower respiratory tract infections (LRTIs) during treatment. The study had an ambispective study design, with data collected retrospectively from medical records and prospectively. Patients aged > 18 years who received radiation with curative intent were included. Patients treated with palliative intent, incomplete records, underlying immunosuppressive illnesses such as HIV/AIDS, organ transplantation, or chronic immunosuppressive treatments were excluded.
In our study, lower respiratory tract infections (LRTIs) were diagnosed using a combination of clinical, radiological, and microbiological criteria to ensure diagnostic accuracy. Clinically, patients presented with symptoms such as fever, cough with expectoration, dyspnea, and an increased respiratory rate. Radiological evaluation, including chest X-rays or CT scans, revealed findings consistent with pneumonia or bronchopulmonary infection. Microbiological confirmation was obtained through positive sputum cultures showing significant bacterial growth from validated sputum samples, thereby corroborating the clinical and imaging findings. Sputum culture and sensitivity tests were performed for bacterial pathogen identification in these patients. Suction tip cultures were obtained from the tracheostomy patients for microbiological examination.
Sample collection and handling
To ensure that bacterial isolates and resistance patterns were not influenced by prior treatment, sputum samples were collected only from patients who had not yet been started on antibiotics. For suspected lower respiratory tract infections, early morning sputum samples were obtained in sterile containers with aseptic precautions. In patients for whom spontaneous expectoration of sputum was not possible, saline nebulization was used to aid sputum collection. In patients who underwent tracheostomy, suction-tip cultures were obtained as specimens for analysis. All specimens were sent within one hour of collection to the microbiology laboratory to enable maximum recovery.
To ensure sample quality, sputum samples were validated using Gram stain criteria, including only those with <10 epithelial cells and >25 polymorphonuclear leukocytes per low-power field. Accepted samples were cultured in a routine aerobic culture and incubated at 37°C for 24–48 h under suitable atmospheric conditions. Bacterial isolates were typed using routine biochemical tests, including catalase, oxidase, coagulase, and carbohydrate fermentation tests. Automated systems, such as VITEK®2 (bioMérieux, Inc), were employed for correct species-level identification.
Antimicrobial susceptibility testing
Antibiotic sensitivity testing was performed using the Kirby-Bauer disk diffusion and VITEK 2 systems. The results were interpreted and reported based on the standards of the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines.17,18 The bacterial isolates were tested against a panel of commonly used antimicrobial agents, including beta-lactams (penicillin, cephalosporins, and carbapenems), fluoroquinolones, aminoglycosides, and other classes suitable for the identified pathogen. The sensitivity results were reported as sensitive (S), intermediate (I), or resistant (R) based on the CLSI 2023 guidelines.17
Assessed clinical and therapeutic parameters
Demographic information, such as age and sex, and appropriate comorbid diseases, such as diabetes mellitus, hypertension, and COPD, were collected. The time gap between the initiation of treatment and the development of LRTI was recorded. Tumor-related characteristics such as location of the primary tumor, stage as per TNM staging system, and the treatment received, including radiotherapy parameters (i.e., total dose, fractionation schedule, and treatment duration) were noted. Chemotherapy-related characteristics, such as the type of agents administered and the total number of courses of chemotherapy administered, were documented.
Statistical analysis
All statistical analyses were performed using SPSS version 23.0.19 Descriptive and inferential statistical methods were used to examine the trends, associations, and resistance patterns among the bacterial isolates.
Categorical variables were reported as frequencies and proportions. Continuous variables are reported as mean values with corresponding standard deviations (SDs). The range of bacterial isolates from sputum and tracheal aspirate cultures was compared, and the most prevalent pathogens were identified. Antibiotic resistance patterns were ascertained by classifying bacterial isolates into sensitive (S), intermediate (I), and resistant (R) groups, and the proportion of resistant isolates for each of the tested antibiotics was approximated. Chi-square tests (χ2) were used to examine categorical variables such as the distribution of bacterial species according to age group, sex, chemotherapy regimen, and radiation dose. Independent t-tests or Mann-Whitney U tests were used to compare continuous variables, such as age and days of treatment, between non-infected and infected patients. One-way ANOVA was employed to compare several group means of infection rates and antibiotic resistance patterns. To determine independent risk factors for LRTI development, a logistic model was developed to include age, sex, chemotherapy regimen, radiation dose, and timing of onset of infection. Statistical significance was determined at a p-value <0.05.
