Keywords
SIRR model, delay differential equation, Equilibrium point \sep stability, center manifold theory, Bifurcation Analysis
This article is included in the Pathogens gateway.
This article is included in the Emerging Diseases and Outbreaks gateway.
Ebola virus disease (EVD) is a severe and often fatal illness with high transmission potential and recurring outbreaks. Traditional compartmental models often neglect biologically important delays, such as the latent period before an infected individual becomes infectious, limiting their ability to capture real-world epidemic patterns. Including such delays can provide a more accurate understanding of outbreak persistence and control strategies.
In this study, we develop and analyze a novel deterministic SIRR model that captures the complex transmission dynamics of Ebola by explicitly combining nonlinear incidence rates with a delay differential equation framework. Unlike traditional models, this approach integrates a biologically motivated delay to represent the latent period before infectiousness, providing a more realistic depiction of disease spread. The basic reproduction number (R0) is derived using the next-generation matrix, and local stability for disease-free and endemic equilibria is established. Using center manifold theory, we investigate transcritical bifurcation at R0 = 1, while Hopf bifurcation analysis determines when delays trigger oscillatory epidemics. Sensitivity analysis identifies parameters most influencing R0, and numerical simulations are performed using the fourth-order Runge–Kutta method.
The main novelty of this work lies in its detailed investigation of how delays influence outbreak persistence and can trigger oscillatory epidemics, patterns often observed in practice but rarely captured by classic models. For R0< 1, the disease-free equilibrium is locally asymptotically stable; for R0> 1, an endemic equilibrium emerges. Increasing delays destabilizes the system, amplifying peak infections, prolonging outbreaks, and producing sustained oscillations. Isolation of recovered individuals (c) significantly reduces R_0, while transmission rate (β), recruitment rate (Λ), and isolation transition rate (ρ) are identified as the most sensitive parameters.
Accounting for delayed recovery dynamics is crucial for accurately predicting outbreak patterns and designing effective interventions. This delay-based, nonlinear-incidence model offers a robust analytical and computational framework for guiding public health strategies, with direct implications for reducing transmission, shortening outbreak duration, and preventing epidemic resurgence.
SIRR model, delay differential equation, Equilibrium point \sep stability, center manifold theory, Bifurcation Analysis
Epidemiological modeling plays a pivotal role in understanding and controlling the spread of infectious diseases.1 These models enable researchers and policymakers to analyze disease transmission dynamics, predict outbreaks, and design effective intervention strategies. Among the earliest and most influential frameworks is the SIR model, introduced by Kermack and McKendrick in 1927.1 This simple yet profound model divided the population into three compartments: susceptible, infected, and recovered, and demonstrated the concept of a threshold level of population immunity required to stop an epidemic. The SIR model not only explained how epidemics unfold but also underscored the importance of early interventions. Over the decades, it has remained a cornerstone of infectious disease modeling and public health strategies, forming the basis for countless extensions and refinements in mathematical epidemiology.1,2
In the mid to late 20th century, the field underwent significant expansion, driven by the work of researchers like Anderson and May.3 They broadened the scope of epidemiological models by incorporating critical factors such as heterogeneous mixing patterns, disease vectors, and variable transmission rates. These innovations made the models more applicable to real-world scenarios, providing insights into the dynamics of diseases like malaria, HIV, and schistosomiasis. Their studies emphasized the importance of understanding transmission dynamics, host-vector relationships, and the role of immunity in shaping the persistence and control of endemic diseases. Additionally, they highlighted the potential impact of control measures such as vaccination and vector management on disease eradication efforts.4
Building on these advancements, Hethcote5 made substantial contributions by addressing nonlinear dynamics, seasonality, and spatial heterogeneity in epidemiological models. His work introduced new dimensions to understanding disease outbreaks, particularly in cases where environmental or seasonal factors play a significant role. These refinements underscored the complexity of real-world disease transmission and the need for models capable of capturing multifaceted interactions among biological, social, and environmental variables. The concept of the basic reproduction number R0, as discussed by Heesterbeek and Dietz,6 further formalized how to quantify the potential of a pathogen to spread in a population.
