Keywords
Transcutaneous bilirubinometer; bilirubin; correlation analysis; phototherapy; Bland Altman analysis; noninvasive; Neonatal jaundice
This article is included in the Health Services gateway.
This article is included in the Manipal Academy of Higher Education gateway.
Neonatal jaundice is one of the most prevalent condition during first week of life causing morbidity and even mortality in few, especially in low – middle income countries. Although visual inspection for jaundice has been a time tested method, serum bilirubin is the gold standard investigation of choice. Due to this newborn receive many heel or vein pricks for testing, hence transcutaneous bilirubinometer can be a helpful non-invasive tool for diagnosing jaundice requiring phototherapy.
This prospective study was carried out in a tertiary care hospital in Mangalore, Karnataka to compare a non invasive method of detecting bilirubin levels and serum bilirubin levels. Performance of a transcutaneous bilirubinometer Dräger Jaundice Meter JM-105 was assessed against routine venous serum bilirubin testing before phototherapy during neonatal care in the first two weeks of life. Results were derived by analysing the correlation coefficient between two methods and direct agreement was analysed using Bland Altman analysis.
Total of 271 neonates (>35 weeks) were included in the study. Transcutaneous bilirubinometry and serum bilirubin values were done on all of them in the first week of life. Correlation analysis showed significant relationship with a Pearson correlation coefficient of 0.629. Values of transcutaneous bilirubinometer showed excellent agreement with venous serum bilirubin concentration in Bland Altman analysis.
The transcutaneous bilirubinometer is a reliable tool to screen neonates and identify those needing phototherapy there by reducing invasive blood sampling.
Transcutaneous bilirubinometer; bilirubin; correlation analysis; phototherapy; Bland Altman analysis; noninvasive; Neonatal jaundice
This revised manuscript (Version 2) reflects substantial improvements made in response to peer reviewer feedback. The Introduction has been expanded to better articulate the research gap and the rationale for the study, particularly in the context of limited Indian data on transcutaneous bilirubin (TCB) screening. We have clarified the novelty of our work, which includes a large single-center data set from a coastal city of South India, correlation of TCB with both total serum bilirubin (TSB) and clinical parameters such as gestational age and hours of life, and population-specific insights relevant to darker-skinned neonates. We have also emphasized role of skin pigmentation and skin hydration on TCB measurements which was our concern due to majority of subjects belonging to Dravidian race and coastal environment.
Additionally, the Discussion now includes a detailed ethical justification for the use of invasive sampling, acknowledging the limitations and contextualizing the methodology within local clinical practice. We have also strengthened the manuscript by incorporating additional references to support our findings and ensure alignment with current literature. These revisions aim to enhance the clarity, relevance, and scientific rigor of the study, making it more informative for clinicians and researchers working in similar healthcare settings.
See the authors' detailed response to the review by Sanjoy Kumer Dey
Neonatal hyperbilirubinemia is a prevalent condition in newborns, marked by the appearance of jaundice within the first week after birth. It affects approximately 60% of term neonates and up to 80% of preterm neonates. This condition occurs due to the accumulation of unconjugated bilirubin, a lipid-soluble pigment, in the skin and mucous membranes, leading to a yellowish discolouration.1 Neonatal hyperbilirubinemia, while generally benign, is commonly seen postnatally in newborns. However, premature neonates and certain high-risk groups are more susceptible to severe forms, which, if not managed, can progress to complications like kernicterus.2 Neonatal jaundice is clinically identified by a yellowish discolouration of the sclera, skin, and mucous membranes resulting from increased bilirubin levels in the bloodstream. The condition is categorised into two types: Unconjugated Hyperbilirubinemia and Conjugated Hyperbilirubinemia.3 Phototherapy and exchange transfusion are the primary interventions for the prevention and management of bilirubin encephalopathy.
