Keywords
Encapsulated papillary carcinoma, elderly breast cancer, metastasis, centenarian, CDK4/6 inhibitors, case report.
This article is included in the Oncology gateway.
Breast cancer in elderly patients, particularly in centenarians, presents unique diagnostic and therapeutic challenges. Encapsulated papillary carcinoma (EPC) is a rare subtype that typically exhibits indolent behavior, but its aggressive course remains poorly understood. This report describes an exceptional case of EPC in a 102-year-old woman with a rapid metastatic progression.
A 102-year-old female with hypertension presented with a one-year history of a progressively enlarging, left-sided breast mass. Imaging revealed a 28 cm cystic mass with a 14 cm intracystic nodule. Biopsy confirmed ER-positive, PR-negative, HER2-negative encapsulated papillary carcinoma (T3N0M0). Mastectomy with axillary dissection revealed a 13 cm tumor with close surgical margins (<0.5 mm). The final histologic examination concluded to a pure EPC. Three months postoperatively, metastatic lesions in the sternum and liver were identified. Despite initiating letrozole and CDK4/6 inhibitor therapy, the patient succumbed to cardiac arrest.
The case of a 102-year-old female with EPC of the breast illustrates several unique and clinically significant features, including the rarity of metastasis, exceptional tumor size, and implications of advanced age on the disease course and management. This case study in accordance with the CARE guidelines.
Encapsulated papillary carcinoma, elderly breast cancer, metastasis, centenarian, CDK4/6 inhibitors, case report.
First reported case of metastatic EPC in a centenarian, emphasizing age-specific therapeutic challenges.
Positioning the case as a catalyst to re-evaluate risk stratification in elderly patients with breast cancer.
The incidence of breast cancer increases with age. However, clinical management data for patients over 100 years of age remain limited owing to their exclusion from clinical trials.1–3 Encapsulated papillary carcinoma (EPC), a rare entity comprising 1–2% of breast cancers, is histologically characterized by a fibrous capsule and loss of peripheral myoepithelial cells. EPCs typically display indolent behavior, and are classified as in situ lesion.3–5
The pathological classification of EPC, either in situ or invasive, remains controversial, with persistent discrepancies across studies.6 Although uncommon, high-grade EPC exhibiting aggressive histopathological features and clinical progression has been reported.3,6 A clinically utilized framework categorizes EPC into three groups: pure EPC (isolated lesions), EPC with concurrent ductal carcinoma in situ (DCIS), and EPC with invasive carcinoma.7
Prognosis is generally favorable, with metastatic dissemination occurring in fewer in 4.3% (ranging from 0% to12%),3 though outcomes may correlate with tumor size and margin status. EPC predominantly affects postmenopausal women, with a median age of 69.5 years.3
First-line treatment entails complete surgical resection with negative margins (breast-conserving surgery or simple mastectomy), whereas adjuvant therapy is restricted to tumors with invasive components, high-grade morphology, or nodal metastasis.8 Axillary lymph node involvement is exceedingly rare, with an incidence of 1.1 to 3%.3,9 Giant breast tumors (≥10 cm) are infrequent and usually represent phyllodes tumors. However, documentation of giant EPCs remains virtually absent in the literature.3
In centenarians, management decisions are complicated by frailty and comorbidities, requiring tailored therapeutic strategies.1
To the best of our knowledge, this is the first report of EPC in a centenarian female patient with a 30 cm giant EPC and rapid post-surgical metastatic spread, challenging the traditional view of EPC as being universally indolent. This case emphasizes the necessity of individualized risk assessment in elderly populations, even when classical prognostic markers suggest a low-risk disease.
This case was conducted according Care guidelines.
We present the case of a 102-year-old female (10G-8P, 165 cm, 73 kg) with a history of hypertension who presented with a progressively enlarging left breast mass over the course of one year. The patient had no personal or family history of malignancy of breast cancer.
Physical examination revealed a cystic-appearing mass measuring 30 cm in its greatest dimension, deforming the left breast (Figure 1). The mass did not invade the overlying skin, and no palpable ipsilateral axillary lymphadenopathy was noted. There wasn’t any nipple discharge. The contralateral breast was unremarkable.