The urgency and complexity of Ebola virus outbreaks have driven widespread interdisciplinary research, especially in mathematical modeling, to guide timely and practical intervention strategies. Ebola virus disease (EVD) is particularly challenging due to its high fatality rate, rapid symptom development, and potential for international spread. These factors make it not only a medical emergency but also a complex modeling problem that requires input from biology, sociology, and public health policy. Over the years, scientists have refined models to include not just disease transmission but also the societal, ecological, and decision-making contexts in which these outbreaks unfold. One of the earlier efforts to understand the spread of Ebola was made by Lekone and Finkenstädt.7 They used a stochastic SEIR model, which stands for Susceptible, Exposed, Infectious, and Recovered, to simulate how interventions like isolating patients and tracing their contacts could slow down the spread. What made their approach notable was how they factored in the randomness of real-world disease spread, something that becomes even more important in smaller populations where each case can dramatically change the outbreak's trajectory. Soon after, Ndanguza and colleagues8 focused on data from the 1995 outbreak in the Democratic Republic of Congo. They used statistical tools to dig into what actually happened during that outbreak. This kind of retrospective analysis helped build more accurate models for future use by highlighting how local healthcare quality and contact patterns influenced disease transmission over time. In 2014, Nigeria's experience with Ebola became a model for successful containment. Fasina et al.9 built a model to understand how early diagnosis, rapid response, and effective isolation measures helped stop the virus from spreading widely. Their work showed that local readiness, like good communication and coordination between agencies, can dramatically change the outcome of an epidemic, even in highly populated cities. Agusto et al.10,11 added a cultural layer to traditional epidemiological models. They looked at how local customs, such as burial practices, affected transmission. For instance, traditional funerals often involve close physical contact with the deceased, which can unknowingly contribute to further infections. Their model demonstrated that overlooking these behaviors could lead to significant underestimation of outbreak severity. This idea was supported by Weitz and Dushoff,12 who explored how the virus can still be contagious even after the host has died, making safe burial practices a critical public health intervention. On a broader scale, Ivorra and colleagues13 developed the Be-CoDiS model, short for Between-Countries Disease Spread. This model wasn't just about one community or one country, it was about how people, goods, and even the virus itself move across borders. Their model helped global health agencies better predict which countries might be at risk next and what steps could be taken to prepare. Berge et al.14 focused on making models that are both simple and useful, especially for countries with limited data and resources. They showed that even basic models could provide valuable guidance if they focused on the right variables, like how long someone remains infectious or how quickly they can be isolated. These small adjustments in the model had a big impact on the accuracy of predictions. Rachah and Torres15 approached the problem using control theory. Their models helped answer questions like: "How much should we spend on vaccinations? When should we focus more on public education?" Their findings suggested that adjusting strategies over time, rather than sticking to a fixed plan, often leads to better health outcomes and a more efficient use of resources. Abo and Smith16 explored what happens when vaccines are either available or unavailable. They built a model that accounted for changes in vaccine supply and how public acceptance might vary. Their work showed that even short interruptions in vaccination campaigns can drastically increase risk, highlighting the importance of consistent public health messaging and resource allocation. At the community level, Marais et al.17 stressed the need for cultural sensitivity in designing infection control programs. Their research showed that people are more likely to follow health guidelines when local leaders and traditions are involved in planning and communication. For example, religious leaders could help shape messages in a way that respects local customs while still promoting safe behavior. On the ecological side, Leroy et al.18 discovered that fruit bats likely serve as a natural reservoir for the Ebola virus. This means the virus can survive in these animals without making them sick, only to spill over to humans under certain conditions. Their work reinforced the idea that human encroachment into wildlife habitats, like forests, isn't just an environmental issue; it's a public health risk too. Burkett-Cadena and Vittor19 expanded on this by modeling how environmental changes, like deforestation, bring humans and wildlife into closer contact. Their models demonstrated how clearing forests or altering ecosystems can create new opportunities for viruses to jump from animals to people, making future outbreaks more likely. In terms of data-driven modeling, Kucharski et al.20 used real-time data from Sierra Leone to adjust their models as the epidemic unfolded. This approach helped public health officials make quicker and better-informed decisions, such as when to deploy more resources or change intervention strategies. Their method marked a shift toward more responsive, real-time modeling that adapts to the outbreak as it evolves. Nishiura and Chowell21 brought all these ideas together in a comprehensive review. They evaluated dozens of different models to see what worked and what didn't. One of their key takeaways was that modeling accuracy improves dramatically when uncertainty, cultural diversity, and differences in healthcare access are taken seriously. They also called for better data-sharing practices to help researchers and governments act more cohesively during crises. Altogether, these studies reflect just how multifaceted Ebola modeling has become. Today's models aim to do more than just predict numbers, they try to capture the human, social, and ecological realities behind those numbers. Whether it's the behavior of a virus, a person, or a government, integrating all these layers gives us a better shot at preventing the next outbreak. The evolution of these modeling approaches shows that science and society must work hand-in-hand to effectively combat emerging infectious diseases. Agbomola and Loyinmi22,23 analyzed the impact of human-bat interactions and control strategies on Ebola transmission using optimal control models, emphasizing the role of vector management and safe burial practices. Legrand et al.24 provided critical insights into the effectiveness of public health interventions such as isolation, contact tracing, and the promotion of safe burial practices, which are crucial in breaking chains of transmission during outbreaks. Pandey et al.25 explored the combined effects of vaccination campaigns, public awareness, and behavioral changes in reducing the spread of Ebola, offering a framework for understanding how early interventions could mitigate future outbreaks. Rivers et al.23,26 demonstrated the power of real-time modeling in predicting outbreak trajectories, providing policymakers with tools to allocate resources effectively during the West African Ebola epidemic. Similarly, Althaus27,28 employed dynamic transmission models to estimate key epidemiological parameters, such as the basic reproduction number R0, for different stages of the epidemic. These models offered vital insights into the rate of spread and the effectiveness of interventions over time. Chretien et al.29 underscored the role of environmental and ecological factors in Ebola outbreaks, highlighting how climatic conditions and land-use patterns influence disease emergence and persistence. While traditional models like the SIR and its derivatives provided a robust framework, they initially lacked the capacity to account for delays inherent in biological and societal processes. Delays, such as the incubation period of a disease, the time lag in symptom onset, or delays in implementing public health interventions, are crucial to accurately capturing disease dynamics. Addressing this limitation, delay differential equations (DDEs) emerged as a powerful mathematical tool to incorporate time-dependent processes into epidemiological models. Early contributions by Cooke and van den Driessche30 demonstrated the utility of integrating delays into compartmental models, allowing for more realistic representations of disease progression and intervention outcomes. These models could, for example, simulate the effects of latent periods or the time-dependent impact of treatment protocols. Epidemiological modeling has long relied on ordinary differential equations (ODEs) to represent the dynamic spread of infectious diseases. These models, typified by the classical SIR framework, offer mathematical simplicity and analytical tractability. However, they inherently assume that all changes in a population's disease status occur instantaneously, overlooking the biological reality that many processes in disease transmission involve time delays. For instance, incubation periods, delays in symptom onset, and the lag between infection and the implementation of control measures are all time-dependent factors that shape disease dynamics in fundamental ways.5 By treating such processes as instantaneous, ODE-based models risk oversimplifying complex epidemiological phenomena. Delay differential equations (DDEs) address this critical limitation by explicitly incorporating time lags into the modeling framework.30–32 This refinement allows for the representation of delayed infection responses, delayed treatment outcomes, and other temporally structured processes, offering a more biologically realistic depiction of disease progression.