Results
In total, 120 patients were included in the analysis. The median age of the study population was 54.2 years. The study population was predominantly male (75.0%), with females accounting for only 25.0%. The most commonly administered radiotherapy dose was 70 Gy (45.0%), followed by 66 Gy (35.0%) and 60 Gy (20.0%). Cisplatin was the most frequently used chemotherapeutic drug (60.8%). Most patients (83.3%) received between 1-5 cycles of chemotherapy, while only 6.7% underwent more than five cycles. The sputum was the primary specimen collected (90.0%), with suction tip samples in 10.0%. More than half of the specimens (52.5%) were collected between 15-22 days after treatment initiation. The detailed patient characteristics are shown in
Table 1.
Table 1. Baseline patient characteristics.
| Base line data | Number |
Percentage |
|---|
| Age | 18-37 years | 8 | 6.7 |
| 38-57 years | 67 | 55.8 |
| 58-77 years | 41 | 34.2 |
| 78 years and above | 4 | 3.3 |
| Gender | Female | 30 | 25.0 |
| Male | 90 | 75.0 |
| Radio therapy dose | 60 Gy | 24 | 20.0 |
| 66 Gy | 42 | 35.0 |
| 70 Gy | 54 | 45.0 |
| Chemotherapy used | Carboplatin | 12 | 10.0 |
| Cisplatin | 73 | 60.8 |
| Cisplatin + Capecitabine | 23 | 19.2 |
| No Chemotherapy | 12 | 10.0 |
| Number of chemotherapy cycle | 0 | 12 | 10.0 |
| 1-5 cycle | 100 | 83.3 |
| >5 cycle | 8 | 6.7 |
| Specimen type | Sputum | 108 | 90.0 |
| Suction Tip | 12 | 10.0 |
| Days between start of treatment and sputum collection | 7-14 days | 21 | 17.5 |
| 15-22 days | 63 | 52.5 |
| 23-30 days | 32 | 26.7 |
| 31-38 days | 4 | 3.3 |
The most commonly isolated organisms were Pseudomonas aeruginosa (35.0%),Klebsiella pneumoniae (16.7%), Acinetobacter baumannii (10.0%), Staphylococcus aureus (9.2%), Escherichia coli (6.7%), Haemophilus influenzae, and Streptococcus pneumoniae (5.0% each) (
Table 2).
Table 2. Details of organisms isolated.
| Organisms | Frequency |
Percentage |
|---|
| Achromobacter Xylosoxidans | 2 | 1.7 |
| Acinetobacter Baumannii | 12 | 10.0 |
| Acinetobacter lwoffii | 2 | 1.7 |
| Burkholderia Cepacia | 1 | .8 |
| Citrobacter Koseri | 1 | .8 |
| Corynebacterium Striatum | 1 | .8 |
| Diphtheroid species | 2 | 1.7 |
| Enterobacter Aerogenes | 1 | .8 |
| Enterobacter species | 1 | .8 |
| Escherichia Coli | 8 | 6.7 |
| Haemophilus Influenzae | 6 | 5.0 |
| Klebsiella Pneumoniae | 20 | 16.7 |
| Moraxella species | 1 | .8 |
| Proteus Vulgaris | 1 | .8 |
| Pseudomonas Aeruginosa | 42 | 35.0 |
| Staphylococcus Aureus | 11 | 9.2 |
| Stenotrophomonas Maltophilia | 2 | 1.7 |
| Streptococcus Pneumoniae | 6 | 5.0 |
Klebsiella pneumoniae showed the highest resistance to ampicillin (40%), ceftriaxone (30%), and ciprofloxacin (25%), limiting their effectiveness. In contrast, amikacin (100%), meropenem (95%), Cefoperazone+Sulbactam, Gentamicin, and Imipenem (90%) exhibited high sensitivity, making them preferred treatment options.