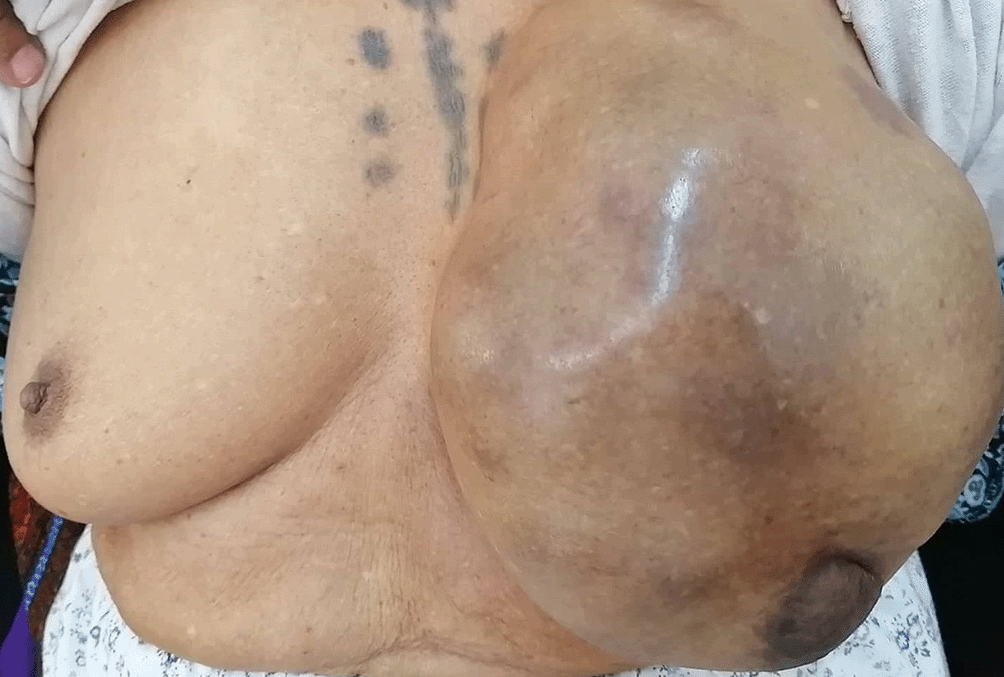
Ultrasound revealed a 28 cm cystic mass with a large intracystic nodule measuring 14 cm was identified. Color Doppler imaging revealed vascularization within the cyst. No pathological axillary lymph nodes were detected.
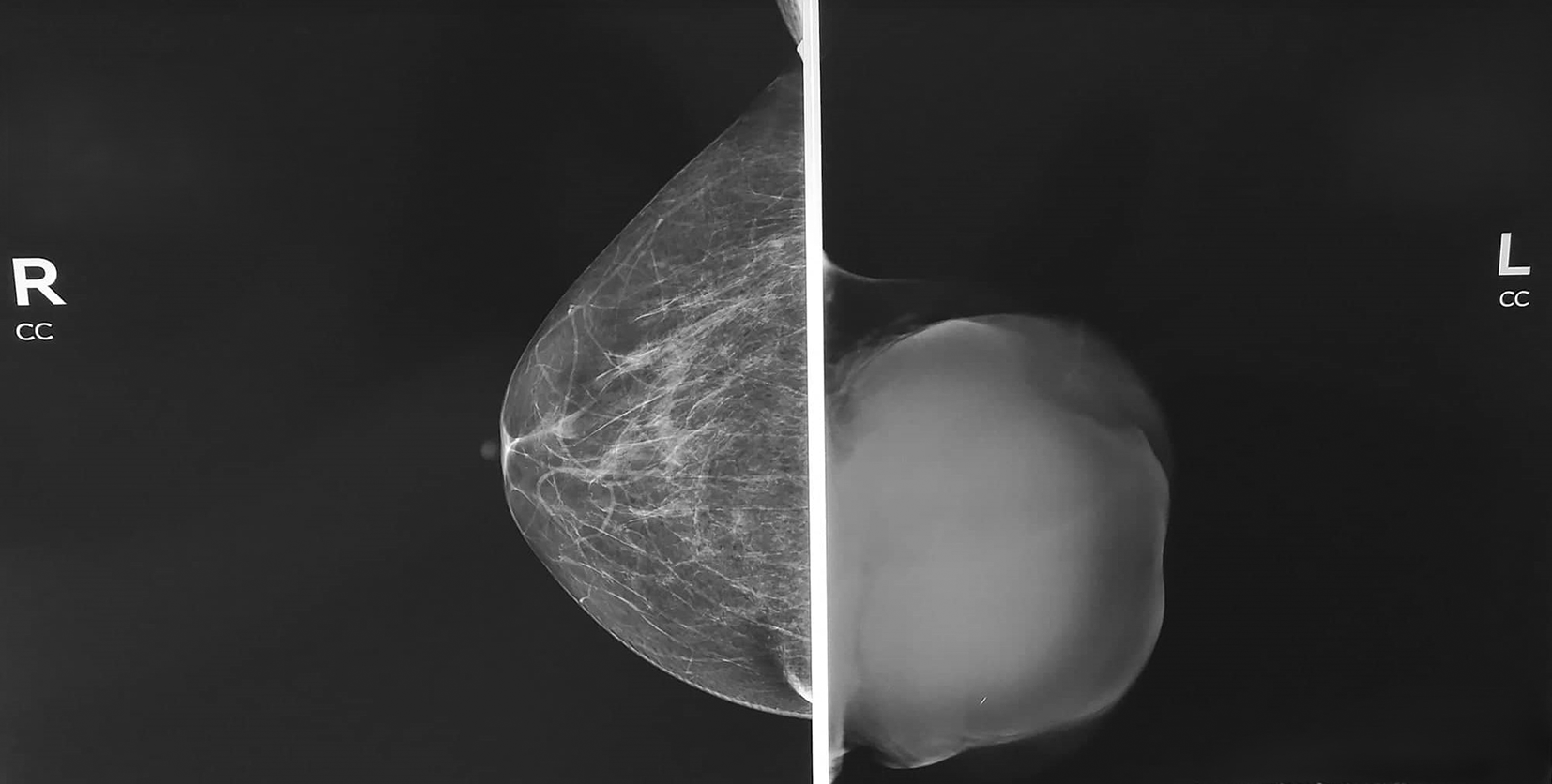
Mammography showed A homogeneous opacity occupying the entire left breast was observed (Figure 2).
