Keywords
Arterio-venous malformation, enhanced myometrial vascularity, uterine artery embolization, Progesterone
This article is included in the Global Public Health gateway.
This article is included in the Manipal Academy of Higher Education gateway.
This article is included in the Médecins Sans Frontières gateway.
Uterine arteriovenous malformation (AVM) is a rare cause of chronic to catastrophic vaginal bleeding. The basic pathology is an abnormal arteriovenous connection in the uterine myometrium, which is observed as an entangled serpentine vascular architecture on ultrasound. Color Doppler and higher imaging modalities with angiography are usually used to confirm a diagnosis.
An electronic database of Obstetrics and Gynecology departments was searched for cases of arteriovenous malformation (AVM) between 2021 and 2025. Only cases of AVM confirmed by ultrasound or higher imaging were considered.
We comprehensively reported 15 cases of arteriovenous malformations. All patients had acquired AVMs, with abnormal uterine bleeding as the main symptom in 93% of cases. The treatment provided was medical management in 26% of patients, conservative management by transcatheter embolization in 66%, and hysterectomy in 6.6%.
AVMs are rare in clinical practice, and an understanding of their pathophysiology and prompt diagnosis are highly important for managing this emergency. Fertility-preserving treatments include medical therapy and uterine artery embolization. Hysterectomy may be the definitive treatment for selected cases.
Arterio-venous malformation, enhanced myometrial vascularity, uterine artery embolization, Progesterone
Version 2 provides further insight into the topic of arterio-venous malformation (AVM) in uterine myometrium. Since there is still ignorance about the Enhanced Myometrial Vascularity (EMV) and AVM in obstetric population, there is a possibility of suboptimal or improper treatment. This article sensitizes all the readers about the type of AVMs, aetiology and pathology of the condition, detection methods and differentiation from EMV, with different clinical presentations and management options.
The differentiation between AVM of uterus and enhanced myometrial vascularity in presence of retained products of conception is essential. It is not an uncommon finding after pregnancy or abortion. The presence of retained products of conception needs further evaluation with B hCG and removal of RPOC is the treatment in cases of enhanced myometrial vascularity with or without features of AVM. Presence of AVM requires due caution while evacuation of uterus and backup strategy to prevent catastrophic hemorrhage.
Here, we also mention about the diagnosis by ultrasound and doppler. The color doppler also acts as management guide for the treatment. For example, many centers prefer conservative therapy with low pulsatility index (PI) but uterine artery embolization with high PI on doppler. The angiography of uterine vessels is the gold-standard diagnostic modality. Many a times, presenting symptoms of the patient and availability of the treatment facility determines the mode of management.
Emphasizing that due to the rarity of the condition and unavailability of the treatment guidelines, multidisciplinary decision for the patient care is of utmost importance. The present study mentions about the management provided at tertiary center. In a referral center, the patients are referred after suboptimal or after the failure of primary treatment at private clinics or other health care centers. Hence, in this study, we emphasize the need of interventional radiologist for decision of uterine artery embolization (UAE).
See the authors' detailed response to the review by Antonella Vimercati
Uterine arteriovenous malformation (AVM) is a rare, life-threatening condition. It is characterized by abnormal connections between the arteries and veins of the uterine myometrium bypassing capillary network. The exact prevalence of this condition remains unknown. These can be congenital or acquired. Acquired uterine AVMs are sometimes recognized as having enhanced myometrial vascularity (EVM), which is a preferred terminology nowadays. It refers to any uterine pathology resulting in increased myometrial vascularity regardless of presence or absence of residual tissue of conception.1,2 Uterine acquired AVMs are typically noticed in a multipara woman in her thirties.3 The common manifestations are abnormal uterine bleeding, anemia and catastrophic hemorrhage, which can lead to significant morbidity and, rarely, mortality. Ultrasound and CT angiography are the main diagnostic tests used in the clinical practice. Treatment is generally multidisciplinary and involves obstetricians, radiologists, transfusion medicine professionals, and interventional radiologists. Medical, interventional (uterine artery embolization) and surgical methods are the different management modalities.
The objective of this study was to systematically study cases of acquired arteriovenous malformation and their impact on health. The article has followed strobe guidelines for the reporting of data.