Pseudomonas aeruginosa showed the highest resistance to levofloxacin (33.3%), followed by trimethoprim/sulfamethoxazole (26.2%) and ciprofloxacin (26.2%). High sensitivities were observed for Cefoperazone+Sulbactam (81.0%), imipenem (78.6%), ceftazidime (76.2%), and meropenem (73.8%). Intermediate susceptibility to cefepime (14.3%), minocycline (7.1%), and Ticarcillin/Clavulanic Acid (7.1%) was noted.
Among Acinetobacter baumannii isolates, the highest resistance was observed with Piperacillin+Tazobactam (33.3%), cefepime resistance was also notable at 25.0%, although 75.0% of isolates remained sensitive, ciprofloxacin exhibited a 25.0% resistance rate, and trimethoprim/sulfamethoxazole showed 25.0% resistance. Among Escherichia coli, ceftriaxone showed the highest resistance (62.5%), followed by ampicillin (50.0%). Ciprofloxacin showed 50.0% resistance with 25.0% intermediate sensitivity, while trimethoprim/sulfamethoxazole exhibited 37.5% resistance. For H influenzae, cotrimoxazole showed the highest resistance (66.7%), followed by ampicillin (50.0%). Cefuroxime and Ciprofloxacin both showed 33.3% resistance, while Amoxycillin/Clavulanic Acid showed 16.7% resistance. In contrast, azithromycin (100%) and cefotaxime (83.3%) showed the highest sensitivities, making them the preferred treatment options. Levofloxacin, Meropenem, and Cefuroxime were effective (66.7% sensitivity).
The resistance rates to higher-end antibiotics revealed that Piperacillin+Tazobactam exhibited the highest resistance at 15.0% (18 cases), followed by imipenem at 10.0% (12 cases). Cefoperazone+ sulbactam showed a resistance rate of 5.8% (seven cases), while tigecycline had the lowest resistance at 1.7% (two cases) (
Table 3).
Table 3. Resistance rates for higher end antibiotics.
| Antibiotics | Frequency |
Percentage |
|---|
| Cefoperazone+Sulbactum | 7 | 5.8 |
| Imipenem | 12 | 10.0 |
| Piperacillin+Tazobactam | 18 | 15.0 |
| Tigecycline | 2 | 1.7 |
Organisms and age
Although not statistically significant in the 18-37 years age group, the most common organisms were Pseudomonas aeruginosa and Staphylococcus aureus, both found in 25.0% of the cases (p=0.36). In the 38-57 years age group, Pseudomonas aeruginosa was the most prevalent organism, detected in 32.8% of cases, followed by Klebsiella pneumoniae (17.9%) (p=0.44) For the 58-77 years age group, Pseudomonas aeruginosa remained the most common organism (34.1%), followed by Klebsiella pneumoniae (17.1%) (p=0.56). Pseudomonas aeruginosa was the only organism detected in the 78 years and above category, accounting for 100% of the cases (
Figure 1).