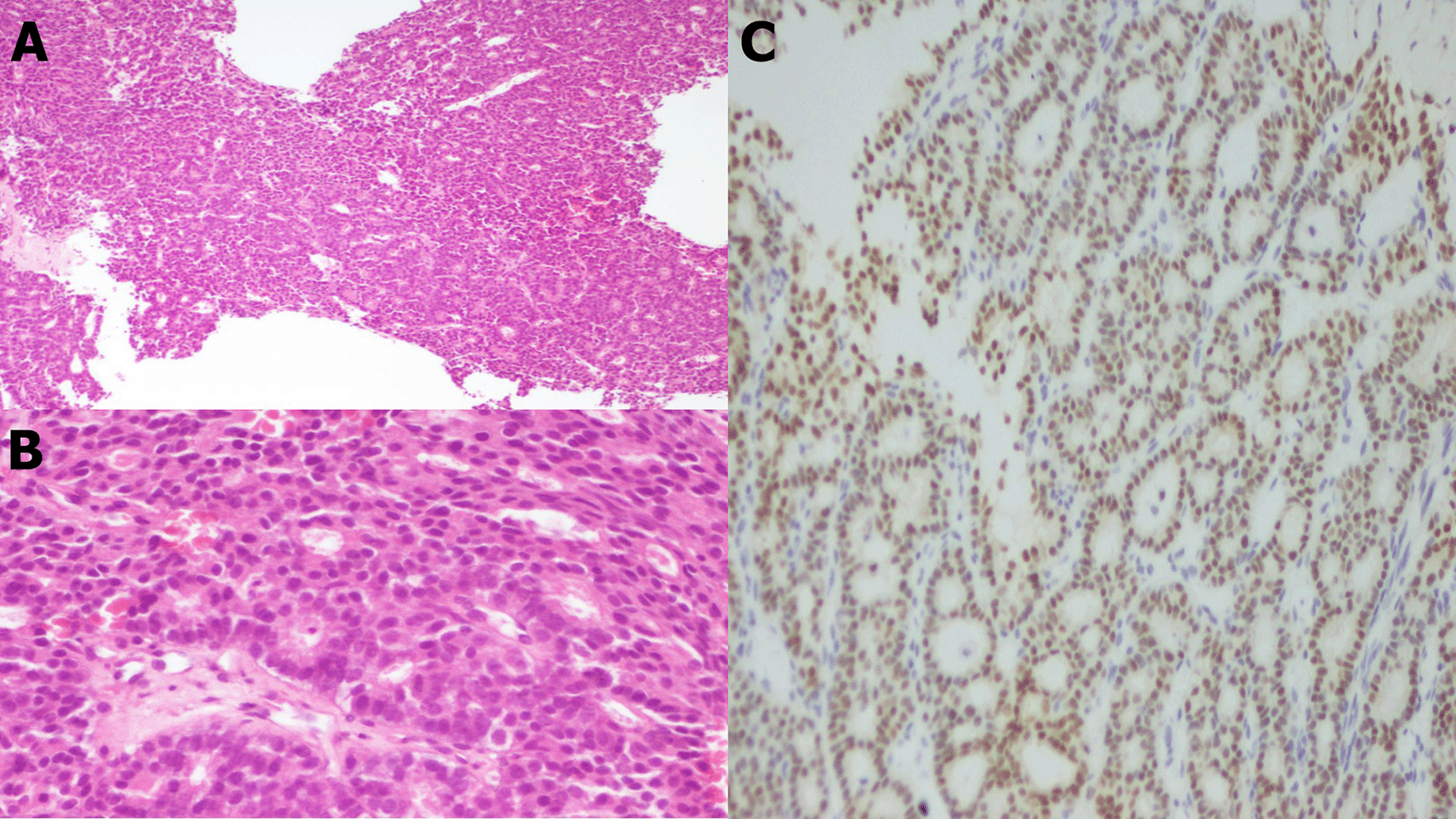
A core needle biopsy under ultrasound of the solid component of the cyst revealed papillary carcinoma (Figure 3). Immunohistochemical analysis showed estrogen receptor (ER) positivity (60%), progesterone receptor (PR) negativity, a HER2 score of 0, and Ki-67 proliferation rate was 10% (Figure 4).