First, this was a retrospective study. Ethical committee clearance was also obtained in 23rd April 2025. (IEC1-166/2025). The search was performed from January 2021 to February 2025 for all patients admitted with an AVM diagnosis. Details of the AVM cases were obtained from the electronic database system or from the medical records section of the Kasturba Medical College, Manipal, Karnataka. Patient enrollment was performed only after confirmation of the diagnosis by uterine power Doppler or after imaging with angiography. Informed written consent was obtained. Data concerning the patient’s age, presenting symptoms, obstetric history, comorbid conditions, detection modality, and treatment provided were obtained. Patients with inconclusive diagnoses were excluded from the study.
During the abovementioned time frame, a total of 19 cases of AV malformation were found, and 15 cases had appropriate doppler or higher imaging diagnosis. Patient characteristics are summarized in Table 1. The age range of the patients was 24–40 years. The presenting symptom was bleeding or spotting per vagina in 93.3% of patients. A total of 86.8% of patients were referred, and a referral was observed 4 days to 10 weeks after the trigger event. A total of 13.2% of patients had severe multiple system involvement. The distribution of gravidae was as follows: primi (33.3%), second (26.6%), third (19.9%), fourth (13.2%), and fifth (6.6%) gravidae. A total of 46.2% had previous cesarean sections and 19% had undergone vaginal delivery. A total of 19.98% of women had a history of previous pregnancy mishaps in the form of first-trimester abortions. A total of 93.34% of women had early pregnancy loss or MTP as an inciting event between 5 and 14 weeks. A total of 79.9% of patients required dilatation and curettage (D&C) or MVA before or after AVM diagnosis. One patient presented after normal delivery. The reported hemoglobin level was between 4.2 and 11 during treatment, and 46.2% of the patients received blood ± blood product transfusions. B hCG observed at the time of admission varied between 0.1 and 109 mIU/ml, which became negative during follow-up visits. Two patients delivered after treatment (Uterine Artery Embolization), one of whom had stage 1 gestational trophoblastic neoplasia.
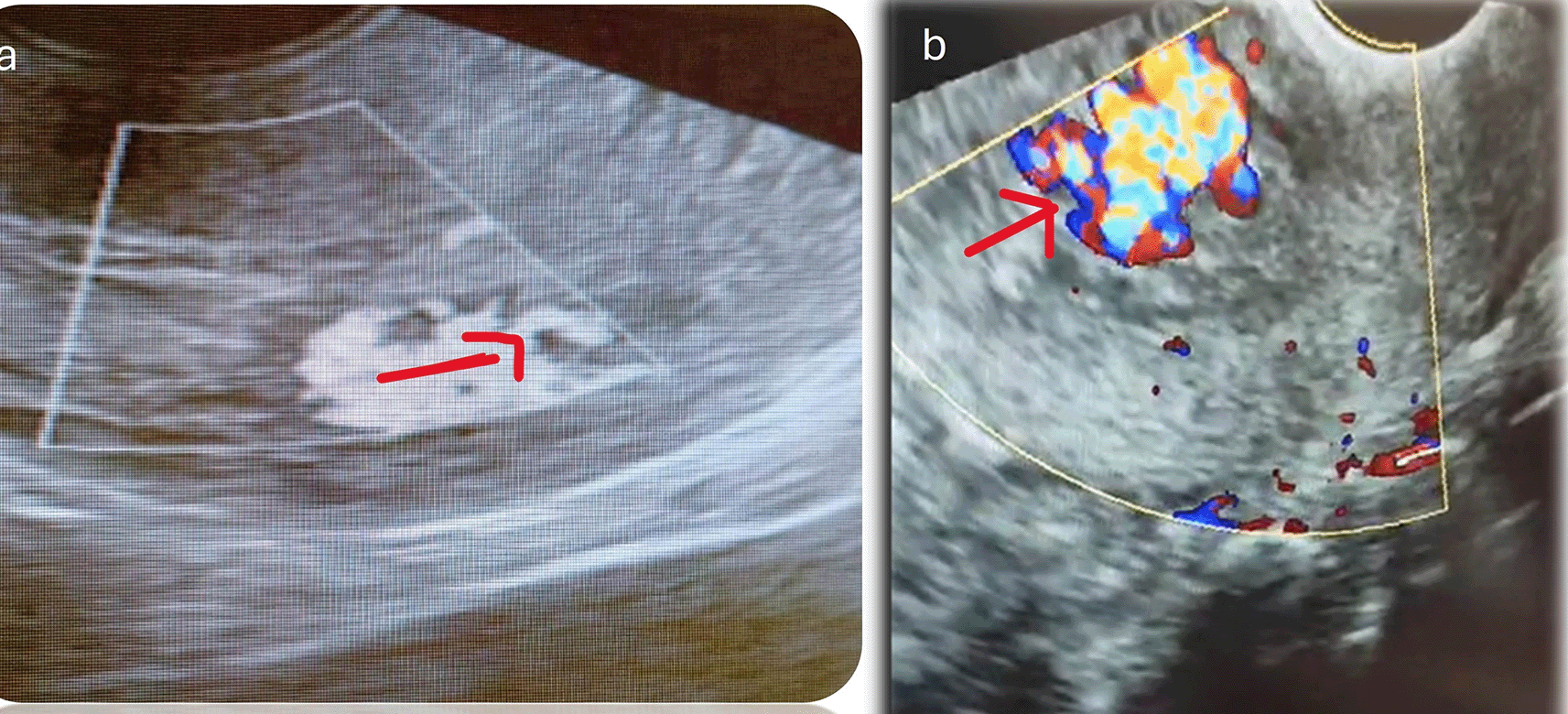
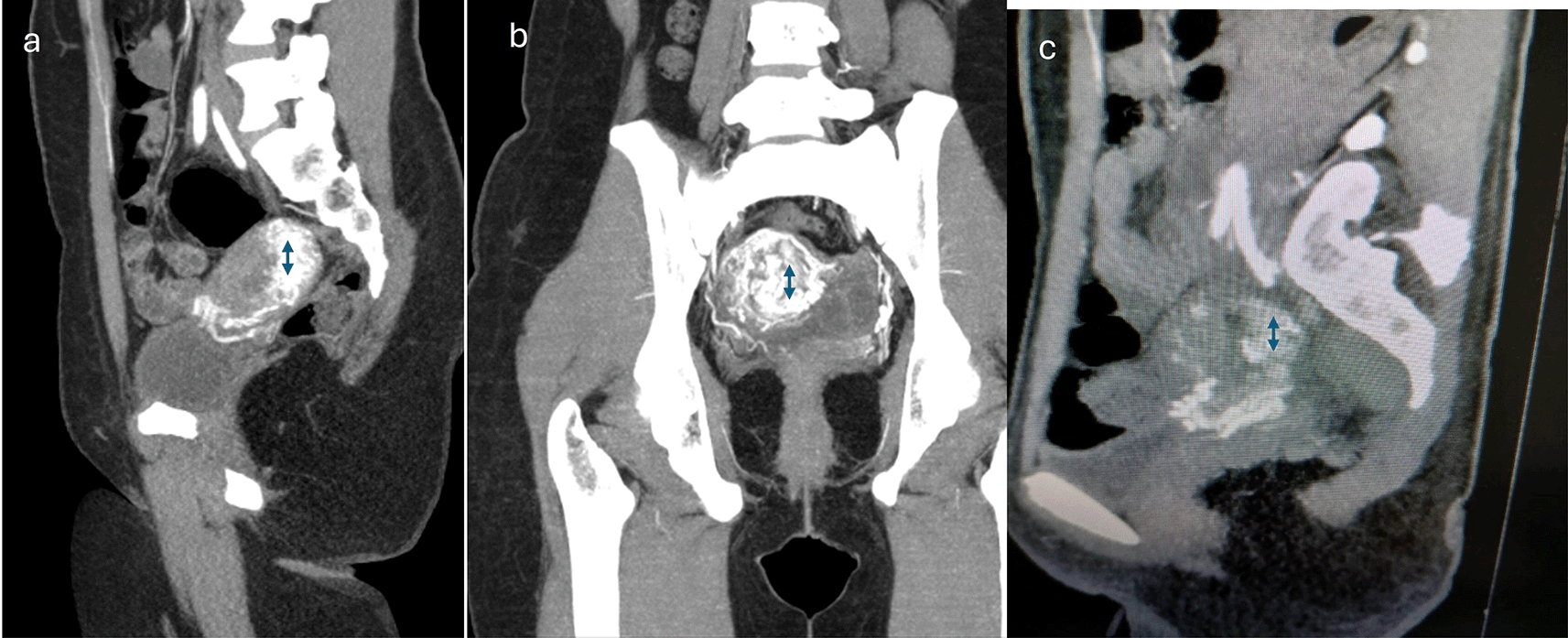
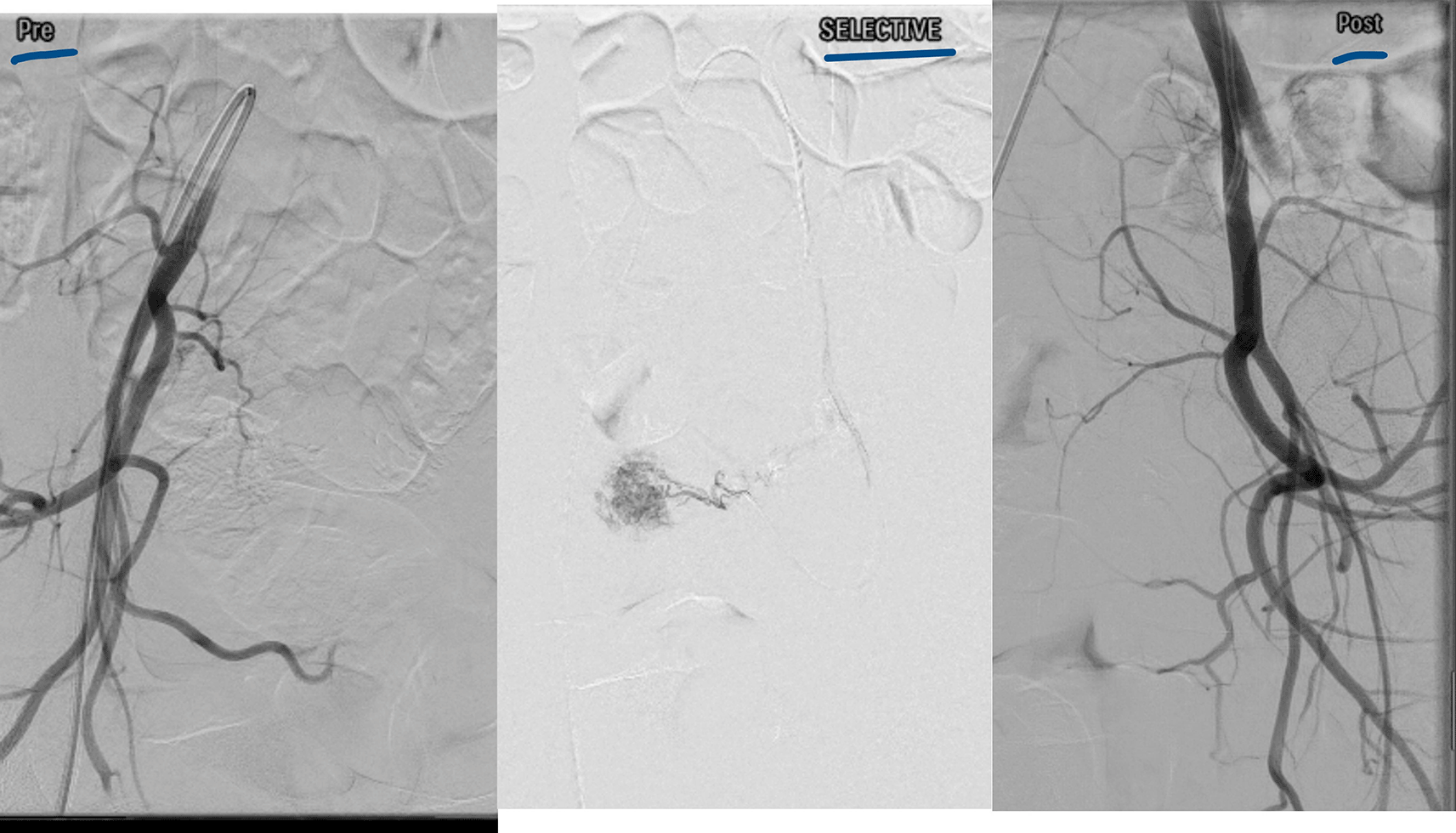
The diagnostic modalities and treatments are summarized in Table 2. All patients underwent transvaginal ultrasound, and confirmation was provided by CT angiography in 59.4%, MRI angiography in 19.9%, digital subtraction angiography (DSA) in 13.3%, and contrast-enhanced computed tomography (CECT) in 6.6%. We observed that posterior uterine wall lesions were more common than anterior uterine wall lesions. The final treatment options included uterine artery embolization/transcatheter embolization (TCE) (66%), surgical hysterectomy (6.6%), and conservative management (26.6%). Failure of conservative management was observed in 2 patients (one after treatment with OC pills and one after treatment with progesterone). Injectable leuprolide (2 patients) and progesterone (2 patients) treatment resulted in satisfactory control of bleeding. One patient with medical management required an additional balloon tamponade for symptom control. Four procedures involved the use of PVA exclusively as embolization material (500–710 μm), one involved the use of glue, and five procedures involved the combination of both as embolization materials. The glue used was NCBA with a concentration between 20 and 40% and 2–3 cc used for a single artery, and is often used with a lipiodol emulsion. All the patients underwent bilateral uterine artery embolization (UAE). Figure 1 shows an ultrasound depiction of the AVM. MRI and Angiography findings of the AVM are depicted in Figures 2 and 3, respectively. Figure 4 illustrates the UAE procedure.