Beyond epidemiology, the mathematical foundation of DDEs is well-established in dynamical systems theory, with results on stability analysis, bifurcation theory, and solution behavior extensively developed by researchers such as Hale and Lunel33 and Beretta and Kuang.34 These advances provide rigorous tools for understanding threshold phenomena, long-term behavior, and the conditions under which diseases can be controlled or eradicated. The flexibility of DDEs also extends to computational modeling. Shampine and Thompson35 have shown how modern numerical methods can efficiently solve DDE systems, enabling the simulation of complex scenarios with time delays, such as delayed response to interventions or the accumulation of immunity in a population over time.
In addition, while ODE models offer foundational insights, DDEs provide a more accurate, realistic, and mathematically robust framework for modeling infectious diseases.36,37 The explicit incorporation of delays bridges the gap between theoretical constructs and the biological realities of disease transmission, offering policymakers and public health experts more reliable tools for predicting outbreaks and designing effective interventions.30–32,38
We examine the SEIRR model presented by Ref. 39, whose structure is illustrated in the schematic diagram below Figure 1 and Parameters Description are contained in Table 1. The model focuses on the host compartment of the epidemiological system, where the total population is divided into distinct epidemiological classes:
• Susceptible ( ): Individuals at risk of infection
• Exposed ( ): Infected individuals not yet infectious
• Infectious ( ): Individuals capable of transmitting the disease
The model further distinguishes between two types of Recovered Individuals: Even after recovering from Ebola with medical treatment, patients may still carry traces of the virus in certain parts of their bodies. Research shows the virus can linger in places like semen, eye fluid, breast milk, and spinal fluid - sometimes for weeks or months after symptoms disappear. The subdivision of the Recovered Individuals is:
• Recovered in Isolation ( ): Individuals who have recovered but remain under medical isolation due to potential viral persistence in body fluids
• Recovered without Isolation ( ): Individuals who have recovered and are not under isolation protocols
It is common to observe that infected individuals do not transmit the infection to others immediately. There is a time delay before any confirmed case can occur after the exposure. This delay is known as the latent period. This introduces a gap between exposure and when the infection becomes transmissible. In mathematical form, this latent period is modeled as a time delay in the infection process. Time delays can destabilize the equilibrium point of the system, leading to a Hopf bifurcation. This results in periodic oscillations. This motivates the inclusion of the delay in the model reflecting that individuals infected at time can spend at time .
Let’s take into consideration a delayed SIRR model with a saturation incidence rate and exponential birth rate:
where is a constant related to the saturation effect.
The system of equations below represents the delay differential equations from this model:
From the system of equations, we have in (4)
We can see that and compartments are independent of and .
Thus, it’s enough to consider the following reduced system of equations for our study:
The equilibria of system (5) are found by setting the right-hand side of the system to zero. A stable endemic equilibrium point is a fixed point in a system where if the system is slightly disturbed, it will eventually return to that point. Therefore, the equilibrium solutions of a system with a time delay are the same as those of the corresponding system without delay.
The D.F.E can be found by setting the system of equation in to zero. Which is i.e.
The basic reproductive number : This is the number of secondary infections caused by a single infected individual in a completely susceptible population.
From system (4), we compute by setting
3. Theorem
The disease-free equilibrium is locally asymptotically stable if and unstable if
Let and be matrices representing the infection and transition dynamics, respectively, evaluated at the DFE. Assume that (non-negative matrix) and is a nonsingular -matrix (i.e., a matrix whose inverse is non-negative and has eigenvalues with positive real parts).
When R 1
We know that and is a non-singular -matrix. Hence, , and all eigenvalues of have negative real parts.
If , then . Recall that .
Therefore, is a non-singular -matrix, and by the characteristics of non-singular -matrices, all the eigenvalues of have positive real parts.
Consequently, all eigenvalues of have negative real parts, implying that the DFE is locally asymptotically stable.
When
We can prove that if , the DFE is unstable. Assume and suppose by contradiction that all eigenvalues of have non-negative real parts.
For any , this matrix remains a non-singular -matrix. Hence, it has all positive eigenvalues.
Thus, when , the matrix has at least one eigenvalue with a positive real part, which implies the DFE is unstable.
When
When we can see that
When is the bifurcation parameter, hence the system has a zero eigenvalue (simple eigenvalue) and the other is a negative eigenvalue, i.e. nonhyperbolic equilibrium.
To study the thermal stability of the nonhyperbolic equilibrium, we will need to apply the center manifold theory [Sastry(1999)]42 and Theorem 4.1 of [castillo-chaves and song (2004)]40
To describe this, let’s consider the system a general system for ordinary differential equations.