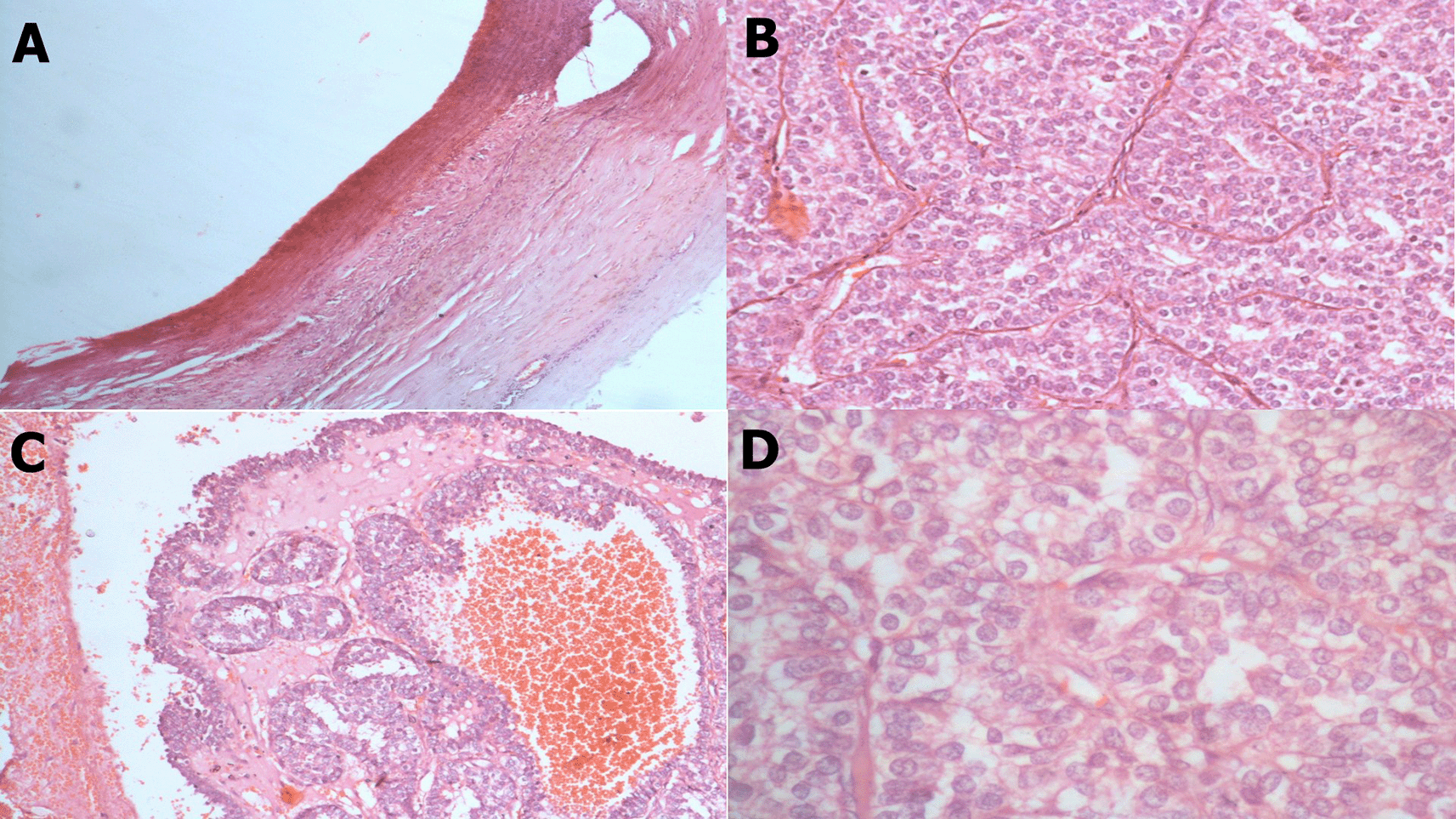
(A) x40: Cyst wall with papillary structures. (B) x100: Complex papillary architecture. (C) x200: Cellular papillae with crowded, back-to-back arrangement. (D) x400: Moderate nuclear atypia.