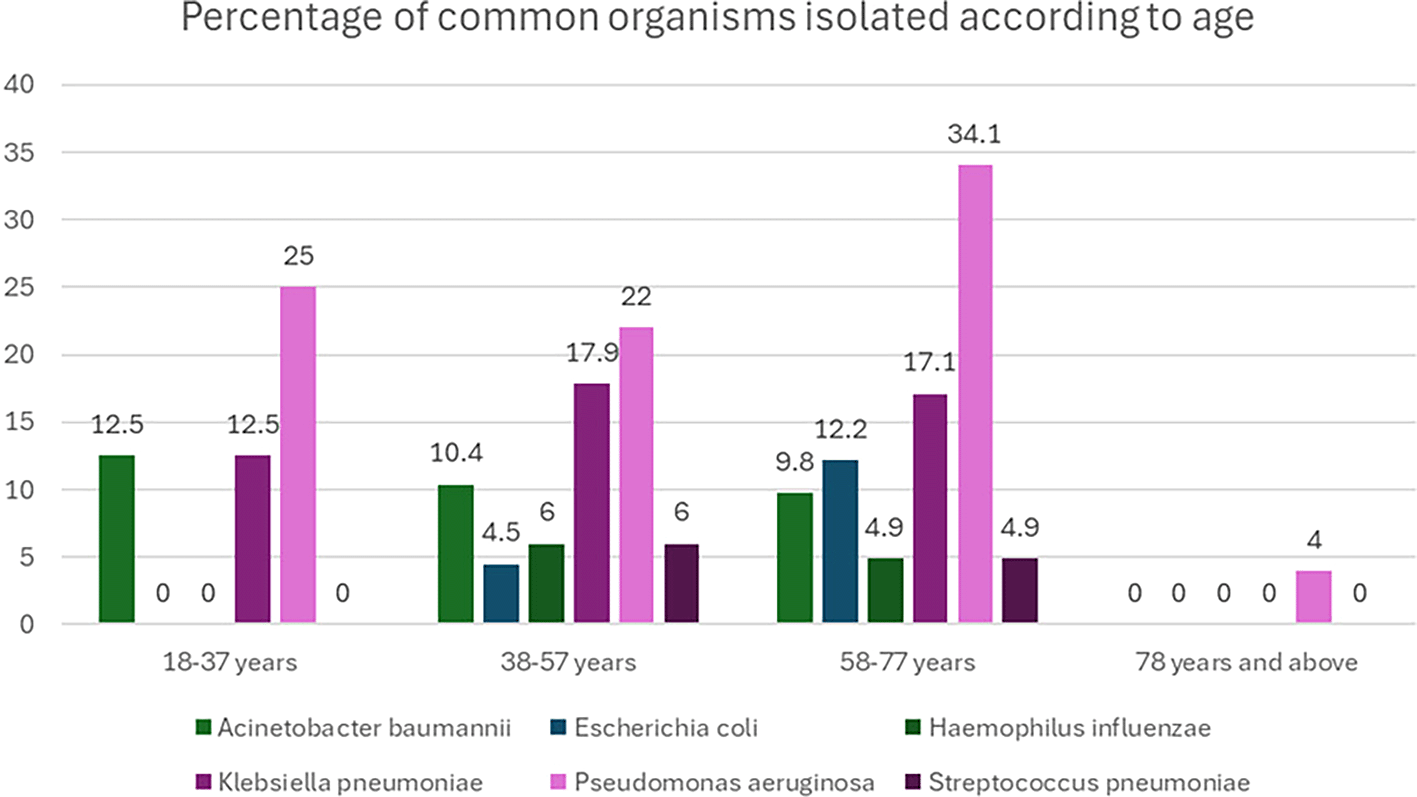
Figure 1. Percentage of common organisms isolated according to age.
(The figure is created by the authors based on original data and hence does not require permission for reproduction).
Organism and onset of LRTI
Between 7-14 days, Pseudomonas aeruginosa (33.3%) was the most common isolate, followed by Klebsiella pneumoniae (14.3%), Streptococcus pneumoniae (14.3%), and Staphylococcus aureus (9.5%) (p <0.001). In 15-22 days, Pseudomonas aeruginosa (31.7%) remained dominant, followed by Klebsiella pneumoniae (17.5%), Staphylococcus aureus (9.5%), and Escherichia coli (6.3%). By 23-30 days, Pseudomonas aeruginosa (40.6%) increased, while Klebsiella pneumoniae (15.6%) and Acinetobacter baumannii (15.6%) increased significantly (p<0.001). In 31-38 days, Pseudomonas aeruginosa (50.0%) was the highest, followed by Klebsiella pneumoniae (25.0%) and Staphylococcus aureus (25.0%) (p=0.34) (
Figure 2).