Let , the system in (5) becomes:
At
at
The jacobian matrix of the system above around
Let’s consider the right eigenvector associated with 0 eigenvalue which is and left eigen vector associated with the zero eigenvalue (simple eigenvalue is )
When we consider the partial derivative associated with the system.
Now we can apply Theorem 4.1[Castillo-chaves and song[2004]40
1. Remarks:
From Theorem 4.1[Castillo chaves and song [2004].40 Since and the system undergoes a transcritical bifurcation at as the bifurcation parameter is varied.
To establish the conditions for the existence of endemic equilibrium , when the disease remains present in the population, the system of equation (5) is reformulated to derive the fixed points for and yielding the following results;
is given by the quadratic equation;
Where
By Rene Descarte’s rule of sign we can say that the quadratic equation have a unique positive real root if the following condition holds:
Local stability of the endemic equilibrium is analysed as follows; The characteristic equation of system evaluated at
To ensure that the endemic equilibrium is locally asymptotically stable, we need both eigenvalues to have negative real parts.
According to the routh-Hurwitz criterion, this happens if and only if;
Hence the theorem:
4. Theorem
for the endemic equilibrium is locally asymptotically stable if both of the following conditions hold simultaneously;
Hence and satisfies Routh-Hurwitz criterion.
The characteristic equation is given by:
Separating real and imaginary parts:
Squaring and adding these parts eliminates and , yielding:
Replacing this in the equation.
Given the condition , it follows that:
From equation (5), we have