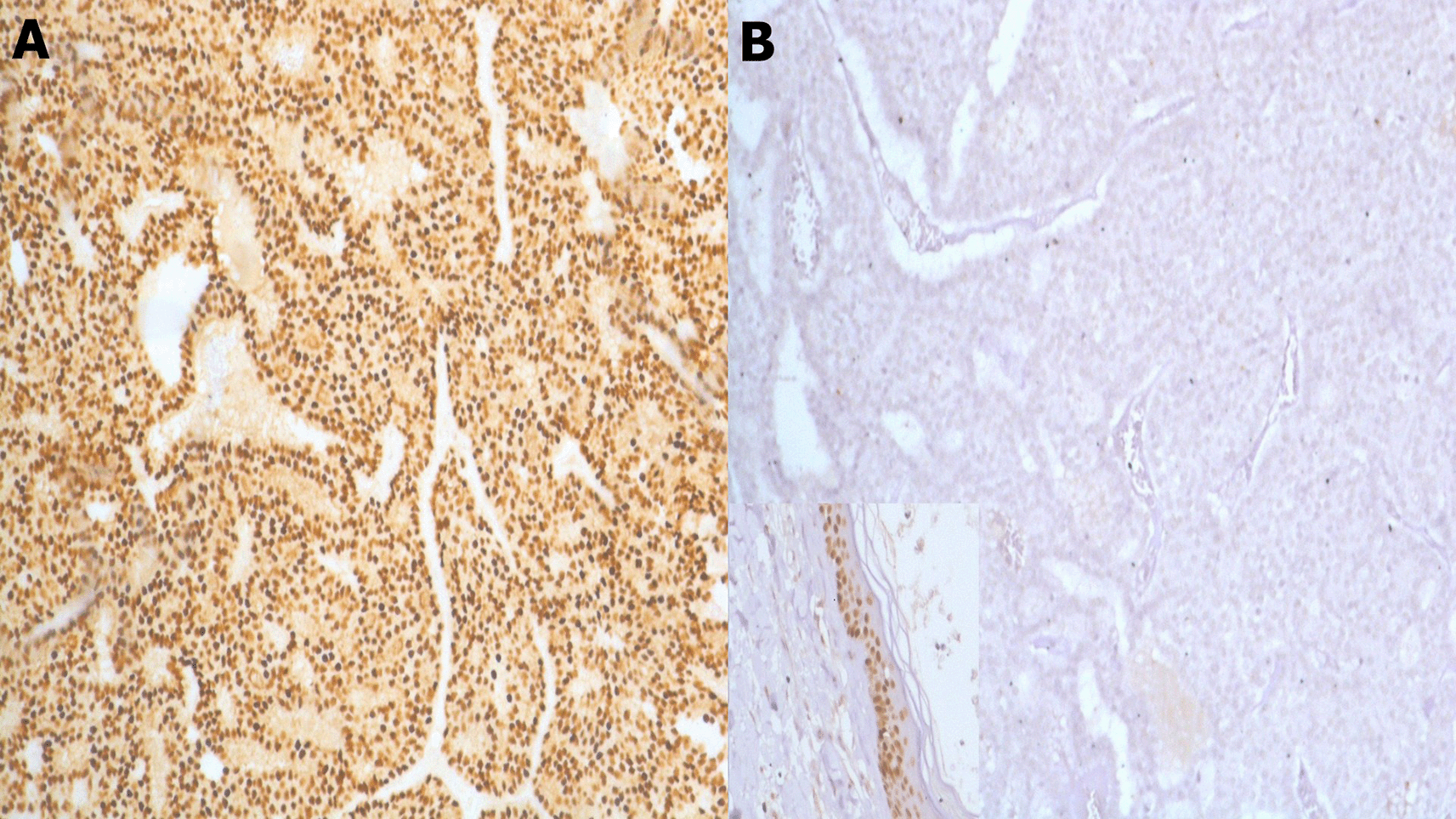
(B) x100 p63 with positive control at the bottom left (epidermis).
Thoraco-abdomino-pelvic computed tomography (CT) scan showed no evidence of distant metastases at the time of initial diagnosis. The tumor was classified as T3N0M0 based on clinical and imaging findings.
Given the large tumor size, thinned overlying skin, and the potential risk of sentinel lymph node biopsy failure, a multidisciplinary team recommended a left mastectomy with axillary lymph node dissection. The patient underwent the procedure without complications.
Pathological Findings showed mastectomy Specimen measuring 23 cm x 21 cm x 3.5 cm. A solid-cystic tumor measuring 13 cm x 14 cm was identified, with a solid component of 7.5 cm x 7 cm and a cystic component of 11.5 cm x 11 cm (Figure 5). The cystic component was septated and occupied the entire breast. The surrounding breast parenchyma was predominantly fatty.