Figure 2. Common Organisms isolated based on the onset of LRTI.
(The figure is created by the authors based on original data and hence does not require permission for reproduction).
Organism and chemotherapy
Based on the chemotherapy regimen used, the most frequently isolated organisms varied across the groups. In the Carboplatin group, Klebsiella pneumoniae (50.0%) was the most common, followed by Haemophilus influenzae (16.7%) and Pseudomonas aeruginosa (16.7%) (p<0.001) The Cisplatin group had a high prevalence of Pseudomonas aeruginosa (37.0%), Escherichia coli (8.2%), Klebsiella pneumoniae (11.0%), and Staphylococcus aureus (11.0%) (p=0.04). Among patients receiving Cisplatin + Capecitabine, Pseudomonas aeruginosa (43.5%) was predominant, followed by Klebsiella pneumoniae (17.4%), and Acinetobacter baumannii (13.0%) (p=0.03). In the group that did not receive chemotherapy, Acinetobacter baumannii (25.0%) and Pseudomonas aeruginosa (25.0%) were the most frequently detected, followed by Escherichia coli (16.7%) and Streptococcus pneumoniae (16.7%) (p=0.23).
Discussion
Respiratory infections are a significant complication in patients undergoing chemoradiation therapy for head and neck cancers, given their immunocompromised status, both from the disease itself and its treatment. This article provides a spectrum of antimicrobial resistance and susceptibility patterns in these patients, which is of critical significance in their management.
The temporal pattern of infections indicated that Pseudomonas aeruginosa prevailed in all periods observed consistently, increasing from 33.3% in the first phase (7-14 days) to 50.0% in later phases (31-38 days). This pattern can be considered an indication of the pathogen’s ability to survive in healthcare settings and as a causative agent of infections later on.20 The presence of Acinetobacter baumannii and Klebsiella pneumoniae in the 23-30 days group might indicate the occurrence of secondary infections, probably as a consequence of prolonged hospitalization, mechanical ventilation, and the effect of antibiotic treatment.21
The higher frequency of Pseudomonas aeruginosa (35.0%), the most frequently isolated pathogen, is in agreement with previous studies that demonstrated immunocompromised patients, particularly those undergoing chemoradiotherapy, to be highly vulnerable to infection by opportunistic pathogens.22,23 Pseudomonas aeruginosa is characterized by inherent resistance mechanisms and the ability to develop further resistance; thus, it is prevalent in this population. The higher rates of Klebsiella pneumoniae (16.7%) and Acinetobacter baumannii (10.0%) further indicate that such patients are highly vulnerable to hospital-acquired infections and ventilation-related infections.
Several factors determine observed microbial diversity and resistance patterns. Patients undergoing chemoradiotherapy tend to develop mucositis, xerostomia, and impaired mucosal immunity, favoring the colonization and invasion of bacteria in the lower respiratory tract.5,6 The widespread use of broad-spectrum antibiotics in these patients has the potential to induce antimicrobial resistance. Antimicrobial resistance (AMR) is a significant issue in oncology, particularly in patients with head and neck cancer (HNC) undergoing chemoradiation therapy. AMR occurs when bacterial pathogens evolve to resist the action of antibiotics, which ultimately renders traditional treatment ineffective.
The implications of AMR in oncology are dire. Studies have proven a direct link between resistant bacterial infections and increased mortality in cancer patients, particularly those with hematologic malignancies or solid tumors undergoing immunosuppressive therapy.24,25 The overuse and misuse of antibiotics, typically triggered by empirical treatment strategies in critically ill cancer patients, are reasons for the rapid emergence of resistant strains.26 The antimicrobial resistance pattern observed in this study is alarming in relation to the effectiveness of commonly used antibiotics. The high resistance of Klebsiella pneumoniae to ampicillin (40%) and ceftriaxone (30%) indicates that beta-lactam antibiotics may not be effective first-line treatment strategies. Similarly, Pseudomonas aeruginosa was highly resistant to levofloxacin (33.3%) and ciprofloxacin (26.2%), most likely because of the widespread empirical use of fluoroquinolones. Conversely, higher sensitivity rates were observed with Amikacin, Meropenem, and Cefoperazone+Sulbactam, which suggests their potential use in the treatment of infections in this patient population.