| Cases | Location of lesion | MRI/CT/DSA | Procedure | Definitive treatment | ICU admission | Hospital stay | Follow up | Other remarks |
|---|---|---|---|---|---|---|---|---|
| 1 | Anterior -Left lateral uterine wall | MRI** Multiple serpentine flow voids with early draining veins 2x2 cm | Nidus occlusion by 33% NBCA glue, 2 cc | B/L UAE | HDU | 7 days | 3 months | Fertility not desired |
| 2 | Anterior uterine wall | *Dilated vessels within myometrium of 2.5x2.5 cm | 3 cc NBCA glue, right UA: PVA particle 350-500 microns | Progesterone & DMPA failure B/L UAE | ICU | 17 days | 4 months | Fertility desired Infection |
| 3 | Antero-lateral uterine wall | *7x5x4.7 cm predominantly cystic area, aneurysmal dilation | 40% NCBA glue, 2cc. additional PVA particles | B/L UAE | HDU | 5 days | 5 months | Fertility not desired |
| 4 | Fundal and Right lateral wall | MRI**3.4x2.5x2.2 cm, arterial enhancement with early drainage | Conservative | Leuprolide for 3 months | Na | 5 days | 6 months | Fertility desired |
| 5 | Fundal and anterior wall | DSA 3x2.5 cm hypervascular area with turbulent flow in fundal and anterior wall | PVA 500-710 microns | Primolute failure B/L UAE | HDU | 10 days | 36 months | Fertility not desired MTP for subsequent pregnancy |
| 6 | Right entire posterolateral wall | CECT: nidus of vessels involving full thickness of myometrium, 5x4.5 cm | Conservative | Norethisterone Tapering doses | Na | 6 days | 29 months | Sterilization done infection and PID |
| 7 | Posterior wall of myometrium | *2x2.3 cm nidus | Deferred UAE | TAH, BS, | Na | 6 days | 28 months | fertility not desired detected with chocolate cyst |
| 8 | Posterior endometrial/8 | *hypervascular area at endomyometial wall | PVA particles and gel foams | B/L UAE | Na | 5 days | 29 months | Fertility not desired afterwards MVA for irregular bleeding |
| 9 | Left fundal posterior wall | *2x2.3 cm nidus at posterior wall | PVA particles | B/L UAE | HDU | 4 days | 36 months | Fertility not desired |
| 10 | Posterior fundal myometrium | *left lateral wall and draining to LUA | B/L UAE with NBCA glue, 20% glue:lipiodol | B/L UAE | Na | 5 days | 12 months | Delivered, had GDM |
| 11 | Fundal myometrium | *multiple tortuous vascular channels | 20% glue-lipodol emulsion | B/L UAE | ICU | 3 days | 24 months | Fertility not desired Had hematometra evacuation after a month |
| 12 | Outer fundal left myometrium | MRI**: nidus at fundus 2x2.1 cm, vascular malformation in B/L adnexa and outer uterine wall | 33%, 3 cc NBCA glue and lipidol emulsion | B/L UAE OC pills failure | no | 3 days | 12 months | One live child and fertility desired |
| 13 | Posterior uterine wall | CECT, DSA Nidus of 3x2.5 cm | PVA particles | B/L UAE | no | 7 days | 42 months | Delivered twice, had HDP and one GTT stage 1 |
| 14 | Right posterior lower body myometrium | *right posterior wall nidus of 2x2 cm | Conservative | Progesterone, cervical tamponade | no | 6 days | 3months | Fertility not desired past history of recent infection |
| 15 | Left posterior myometrial wall | *hyper vascular lesion 3x2.2 cm | Conservative | Leuprolide and aromatase inhibitors | No | 10 days | 3 months | Fertility desired Past history of infection |
The hospital stay was 3–17 days, and nearly 19% of the patients had repeated admissions due to hematometra (n=1) and bleeding per vagina (n=2). ICU admissions were observed in two patients: one for temporal mesial sclerosis and another for multisystem dysfunction. 26% the infected patients had infections in the last 3 months, including hepatitis (n=1), endometritis (n=2), endocarditis, and pancreatitis (n=1).