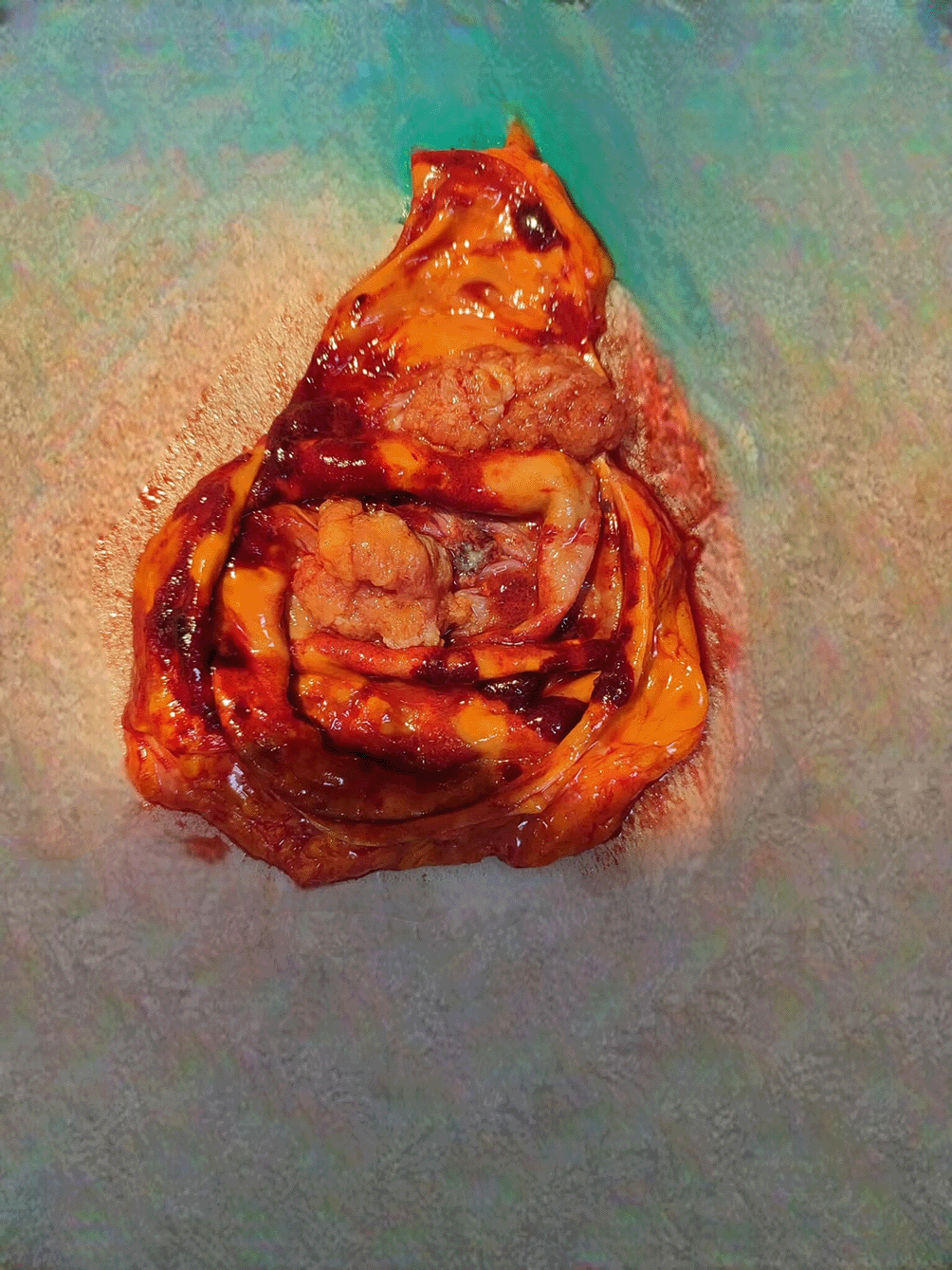
Fifteen axillary lymph nodes were excised, all of which were negative for malignancy.
The tumor exhibited a papillary architecture with mild to moderate cellular atypia. Mitotic activity was 8 mitoses/10 high-power fields (4 mitoses/mm2). The tumor was encapsulated without evidence of infiltration (Figure 3). No associated intraductal component, vascular emboli, or Paget’s disease of the nipple was identified. The surgical margin was <0.5 mm. The tumor cells were negative for p63 (Figure 4).
The patient was presented to the multidisciplinary tumor board, and given the diagnosis of a pure encapsulated papillary carcinoma, no adjuvant treatment was recommended. The therapeutic decision was to plan physical examination 4 times per year during 2 years than 6 months per year for 3 years, and once per year after that.