Radiotherapy is one of the areas of highest importance where treatment gaps can have negative impacts on outcomes. Infection- or treatment-related toxicity-induced delays in chemoradiation have been linked to worse survival outcomes and reduced therapeutic efficacies. Evidence indicates that even brief treatment delays of more than five days may lead to reduced tumor control probabilities and overall survival.27,28
Antibiotic-resistant infections require higher-end antibiotics, longer hospital stays, and intensive care unit hospitalizations, all of which add up to mounting healthcare expenditure.29 In low- and middle-income nations, such as India, where the economic costs of treating cancer are already borne by the patient, the additional cost of more effective newer-generation antibiotics might make them unaffordable, resulting in suboptimal infection treatment and mortality.30,31 In addition, the indirect costs in terms of productivity loss and burden to caregivers add to the economic costs of the enrolled families.
Given the high prevalence of multidrug-resistant organisms in our study, a targeted approach to antimicrobial therapy is crucial. Implementing robust antibiotic stewardship programs is essential for mitigating the spread of resistant pathogens.32 Strategies such as de-escalation based on culture results, restriction of broad-spectrum antibiotics to necessary cases, and rotation of empirical regimens can help to preserve antibiotic effectiveness. Additionally, infection control measures, including stringent hand hygiene, environmental sanitation, and surveillance of resistance patterns, play a critical role in curbing the transmission of multidrug-resistant organisms in oncology settings. Empirical antibiotic selection should be guided by local resistance patterns and periodic surveillance to ensure optimal treatment outcomes. Additionally, the development of new antimicrobial agents and combination therapies may play a vital role in managing infections in this vulnerable population.
This study had certain limitations. Although the sample size is reasonable, it may not be generalizable to the entire patient population with head and neck cancer undergoing chemoradiotherapy. Second, our study mainly used sputum samples, which may not always be generalizable to lower respiratory tract infections. Employing bronchoalveolar lavage or protected specimen brushing would provide more definitive microbiological results. Future studies using these methods in combination may provide a better understanding of the clinical relevance of antimicrobial resistance in this group of patients.
Conclusion
Our study highlights the high prevalence of Pseudomonas aeruginosa and Klebsiella pneumoniae in respiratory infections among patients with head and neck cancer undergoing chemoradiotherapy, with significant resistance patterns to commonly used antibiotics. These findings underscore the importance of antibiotic stewardship, infection control measures, and periodic surveillance to optimize antimicrobial therapy and improve patient outcomes.
Consent
Written informed consent to take part in the research has been obtained from all patients when indicated. Consent for publication is not applicable, as all data are anonymized and no images are being published.
Ethics statement
This study was approved by the Institutional Ethics Committee of Kasturba Medical College, Mangalore, vide Protocol No.- IEC KMC MLR 12/2023/502 dated 21.12.2023. This study was conducted in accordance with the ethical guidelines and regulations set forth by the institution. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Author contributions
All authors contributed equally to the conceptualization, methodology, data collection, analysis, manuscript writing, and revision. All the authors have read and approved the final version of the manuscript.
References
- 1.
Bray F, Laversanne M, Hyuna S, et al.:
Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries.
CA Cancer J. Clin.
74(74): 229–263. PubMed Abstract
| Publisher Full Text
- 2.
Chauhan R, Trivedi V, Rani R, et al.:
A Study of Head and Neck Cancer Patients with Reference to Tobacco Use, Gender, and Subsite Distribution.
South Asian J. Cancer.
2022; 11: 046–051. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 3.