AVMs are rare entities, and two varieties of uterine AVMs have been identified. Congenital AVMs are present from birth and develop during erroneous angiogenesis at the fetal stage, leading to aberrant formation of the primitive capillary plexus with the absence of muscular and collagen tissue.4,5 Congenital or true AVMs are diagnosed either as isolated uterine entities or as a part of extensive pelvic AVMs. Only a few hundred cases have been reported because of the rarity of the condition, and the first case was reported in 1926.4,6
The incidence of acquired AVMs/EMv varies across the studies following abortion or parturition.7 It can be secondary to surgical procedures, pregnancy or other gynaec pathologies. The most common iatrogenic reason is dilation and curettage (D&C), mainly after spontaneous abortion or MTP (medical termination of pregnancy).8 Uterine surgeries, such as myomectomy and cesarean delivery, are iatrogenic causes. It can occur even after a normal delivery. Certain neoplastic conditions, such as gestational trophoblastic neoplasia and endometrial or cervical carcinoma, can even result in AVM.8 It has been reported following infection, fibroid or uterine polyp, intrauterine device placement, cesarean site pregnancy, and in offspring exposed to diethylstilbosterone.1,9,10 The incidence of acquired AVMs has increased due to both, improved diagnosis and increased number of uterine surgeries in recent years.11
In 2015, the international society of ultrasound in obstetrics and gynecology coined the term enhanced myometrial vascularity (EMV) for RPOC-related AVMs.2,12,13 It was almost exclusively meant in the context of recent pregnancy and hence in women of reproductive age.14 In the literature, traumatic AVMs, arteriovenous shunts, EVMs and arteriovenous fistulas are the terms used interchangeably and are difficult to differentiate. All the reported cases of the present study had an event of pregnancy in recent past with vascular web in the myometrium, having early venous filling phase. Hence, we preferred to use term acquired AVMs rather than enhanced myometrial vascularity for the present study.
RPOC-related AVMs are formed between necrotic chorionic villi and venous sinuses of tissue. Histologically, a uterine AVM is an arteriovenous fistula between the intramural arterial branches of the uterine arteries and the myometrial venous plexus, bypassing the normal capillary system, which has an interrupted or absent elastic membrane and completely absent muscular tunica media.15
The spectrum of presenting symptoms varies from asymptomatic detection to catastrophic hemorrhage.16 True AVMs can have additional varicosities in the pelvic or perineal area.17 The usual symptoms are abnormal vaginal bleeding leading to anemia and, sometimes, life-threatening hemorrhage. Other symptoms include pelvic pain, urinary frequency and dyspareunia.18 If not diagnosed and treated in a timely and proper manner, secondary cardiorespiratory or cerebrovascular complications may occur, which can rarely progress to multiple organ failure. The primary complaint was bleeding in 93% (14/15) of patients. Atypical symptoms such as fainting episodes, giddiness, breathlessness, and altered sensorium were present in three patients. One patient had acute kidney injury and another patient had mesial temporal sclerosis. The relationship between AVM and neuronal symptoms, such as temporal sclerosis, is not well established. However, we suggest utmost vigilance during medical therapy for patients with vaginal bleeding to prevent multisystem damage. A referral delay was observed, which needed to be curtailed by appropriate measures to achieve better outcomes.