The primary methods for assessing bilirubin levels in newborns include visual inspection, transcutaneous bilirubinometry, and measurement of total serum bilirubin. Visual assessment is simple using Kramer’s rule but has notable limitations, as it is highly subjective; factors such as the physician's experience, the baby's skin colour, clothing, and lighting conditions can all influence the accuracy of visual estimation. Transcutaneous bilirubinometry provides a non-invasive alternative, whereas total serum bilirubin measurement continues to be the gold standard for accurate assessment. Requiring a blood sample for confirmation, especially in high-risk cases. Transcutaneous bilirubin (TCB) assessment uses a handheld electronic device to measure bilirubin levels non-invasively on the skin's surface, providing a painless and convenient method for screening jaundice in term and near-term neonates. It is increasingly accepted in clinical settings due to its simplicity and effectiveness. The device, Transcutaneous Jaundice Detector (Drager Model MBJ20). utilises optical spectroscopy by emitting light into the skin and analysing the reflected wavelengths to estimate total serum bilirubin levels. This method offers a reliable alternative for early jaundice detection without requiring blood draws.4
The TCB works by correlating the amount of light absorbed by bilirubin with its concentration in the skin, providing an estimate of bilirubin levels. The National Institute for Health and Care Excellence (NICE) guidelines advise against using transcutaneous bilirubin (TCB) measurements within the first day of life or for neonates born before 35 weeks of gestation. Despite these limitations, TCB is a non-invasive screening method used to determine the need for phototherapy. Potentially reducing infection risks. In contrast, total serum bilirubin (TSB) measurement involves drawing a blood sample. The blood sample report is plotted on a nomogram, which is hour-specific to assess the neonatal hyperbilirubinemia risk.2
For monitoring bilirubin levels before and after phototherapy in both term and preterm neonates, total serum bilirubin (TSB) is the most reliable standard. However, Obtaining blood samples via heel stick or venipuncture is not only painful and time-intensive but also elevates the risk of local and systemic infections, particularly in preterm neonates.4
Although TCB is an established screening method worldwide, its reliability may vary depending on ethnicity, skin pigmentation, hydration status and health system context.5,6 In India, available studies are relatively few7,8 and most are from limited sample sizes. This highlights a research gap, as findings from Western and East Asian populations9–13 cannot always be extrapolated to Indian neonates. Moreover, darker skin pigmentation may affect optical bilirubin detection,5 underlining the need for region-specific validation. Against this backdrop, our study provides novel data by evaluating TcB in late preterm and term neonates in South India, with a larger sample size and additional analysis of correlation with hours of life and gestational age. This strengthens the evidence base for TcB adoption in resource-limited neonatal units especially southern India.
This prospective study was carried out at a tertiary care NICU in a tier 2 city of south India following approval from the Institutional Ethics Committee, Kasturba medical college, Mangalore (Reg No. ECR/541/Inst/KA/2014/RR-20) with approval No IEC KMC MLR 08/2024/543 approved on 21/08/2024. The study is done as per STROBE guidelines for cross-sectional observational study. We adhered to all ethical parameters as per Declaration of Helsinki. The primary objective was to examine the correlation between transcutaneous bilirubin (TCB) and total serum bilirubin (TSB) levels in neonates with jaundice who required phototherapy.
The study included 271 neonates admitted to the NICU between August 2024 and December 2024. Both term and preterm neonates (>35 weeks) with clinical jaundice were included, while neonates with major congenital anomalies, skin conditions affecting the forehead or sternum, or those who had already received phototherapy or undergone exchange transfusions were excluded.
For each neonate, demographic details, antenatal history, maternal complications, feeding patterns, and clinical examination findings were documented using a structured pro forma. Bilirubin levels were assessed using two methods:
1. Transcutaneous Bilirubin (TCB): Measurements were taken using a Transcutaneous Jaundice Detector (Model MBJ20). Three readings were taken over the mid-sternum by the duty doctor, and their average was recorded in mg/dL.
2. Total Serum Bilirubin (TSB): Venous blood samples were collected, and TSB levels were measured using standard laboratory methods.
Each neonate underwent TCB and TSB levels at the same time, and these values were compared for correlation analysis. Data was systematically recorded in an Excel sheet.
To detect a mean difference of 0.23 mg/dL between TSB and TCB measurements with a statistical power of 80% and a significance level of p = 0.05, the required sample size was calculated as 271. This value considers the variability in measurements (σ=1.75) and the critical value for a 95% confidence interval (Zα/2=1.96} = 1.96). A design effect 1.17 was incorporated to account for potential clustering or variability across different population subgroups. This adjustment ensures the study is adequately powered to detect clinically significant differences while maintaining the robustness of the results. This calculation aligns with findings from a previous study, which reported a mean TSB of 8.54 mg/dL and highlighted a standard deviation of 1.75 mg/dL in the average difference between TSB and TCB measurements, with negligible variation by gestational age or ethnicity. These findings provide a reliable basis for estimating the sample size required to achieve statistical validity in detecting the specified mean difference.14
Categorical variables were expressed as frequencies and percentages (%), while continuous variables were summarised as means ± standard deviations (SD) and medians with interquartile ranges (25th–75th percentiles). To evaluate the association between TCB and TSB levels, the Pearson correlation coefficient was used, accounting for factors such as hours of life and gestational age. Bland-Altman analysis was performed to assess the agreement limits between TCB and TSB. Data entry was completed using Microsoft Excel, and statistical analyses were carried out using SPSS software (version 29). A p-value below 0.05 was considered statistically significant.