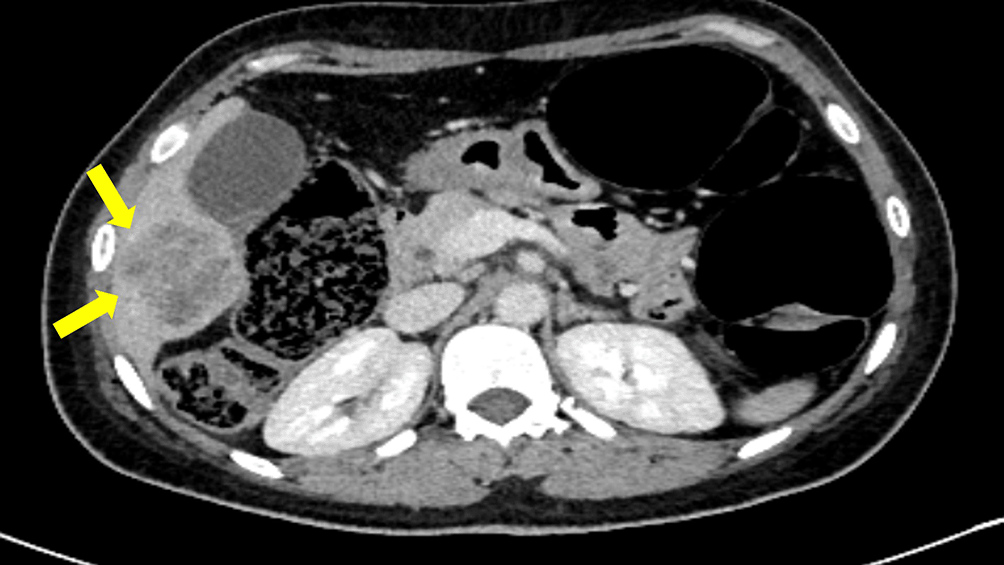
Three months postoperatively, the patient presented with worsening general condition and a sternal swelling. CT imaging revealed a lytic lesion in the sternum and a suspicious liver lesion suggestive of metastasis (Figure 6). A CT-guided liver biopsy confirmed metastatic papillary carcinoma with a cribriform pattern. Tumor cells exhibited abundant eosinophilic cytoplasm and round to oval hyperchromatic nuclei with mild to moderate atypia. Rare mitotic figures were observed. Immunohistochemistry confirmed ER positivity, PR negativity, and HER2 negativity. Neuroendocrine markers (chromogranin and synaptophysin) were negative (Figure 7).
Rare mitotic figures are observed. (C) Immunohistochemestry x20: Nuclear immunostaining of tumor cells by estrogen receptors.
Following multidisciplinary discussion, the patient was started on a combination of anti-CD4/6 therapy and letrozole-based hormonal therapy. Unfortunately, the patient passed away one month later due to cardiac arrest.
EPC is historically classified as an in situ lesion with indolent behavior, though recent evidence suggests it may represent a low-grade invasive carcinoma confined by a fibrous capsule.3,10,11 Metastasis is exceedingly rare, with most studies reporting lymph node involvement in 3% to 18, 8% of cases and even fewer instances of distant spread.12–15 For example, Tan et al. and Gurdal et al. observed no distant metastases in a cohort of 54, and 80 EPC patients respectively.5,16 The median metastasis rate or recurrence rate is 4.3% (ranging from 0% to12%).3,9 The rapid development of sternal and hepatic metastases in the presented patient—three months post-mastectomy—is highly unusual, aligning with rare reports of “late-blooming” aggressive behavior in encapsulated tumors.17 Notably, stromal microinvasion and loss of myoepithelial markers (e.g., p63 negativity, observed here) are now recognized predictors of distant relapse.4,18
The cribriform pattern in the liver metastasis further supports dedifferentiation, which may be indicative of epithelial-mesenchymal transition (EMT) activation, contributing to the tumor’s aggressive progression. However, direct studies linking EMT to EPC are limited.19
The capsule in EPC has been debated as a containment barrier.20 EPC of the breast sits at the intersection of in situ and invasive pathology, with early studies by Carter et al. suggesting it is a DCIS variant, while Collins et al. and Ghannam et al. support its classification as an indolent invasive carcinoma based on myoepithelial loss and capsular characteristics.7,20,21 Esposito et al. counter this with evidence of an intact basement membrane, reinforcing the in situ argument.22 Molecular studies by Duprez et al. and Liu et al. identified shared genomic traits with ER-positive IDC, including PIK3CA and ZFPM1 mutations, underscoring EPC’s unique, less aggressive biology.23,24 Rakha et al. found MMP expression levels in EPC to be intermediate between DCIS and IDC, supporting its transitional status.25 Salvatore et al. and Schwartz et al. further confirmed EPC’s distinct transcriptomic profile and low proliferation potential, suggesting its indolent clinical behavior despite histological ambiguity.26,27 TP53 absence may explain indolence in most cases, this tumor’s sheer bulk likely overwhelmed containment mechanisms. The liver metastasis’s cribriform morphology in the presented case, suggests clonal evolution, with single-cell RNA sequencing identifying ESR1 mutations in metastatic positive hormone receptor, conferring aromatase inhibitor resistance.28
The tumor’s cystic architecture and septations, and the gross tumor size likely created hypoxic niches, fostering angiogenesis (evidenced by Doppler vascularity) and epithelial-mesenchymal transition (EMT). Recent studies have demonstrated that hypoxia-inducible factor-1α (HIF-1α) promotes the transcription of vascular endothelial growth factor A (VEGF-A), which is associated with angiogenesis and metastatic invasion in breast cancer. Furthermore, HIF-1α signaling has been shown to enhance the migration of breast tumor cells by inducing EMT, thereby facilitating metastasis.29–31