A transvaginal scan is a primary, cost-effective, and simple screening modality, and AVMs are suspected on grayscale as dilated enlarged, entangled anechoic vessels in the myometrium/parametrium or as myoendometrial hypervascularity with myometrial thickening.6,19 Endometrial assessment is essential to rule out RPOC. Thickened endometrium, hyperechoic or mixed echogenic area within the endometrial cavity in the background of positive B hCG and bleeding has high positive predictive values for diagnosis of RPOC. Vascularity within RPOC and adjacent myometrium should be evaluated. Further to that, ultrasound helps in identifying other uterine conditions like fibroid, polyp, adenomyosis, endometrial or cervical pathologies, which points towards EVMs. It helps in differentiating pelvic congestion syndrome, trophoblastic tumours and caesarean site pregnancies.
EMV should be differentiated from acquired AVMs as AVMs has inherent high risk of sudden profuse life-threatening condition, whereas EMVs may remain asymptomatic or can present with variable bleeding. EMVs have variable spectral flow on doppler ranging from low to high PI (pulsatility index) and angiographic findings also differ for EMV and AVMs. Acquired AVM requires surgical procedures or selective embolization, while medical or conservative management is mainstay of treatment for RPOC or related EVMs. The author believes that acquired AVMs are part of EMV and they have overlapping aetio-pathology and treatment.
Doppler interrogation is often diagnostic, and AVMs are depicted as enlarged, entangled, hypervascular masses with high turbulence and aliasing. It exhibits a multidirectional flow. On power Doppler, it has a high velocity and low resistance flow. The high peak velocity of >20 cm/sec and a low RI between 0.25 to 0.55 are diagnostic of AVMs. It has prognostic ability, as it can stratify patients at high (PSV>60 cm/sec) or low risk for hemorrhage.4 Thus, ultrasound and color Doppler are valuable tools for identifying AVMs and helps in further management.20 3D color Doppler provides better delineation of feeding and draining vessels. We performed grayscale and color Doppler in all patients; however, power Doppler values were not consistently followed in our study.
MRI or CT is often used for confirmation of the diagnosis6 it helps with pre-procedure planning. MRI/CT angiography is a better diagnostic modality for determining the lesion outlines. Uterine artery angiography (UAA), a specific application of digital subtraction angiography, is the gold standard test.4,6 AVMs are characterized by tangled vessels containing nests, feeding arteries, draining veins, and brisk venous depletion. The utility of UAA is generally restricted for patients who would benefit from UAE because of the invasive nature of the test.19 UAA helps in differentiating AVMs from pseudoaneurysms and other vascular lesions. Overdiagnosis can occur if the disease is diagnosed using ultrasonography alone. Angiography is not always needed for diagnosis, but is essential before planning operative management or arterial embolization. Hysteroscopy may be used to diagnose and manage AVMs.15 We used DSA or CT/MRI angiograms as diagnostic or pretreatment tests in 93.4% of the patients.
The treatment is based on multiple variables like severity of symptoms, hemodynamic stability, ultrasound findings and desire for future fertility and ranges from conservative to uterine artery embolization to the hysterectomy.8,21 Owing to the rarity of this condition, there is a paucity of high-level evidence guiding clinicians with respect to its management, hence, tailormade approach was provided to each patient.21 Spontaneous resolution has been mentioned in asymptomatic women, with a wait-and-watch policy for postpartum/postabortion cases.22 The observational treatment requires serial ultrasound, doppler and B-hCG titres monitoring. Our study had antecedent pregnancy event in all the cases, wherein 9 cases had evacuation of RPOC before the referral was made and majority had medical treatment also. The medications used in the literature vary across the studies and for variable times. Progestins and gonadotropin-releasing hormone analogs are the most frequently used medications. Chemotherapeutic agents such as methotraxate, combined oral contraceptive pills, uterotonic (methyl ergonovine), danazol, ulliprtistol, and aromatase inhibitors, either in combination or alone, are less frequently prescribed.21 The success rate varies between 42% and 100% across the studies. Medical management is prescribed either as primary treatment or after failure of transcatheter embolization (TCE).8,21 A systematic review by Rosen evaluated 32 studies, irrespective of study design; 121 patients were managed with medical treatment, with a success rate of 88%. Progestins, GnRH analogs, and methotrexate are more efficacious than other medical agents are.21 Medical management has the advantage of universal accessibility and is less expensive. It also has a higher fertility-sparing capacity. However, for patients with significant bleeding, medical management failure, and hemodynamic instability, UAE/TCE, internal iliac ligation, or other surgical procedures such as hysterectomy are alternative treatments. We reported 33% failures with medical management in patients who were managed with TCE. The success rate of UAE/TCE is reported to be 71–91%.8,23
No TCE failure was observed. Importantly, repeat embolization, medical management, or surgery are secondary options for patients with UAE failure.8 Mild pelvic pain was observed in 20% of the patients in the present study after TCE, which was controlled by analgesics. Postprocedural fever is another complication of post-embolization syndrome. Iatrogenic vessel dissection, contrast-induced nephrotoxicity, and puncture site hematoma are procedure-related complications.8 The decision for unilateral or bilateral TCE was made by an interventional radiologist in our case.