A total of 271 neonates requiring phototherapy were included in the study to assess the correlation between transcutaneous bilirubin (TCB) and serum bilirubin (TSB) levels. Among the subjects, 140 (51.66%) were male neonates, and 131 (48.34%) were female neonates.
Table 1 provides a summary of the baseline characteristics of the study participants.
Table 2 outlines the descriptive statistics for transcutaneous bilirubin (TCB) and serum bilirubin (TSB) levels ( Figure 1).
| Variable | Mean ± SD | Median (25th–75th percentile) | Range |
|---|---|---|---|
| Transcutaneous bilirubin (mg/dL) | 13.2 ± 3.14 | 13 (11.1–15.85) | 2–19 |
| Serum bilirubin (mg/dL) | 11.4 ± 2.97 | 11.3 (9.5–13.5) | 2.04–18.7 |
There was a significant positive correlation between TCB and TSB levels, with a Pearson correlation coefficient of 0.629 ( Table 3, Figure 2). This correlation was statistically significant, with a p-value of <0.0001.
| Variables | Serum bilirubin (mg/dL) | Transcutaneous bilirubin (mg/dL) |
|---|---|---|
| Pearson Correlation coefficient: | 0.629 | |
| P value: | <0.0001 | |
The correlation of TCB and TSB levels with hours of life and gestational age is summarised in Table 4.
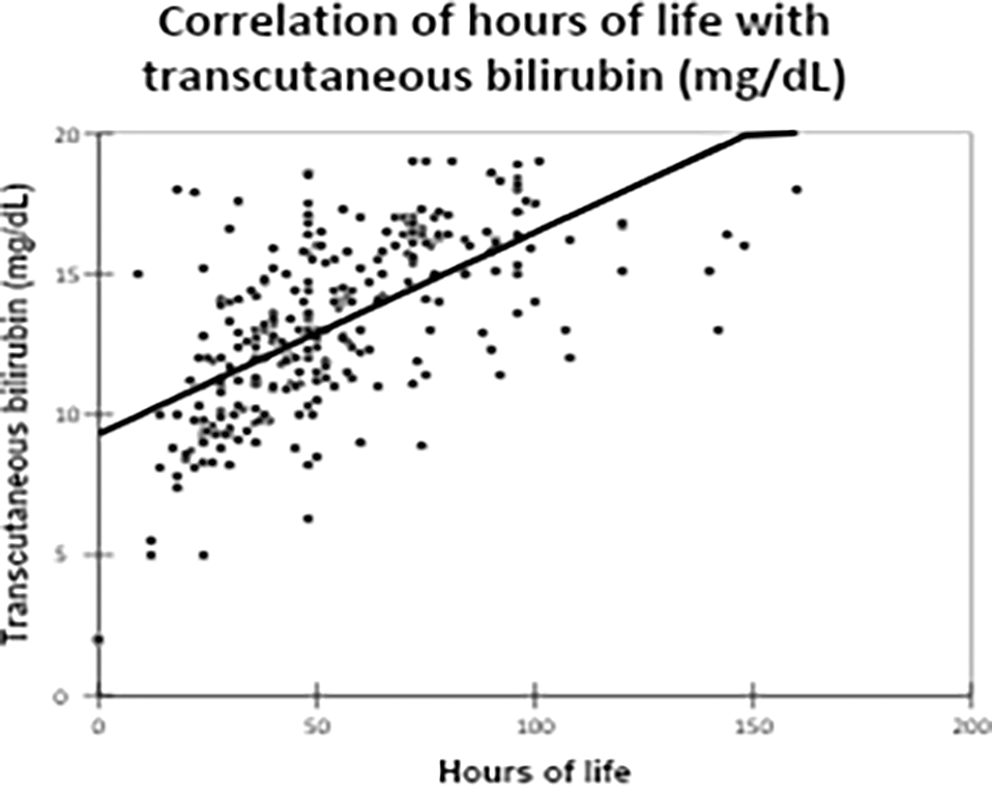
TCB levels demonstrated a strong positive correlation with hours of life (correlation coefficient: 0.620, p-value: <0.0001), indicating that TCB is beneficial during the first five days of life Figure 3.
The Bland-Altman plot ( Figure 4) demonstrates good agreement between TCB and TSB levels, with a mean difference of 1.81 mg/dL between the two values.