The tumor’s massive dimensions (30 cm clinically, 14 cm pathologically) far exceed the T3 classification (>5 cm), challenging conventional prognostic frameworks. While large tumor size is a recognized risk factor for metastasis, EPCs >10 cm are exceptionally rare.3 A 2015 SEER database analysis found that breast tumors >8 cm had a 4.5-fold higher metastatic risk compared to T3 tumors.32
The Extracellular Matrix (ECM) is a dynamic structure composed of proteins such as collagen, fibronectin, and laminin, which provides mechanical and biochemical support to cells. In breast cancer, the ECM undergoes significant remodeling, leading to a stiffened and fibrotic microenvironment that promotes tumor growth and metastasis. Studies have shown that the composition and architecture of the ECM are subtype-specific, with luminal-A and triple-negative breast cancers exhibiting distinct ECM profiles.33 These differences in ECM composition can influence cancer cell behavior, including proliferation, migration, and response to therapy.
Recent studies link bulky tumors to stromal remodeling and immune evasion; exhibit dense fibrotic stroma with reduced CD8+ T-cell infiltration, facilitating immune escape.34 Despite a low Ki-67 index (10%) and modest mitotic rate (8/10 HPF), mechanical stress from tumor bulk and cystic degeneration may have released cells into circulation, overriding typical indolent behavior.
Breast cancer in nonagenarians and centenarians is understudied, with most data extrapolated from octogenarians.1–3,11,34 Kanapuru et al find a one year survival increased from 94.9 to 97.9 %, 93.6 to 96.7 %, and 88.5 to 93.5 % in the 65–74, 75–84, and 85+ age groups often due to vulnerabilities.35 This patient’s rapid decline aligns with findings that metastatic breast cancer in extreme age progresses faster due to immunosenescence and reduced DNA repair capacity.36 The decision to pursue mastectomy reflects evolving geriatric oncology principles, prioritizing symptom control in fit older adults despite limited life expectancy.37
Mastectomy was selected here due to the tumor’s size, thinned overlying skin, and risk of incomplete resection. While breast-conserving surgery is occasionally feasible for smaller EPCs, tumors >5 cm often require mastectomy to achieve clear margins.38
The absence of adjuvant therapy post-mastectomy—consistent with historical guidelines for ER+/HER2- EPCs—may have contributed to metastatic progression. Recent studies suggest that ER+ EPCs with high-risk features (e.g., large size, p63 loss) benefit from endocrine therapy to suppress micrometastases.38 However, adherence to hormonal therapies like letrozole in centenarians is challenging due to polypharmacy and toxicity concerns.39,40
Upon detecting liver and sternal metastases, the patient received letrozole (an aromatase inhibitor) combined with a CDK4/6 inhibitor (e.g., palbociclib), a standard first-line regimen for ER+ metastatic breast cancer.41 However, the presence of ESR1 mutations in metastatic lesions (identified via biopsy) likely conferred resistance to aromatase inhibitors, as these mutations activate ligand-independent ER signaling.42 Emerging therapies, such as selective estrogen receptor degraders (SERDs; e.g., elacestrant), show promise in overcoming ESR1-mediated resistance but were not available in this case.42
Treatment decisions in extreme age require balancing efficacy with quality of life. Comprehensive geriatric assessments (CGAs) are recommended to evaluate functional status, comorbidities, and frailty.43,44 While this patient was deemed fit for surgery, her rapid decline post-metastasis underscores the need for palliative care integration in such cases.37
This case defies the paradigm of EPC as uniformly indolent, highlighting how extreme tumor size and advanced age can override typical biology. Recent molecular insights into stromal interactions, EMT, and clonal evolution provide a framework for understanding aggression in rare EPC variants. Registries capturing extreme presentations (e.g., size >10 cm, metastasis in centenarians) are urgently needed to refine prognostic models and therapeutic strategies.
Written informed consent was obtained from the patient for publication of the case report and accompanying images.
There are no additional data to disclose.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)