Hysterectomy is indicated only if future fertility is not desired and if immediate medical facilities are not available. It should be performed by experienced pelvic surgeons.24 It is the final treatment if conservative medical treatment or UAE fails, leading to life-threatening hemorrhage. Minimally invasive surgical techniques through the laparoscopic uterine artery or internal iliac occlusion through nonabsorbable clips have also been mentioned in the literature, either following failure of UAE or as primary procedures.25 Bipolar coagulation of the uterine arteries is a modality of treatment.26 The resection of myometrial lesions by laparoscopy or hysteroscopy has been advocated in the literature.15,27
Successful pregnancy and delivery have been described in the literature,28 and we achieved a total of three successful pregnancies after TCE in two patients. The complications observed were mild preeclampsia (one patient) and gestational diabetes (one patient); however, all three neonates were normal. One pregnancy was diagnosed as gestational trophoblastic tumor stage I. There are few studies on fertility outcomes after TCE, but a higher risk of placental abnormalities has been described in the literature.
The main limitations of this study are its retrospective nature and the small sample size. The diagnostic method varies across patients; hence, it cannot provide exclusive power Doppler data for diagnosis. Few patients were lost to follow-up; hence, data on the recurrence of symptoms requiring alternate/definitive therapy are not available. However, considering the rarity of this condition, we presented the exclusive details of patients with AVMs, which is the main strength of this study.
AVMs are rare; however, the incidence of acquired AVM is increasing owing to the increase in the number of uterine surgeries and improvements in diagnosis. The primary investigating modality is ultrasound with color and power Doppler imaging. Uterine artery angiography is a gold standard diagnostic method used for the diagnosis of AVMs. Owing to these rare conditions, no treatment guidelines are available. Each case must be individualized. Conservative treatment is an option in mild cases. It also helps retain fertility. Surgical management is the treatment of choice in severe cases without reproductive desire.
The study was approved by Kasturba Medical College and Kasturba Hospital, Institutional Ethical Committee, IEC 1: 166/2025. Date: 23 April 2025.
Ethical standards and research involving human participants: The study involving human participants followed the ethical standards of the institute’s ethical and research committee and the 1964 Helsinki Declaration and its later amendments.
The data generated for the article are depicted in a table format and uploaded to data repository. Relevant images are deposited for data availability. All author agreed to make all data freely available. The dataset can be accessed.
Data file 1
Repository name: AVM repository PP. Power point containing images of AVM cases.
https://figshare.com/articles/figure/AVM_repository_PP/29091842?file=54615845
DOI: 10.6084/m9.figshare.29091842.29
Data file 2
Repository name: case data in table format. Table format data of AVM cases is available in the repository.
https://figshare.com/articles/dataset/case_data_in_table_format/29066567?file=54541703
DOI: 10.6084/m9.figshare.29066567.30
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Vimercati A, Crupano F, Del Vecchio V, Cicinelli E: A Rare Case of an Arteriovenous Malformation Scar Pregnancy Treated With a Combined and Conservative Approach. Journal of Ultrasound in Medicine. 2019; 38 (7): 1921-1924 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: perinatal medicine
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Cavoretto P, Cioffi R, Mangili G, Petrone M, et al.: A Pictorial Ultrasound Essay of Gestational Trophoblastic Disease. Journal of Ultrasound in Medicine. 2020; 39 (3): 597-613 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Obstetrics and gynaecology, prenatal medicine, ultrasound and imaging
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 16 Sep 25 |
||
|
Version 1 03 Jun 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)