The use of TCB measurement is increasingly favoured in hospital postnatal wards and neonatal intensive care units (NICUs) due to its ability to provide early detection, prompt intervention, and timely treatment, ultimately reducing neonatal morbidity and mortality associated with neonatal jaundice. However, its widespread adoption remains limited, particularly in developing countries, due to cost concerns and limited data supporting its use. TCB offers a non-invasive, rapid alternative to TSB tests, reducing the need for painful blood draws. This study evaluated whether TCB measurements reliably correlate with TSB levels.
Our study found a strong positive correlation between TCB levels and TSB levels. A previous multicentric study by James A. et al. shows a correlation of 0.78 was observed, similar to the positive correlation found in our study. The mean difference between TCB and TSB was noted as 0.84 mg/dL,15 while our findings also showed a close approximation between these two measurements. Surana et al. (2017) reported a strong correlation (r = 0.836) among 160 neonates of varying gestational ages, similar to our findings.7
In the study by Arasar Seeralar et al., involving 267 neonates, there was a significant correlation between TCB and TSB levels, which aligns closely with our findings.8 Majid Mansouri et al.14 have also shown that TCB measurement is a reliable estimate of TSB levels in neonates. These studies support the use of TCB as an effective, non-invasive method for monitoring jaundice in newborns. The strong positive correlation between TCB and TSB levels with hours of life observed in this study is consistent with findings by Rahmawati D et al., a study conducted at Dr Soetomo General Hospital among neonates.9
A high correlation between TCB and TSB has also been shown among infants of Asian descent, such as Indonesian,10 Chinese,11 Japanese,12 and Myanmar.13
In the Bland-Altman plot of this study, the bias line indicated the mean difference of 1.81 mg/dl between TCB and TSB. Most data points fell within ±1.96 times the SD of the difference between TSB and TcB values. This corroborates the strong agreement between TCB and TSB.
In resource-limited settings where the prevalence of prematurity is high, it often leads to prolonged NICU stays and phlebotomy-induced blood loss. Given the challenges of access to advanced laboratory techniques, TCB measurement is a more efficient and less invasive screening tool than visual assessment alone. It is quick, painless, and reliable for early identification of hyperbilirubinemia, reducing the reliance on invasive TSB tests. Regular TCB assessments can effectively guide early intervention, thereby improving neonatal outcomes.
The accuracy of TcB is known to be influenced by skin pigmentation, with several studies noting variations across different ethnic populations.15,16 South Indians predominantly belong to the Dravidian ethnic group and typically have darker skin tones compared to North Indian or Caucasian populations. Skin hydration is also another factor which can influence TcB measurements, although this has not yet been extensively studied and validated in literature.17 Our center is in a coastal region where neonatal dehydration is relatively common. Accordingly, we included invasive serum bilirubin measurement as the reference standard for comparison, prioritizing patient safety and keeping in line with our institutional protocol.
While this may appear to increase invasive blood sampling, it provided robust paired data for correlation and Bland–Altman analysis, which is one of the best parameter for external validation.18 Future multicentric studies in Indian settings should focus on validating TcB at recommended cut-offs, which would allow safe reduction of unnecessary blood sampling. Another limitation is the single-center design, which may limit generalizability, though our results provide important baseline evidence for South India.
TCB estimation is a valuable non-invasive screening method for detecting neonatal hyperbilirubinemia. Its simplicity, rapid results, and ability to minimise painful blood sampling make it an excellent tool for monitoring jaundice in neonates, especially in settings with limited resources. This method can aid in timely identification and management, thus reducing morbidity associated with severe hyperbilirubinemia.
This prospective study was carried out at a tertiary care NICU in a tier 2 city of south India following approval from the Institutional Ethics Committee, Kasturba medical college, Mangalore (Reg No. ECR/541/Inst/KA/2014/RR-20) with approval No IEC KMC MLR 08/2024/543 approved on 21/08/2024. The study is done as per STROBE guidelines for cross-sectional observational study. We adhered to all ethical parameters as per Declaraion of Helsinki. Written informed consent was taken from parents of newborns (mother or father).
Figshare: CORRELATION OF TRANSCUTANEOUS AND SERUM BILIRUBIN LEVELS IN LATE PRETERM AND TERM NEONATES AT A TERTIARY CARE CENTER IN SOUTH INDIA. https://doi.org/10.6084/m9.figshare.28514285.v3.19
The project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We are thankful to the department faculty and our patients. Without their support, this study would not have been possible.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Bioengineering, Jaundice
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: neonatal health
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: neonatal health
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 19 Nov 25 |
read | |
|
Version 2 (revision) 02 Sep 25 |
read | read |
|
Version 1 07 Apr 25 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)