Goyal N, Hennessy M, Lehman E, et al.:
Risk factors for head and neck cancer in more and less developed countries: Analysis from the INHANCE consortium.
Oral Dis.
2023; 29: 1565–1578. PubMed Abstract
| Publisher Full Text
- 4.
Mesia R, Iglesias L, Lambea J, et al.:
SEOM clinical guidelines for the treatment of head and neck cancer (2020).
Clin. Transl. Oncol.
2021; 23: 913–921. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 5.
Iqbal MS, Chaw C, Kovarik J, et al.:
Primary Concurrent Chemoradiation in Head and Neck Cancers with Weekly Cisplatin Chemotherapy: Analysis of Compliance, Toxicity and Survival.
Int. Arch. Otorhinolaryngol.
2016; 21: 171–177. Publisher Full Text
- 6.
Mirabile A, Vismara C, Crippa F, et al.:
Health care–associated infections in patients with head and neck cancer treated with chemotherapy and/or radiotherapy.
Head Neck.
2016; 38: E1009–E1013. Publisher Full Text
- 7.
Abdel-Rahman O:
Influenza and pneumonia-attributed deaths among cancer patients in the United States; a population-based study.
Expert Rev. Respir. Med.
2021; 15: 393–401. PubMed Abstract
| Publisher Full Text
- 8.
Nguyen NP, Smith HJ, Dutta S, et al.:
Aspiration Occurence During Chemoradiation For Head and Neck Cancer.
Anticancer Res.
2007; 27: 1669–1672. PubMed Abstract
- 9.
Mirabile A, Numico G, Russi EG, et al.:
Sepsis in head and neck cancer patients treated with chemotherapy and radiation: Literature review and consensus.
Crit. Rev. Oncol. Hematol.
2015; 95: 191–213. PubMed Abstract
| Publisher Full Text
- 10.
Jensen KH, Vogelius I, Moser CE, et al.:
Bloodstream infections in head and neck cancer patients after curative-intent radiotherapy: a population-based study from the Danish Head and Neck Cancer Group database.
Br. J. Cancer.
2021; 125: 458–464. Publisher Full Text
- 11.
Kochhar A, Pronovost PJ, Gourin CG:
Hospital-acquired conditions in head and neck cancer surgery.
Laryngoscope.
2013; 123: 1660–1669. Publisher Full Text
- 12.
Rosi-Schumacher M, Patel S, Phan C, et al.:
Understanding Financial Toxicity in Patients with Head and Neck Cancer: A Systematic Review.
Clin. Med. Insights Oncol.
2023; 17. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 13.
Xu C, Bin YK, Feng RJ, et al.:
Radiotherapy interruption due to holidays adversely affects the survival of patients with nasopharyngeal carcinoma: a joint analysis based on large-scale retrospective data and clinical trials.
Radiat. Oncol.
2022; 17: 1–11. Publisher Full Text
- 14.
Gupta V, Satlin MJ, Yu K, et al.:
Burden of Antimicrobial Resistance in Adult Hospitalized Patients With Cancer: A Multicenter Analysis.
Cancer Med.
2024; 13: e70495. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 15.
Vahedian-Ardakani HA, Moghimi M, Shayestehpour M, et al.:
Bacterial Spectrum and Antimicrobial Resistance Pattern in Cancer Patients with Febrile Neutropenia.
Asian Pac. J. Cancer Prev.
2019; 20: 1471–1474. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 16.
Danielsen AS, Franconeri L, Page S, et al.:
Clinical outcomes of antimicrobial resistance in cancer patients: a systematic review of multivariable models.
BMC Infect. Dis.
2023; 23: 247. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 17.
CLSI:
Performance standards for antimicrobial susceptibil… - Google Scholar.n.d. (accessed March 4, 2025).
Reference Source
- 18.
Kahlmeter G, Brown DFJ, Goldstein FW, et al.:
European Committee on Antimicrobial Susceptibility Testing (EUCAST) Technical Notes on antimicrobial susceptibility testing.
Clin. Microbiol. Infect.
2006; 12: 501–503. PubMed Abstract
| Publisher Full Text
- 19.
IBM Corp:
Released 2023. IBM SPSS Statistics for… - Google Scholar.n.d. (accessed March 4, 2025).
Reference Source
- 20.
Amanati A, Sajedianfard S, Khajeh S, et al.:
Bloodstream infections in adult patients with malignancy, epidemiology, microbiology, and risk factors associated with mortality and multi-drug resistance.
BMC Infect. Dis.
2021; 21: 636. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 21.
Yin Y, Zhao C, Li H, et al.:
Clinical and microbiological characteristics of adults with hospital-acquired pneumonia: a 10-year prospective observational study in China.
Eur. J. Clin. Microbiol. Infect. Dis.
2021; 40: 683–690. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 22.
Azima Hanin SM, Dharman S, Smiline Girija AS:
Association of salivary microbes with oral mucositis among patients undergoing chemoradiotherapy in head and neck cancer: A hospital-based prospective study.
J. Int. Oral Health.
2022; 14: 53–60. Publisher Full Text
- 23.
Soni P, Parihar RS, Soni LK:
Opportunistic Microorganisms in Oral Cavity According to Treatment Status in Head and Neck Cancer Patients.
J. Clin. Diagn. Res.
2017; 11: DC14–DC17. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 24.
Gudiol C, Albasanz-Puig A, Cuervo G, et al.:
Understanding and Managing Sepsis in Patients With Cancer in the Era of Antimicrobial Resistance.
Front. Med (Lausanne).
2021; 8: 636547. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 25.
Bratti VF, Wilson BE, Fazelzad R, et al.:
Scoping review protocol on the impact of antimicrobial resistance on cancer management and outcomes.
BMJ Open.
2023; 13: e068122. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 26.
Nardulli P, Hall GG, Quarta A, et al.:
Antibiotic Abuse and Antimicrobial Resistance in Hospital Environment: A Retrospective Observational Comparative Study.
Medicina (Kaunas).
2022; 58: 58. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 27.
Ferreira BC, Sá-Couto P, Lopes MC, et al.:
Compliance to radiation therapy of head and neck cancer patients and impact on treatment outcome.
Clin. Transl. Oncol.
2016; 18: 677–684. Publisher Full Text
- 28.
Fesinmeyer MD, Mehta V, Blough D, et al.:
Effect of Radiotherapy Interruptions on Survival in Medicare Enrollees With Local and Regional Head-and-Neck Cancer.
Int. J. Radiat. Oncol. Biol. Phys.
2010; 78: 675–681. PubMed Abstract
| Publisher Full Text
- 29.
Michaelidis CI, Fine MJ, Lin CJ, et al.:
The hidden societal cost of antibiotic resistance per antibiotic prescribed in the United States: An exploratory analysis.
BMC Infect. Dis.
2016; 16: 1–8. Publisher Full Text
- 30.
Gedik H:
Antibiotic resistance status and its costs in hematological patients: A two-year analysis. Caspian.
J. Intern. Med.
2017; 8: 276–281. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 31.
Dar MA, Chauhan R, Trivedi V, et al.:
Assessing the Prevalence of Financial Toxicity, its Predictors and Association with Health- Related Quality of Life Among Radiation Oncology Patients in India: A Cross-Sectional Patient Reported Outcome Study.
Int. J. Radiat. Oncol. Biol. Phys.
2023; 116: 157–165. PubMed Abstract
| Publisher Full Text
- 32.
Baubie K, Shaughnessy C, Kostiuk L, et al.:
Evaluating antibiotic stewardship in a tertiary care hospital in Kerala, India: a qualitative interview study.
BMJ Open.
2019; 9: e026193. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 33.
Krishna A:
Datasheet.xlsx. Dataset.
figshare.
2025. Publisher Full Text


Comments on this article Comments (0)