The Lyapunov direct method, as formulated by Huang et al,41 provides a systematic approach for establishing the global stability of equilibria in dynamical systems. This method involves the construction of a suitable scalar-valued function, known as a Lyapunov function, which satisfies specific positivity and derivative conditions along system trajectories. In this section, we construct an appropriate Lyapunov functional adapted to the structure of the model (5) and employ it to thoroughly analyze the global stability of the system’s equilibrium points.
The incidence function is expressed as the product .
1. The functions and satisfy and their first derivatives and are strictly positive for all .
2. Either or for all .
3. The function is concave in , i.e., and for all .
On this premise, we state the following theorem:
5. Theorem
1. Under the conditions 1 and 2, the equilibrium point is globally stable for any delay parameter .
2. If conditions (1)–(3) hold and , the DFE is stable for .
Differentiating by Leibniz’s rule,
Thus, is globally minimum.
Let’s assume
Let’s define the Lyapunov function
This is sufficient for Corollary 5.2 of [Kuang (1993).31 We can then say that the endemic state is globally asymptotically stable.
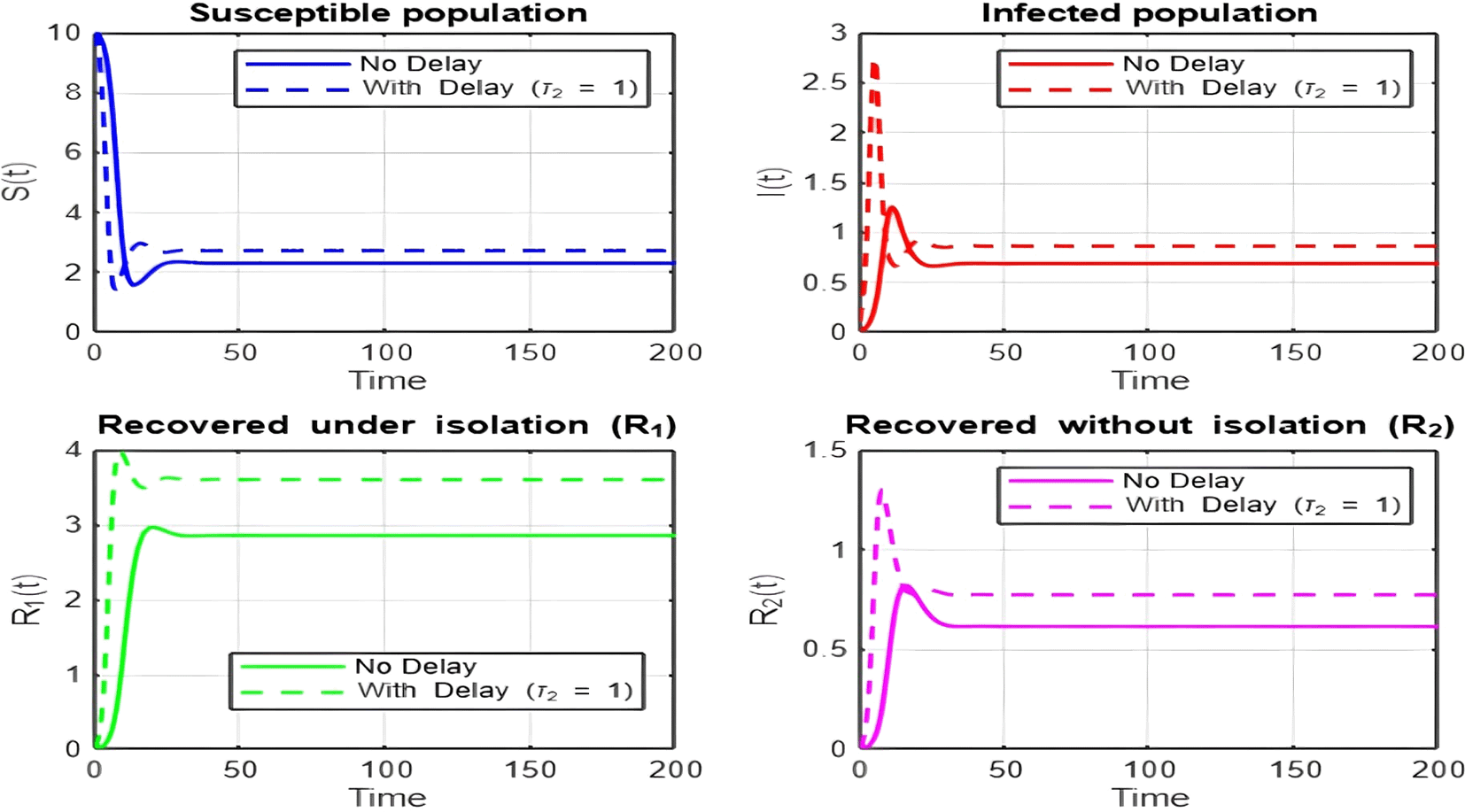
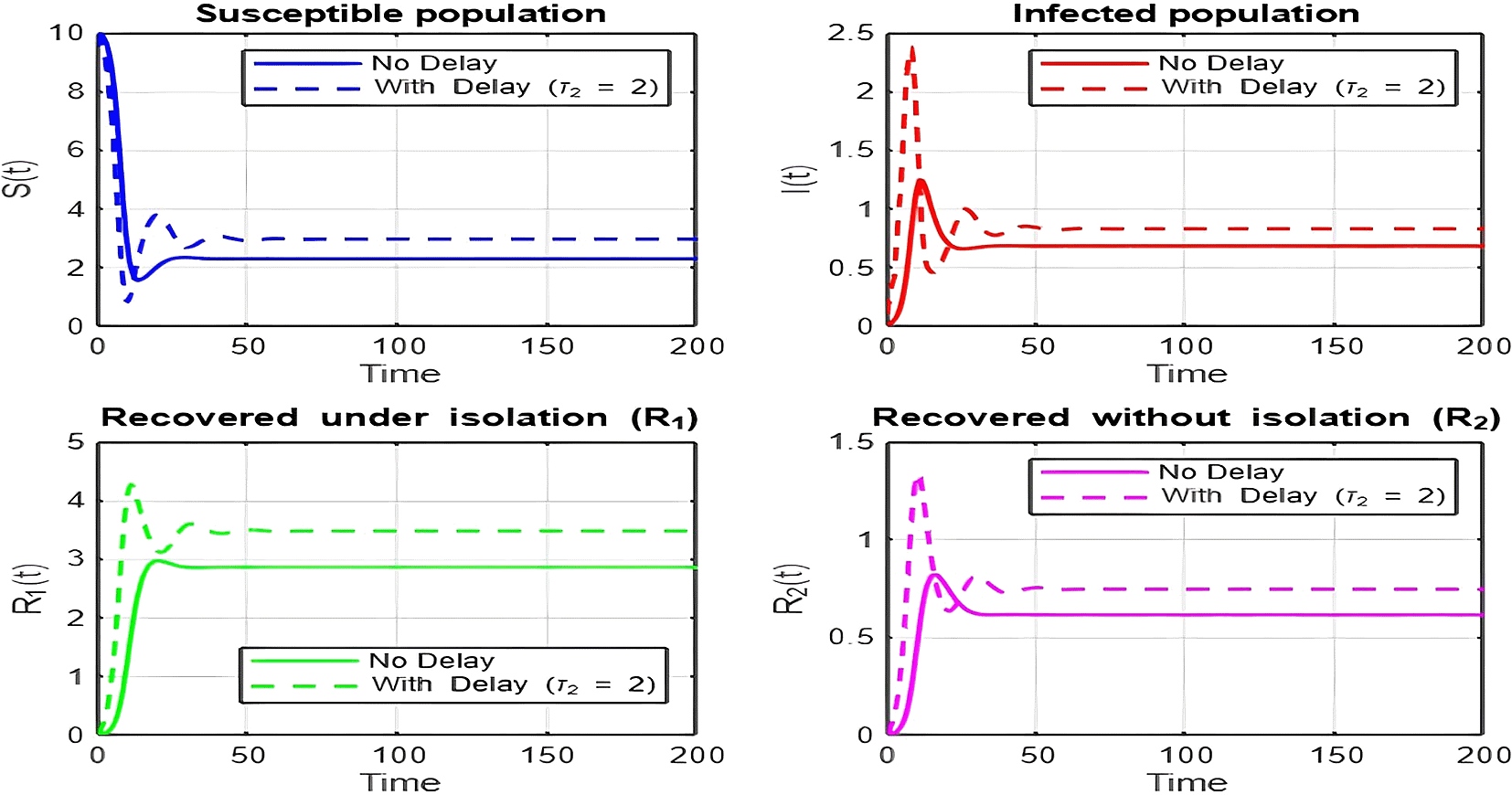
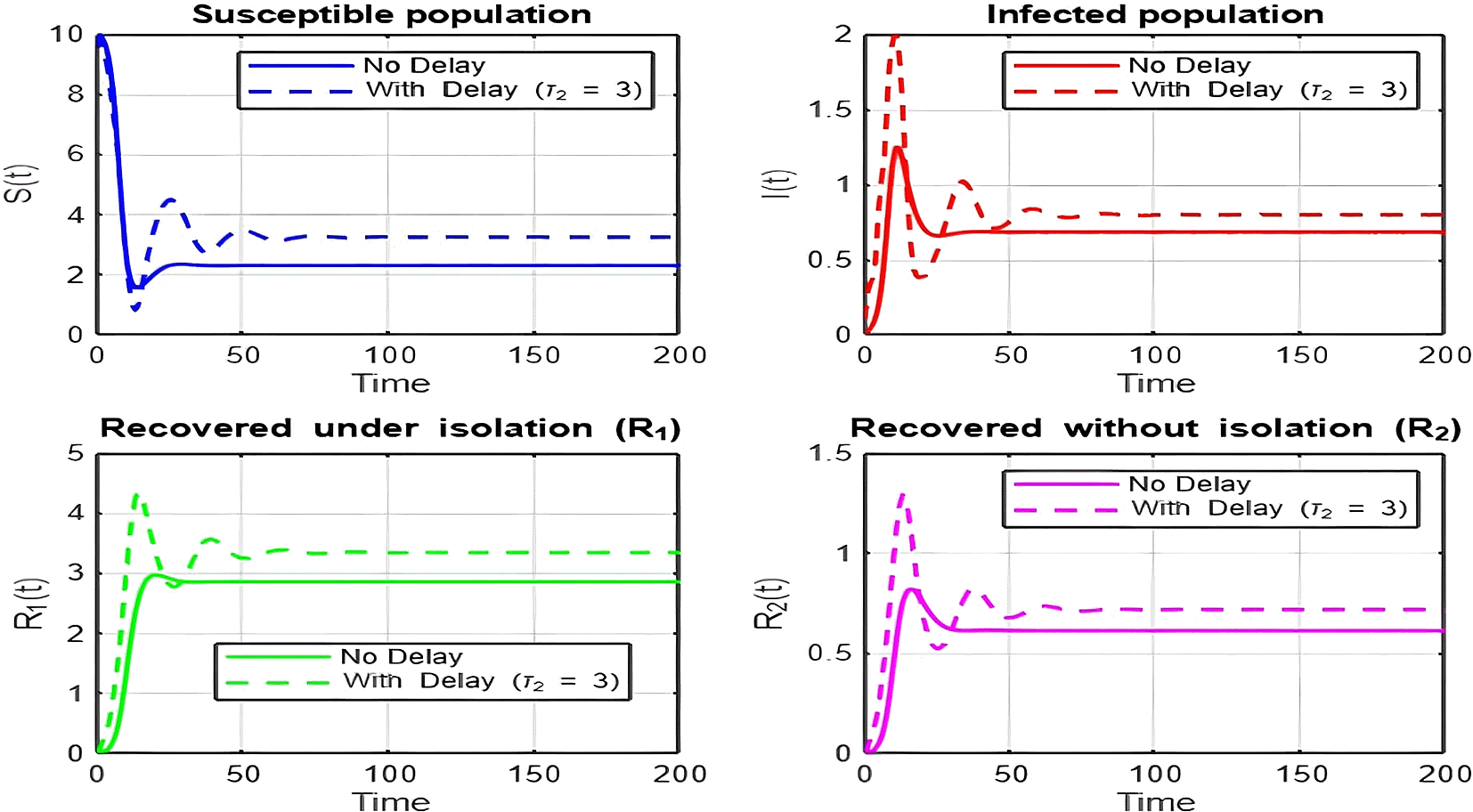
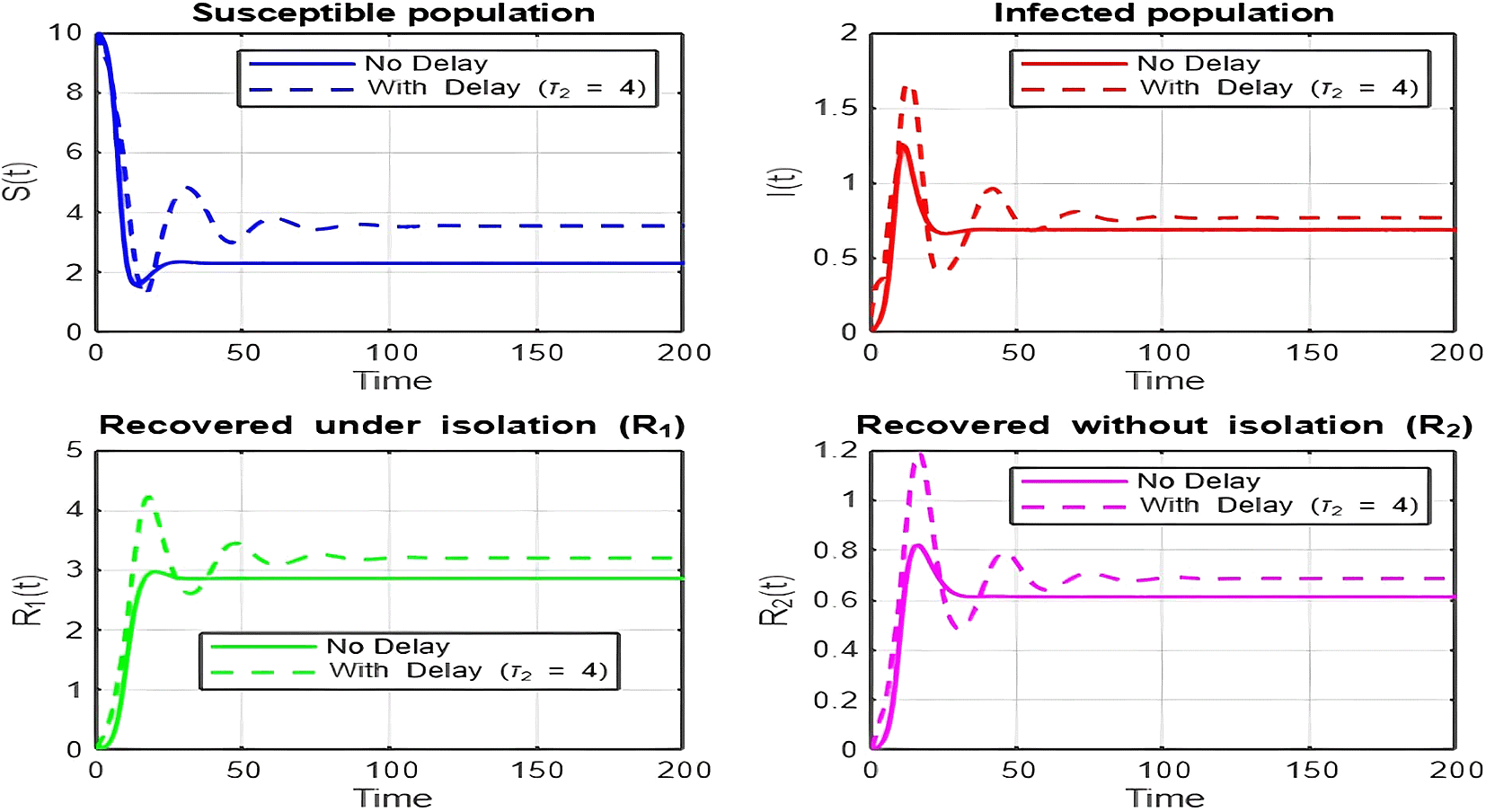
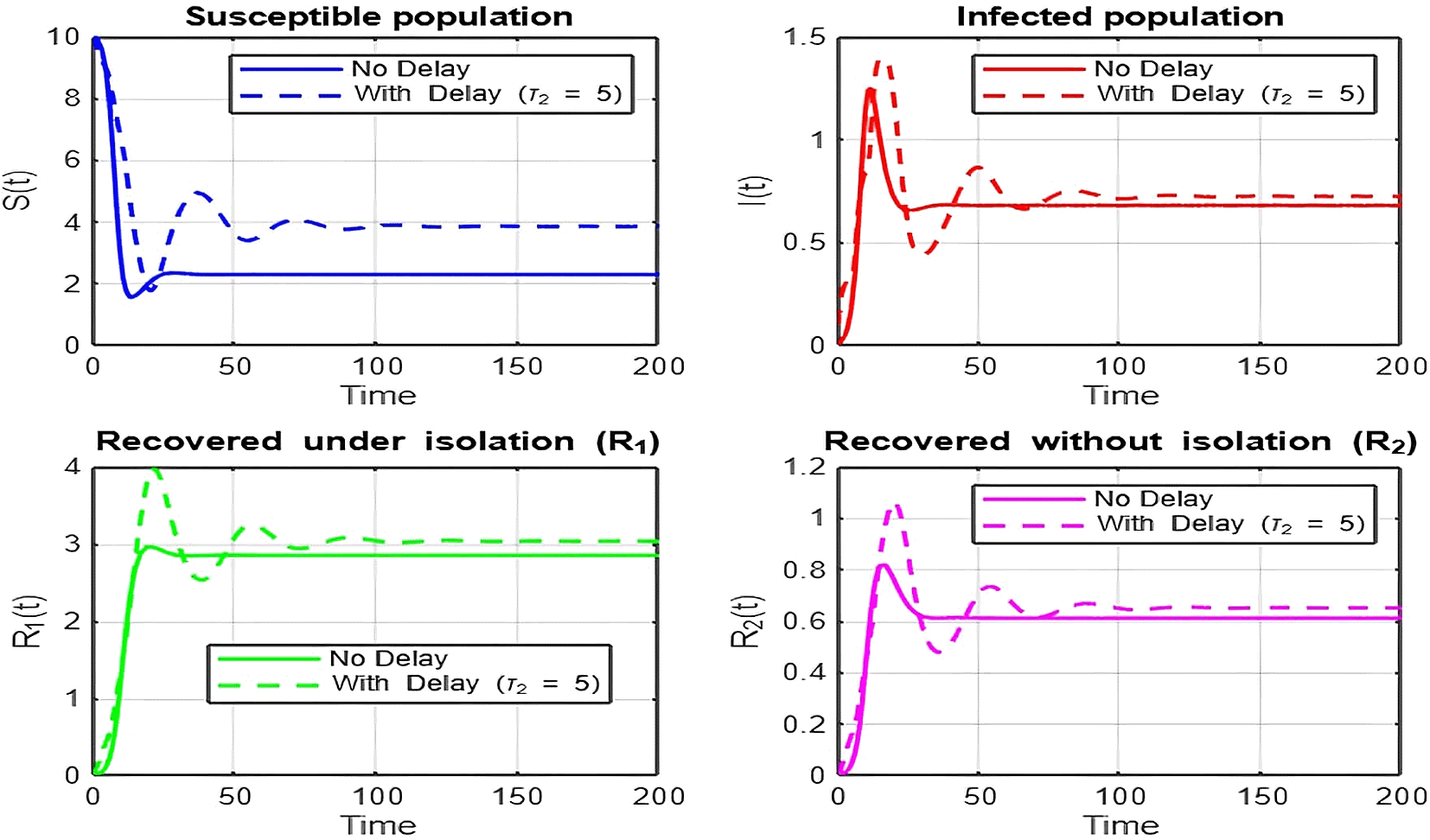
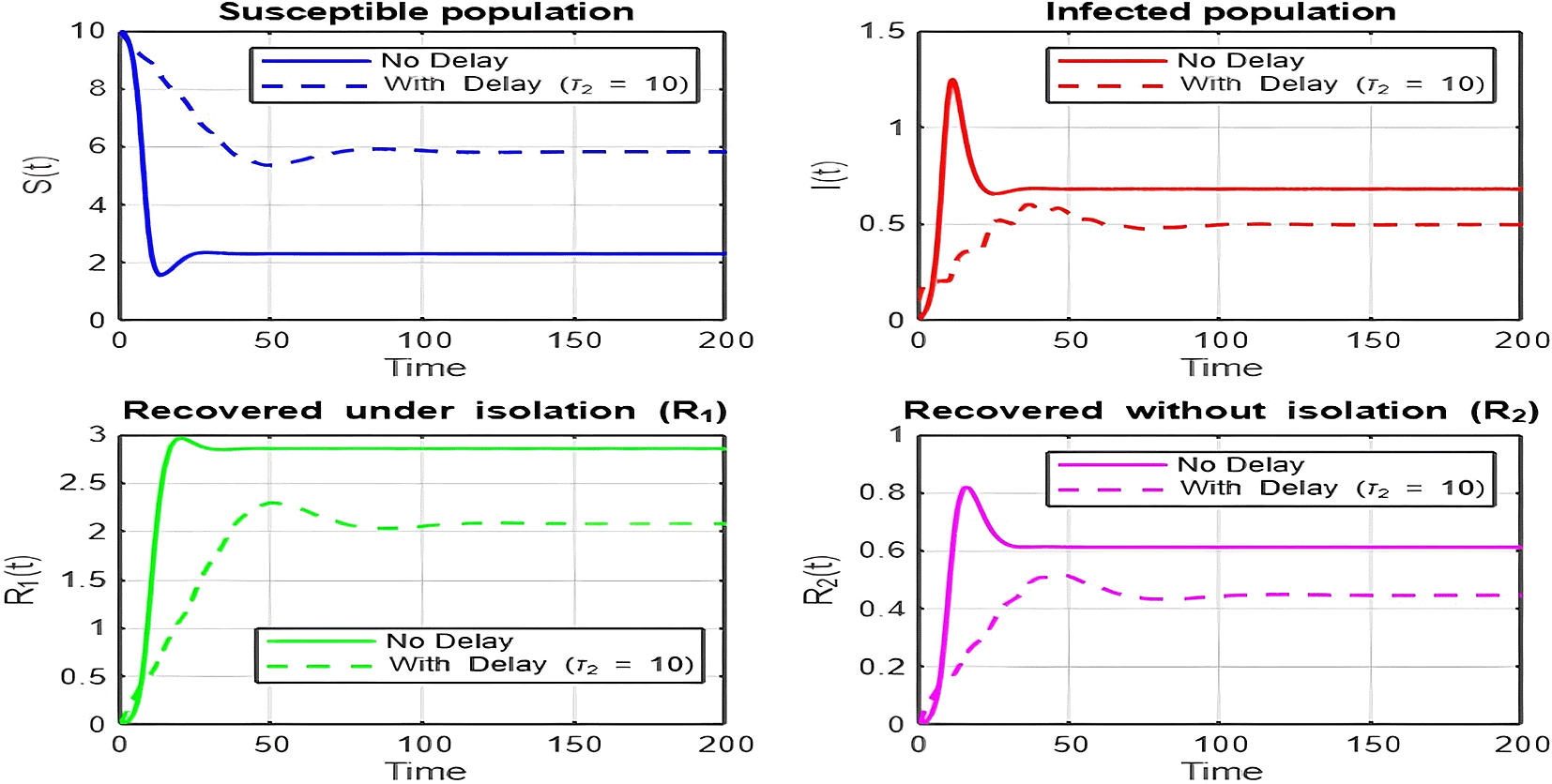
1. Figures (2)-(8) and Figure 17: Dynamics Comparison (With and Without Delay) [ Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 , Figure 8.]
The dynamics comparison plot demonstrates the effect of incorporating a delay ( ) in the recovery process on the population dynamics of susceptible ( ) and infected ( ) individuals.
• Without Delay:
The system without delay ( ) exhibits a faster approach to equilibrium, reflecting a more straightforward trajectory to either disease-free equilibrium (DFE) or endemic equilibrium (EE), depending on parameter values. This is consistent with classical models, where recovery and transitions between compartments occur instantaneously or without delays.
• With Delay:
Introducing a delay ( ) introduces oscillatory behavior in the infected population due to the feedback loop created by the delayed transitions. These oscillations may represent cycles of outbreaks and remissions, commonly observed in zoonotic diseases like Ebola, where external factors such as virus persistence in bodily fluids or immune system delays affect recovery rates.
The presence of delay slows down convergence to equilibrium and may indicate higher disease prevalence over time. These dynamics underscore the critical role of delayed feedback in influencing outbreak persistence and stability in real-world scenarios.
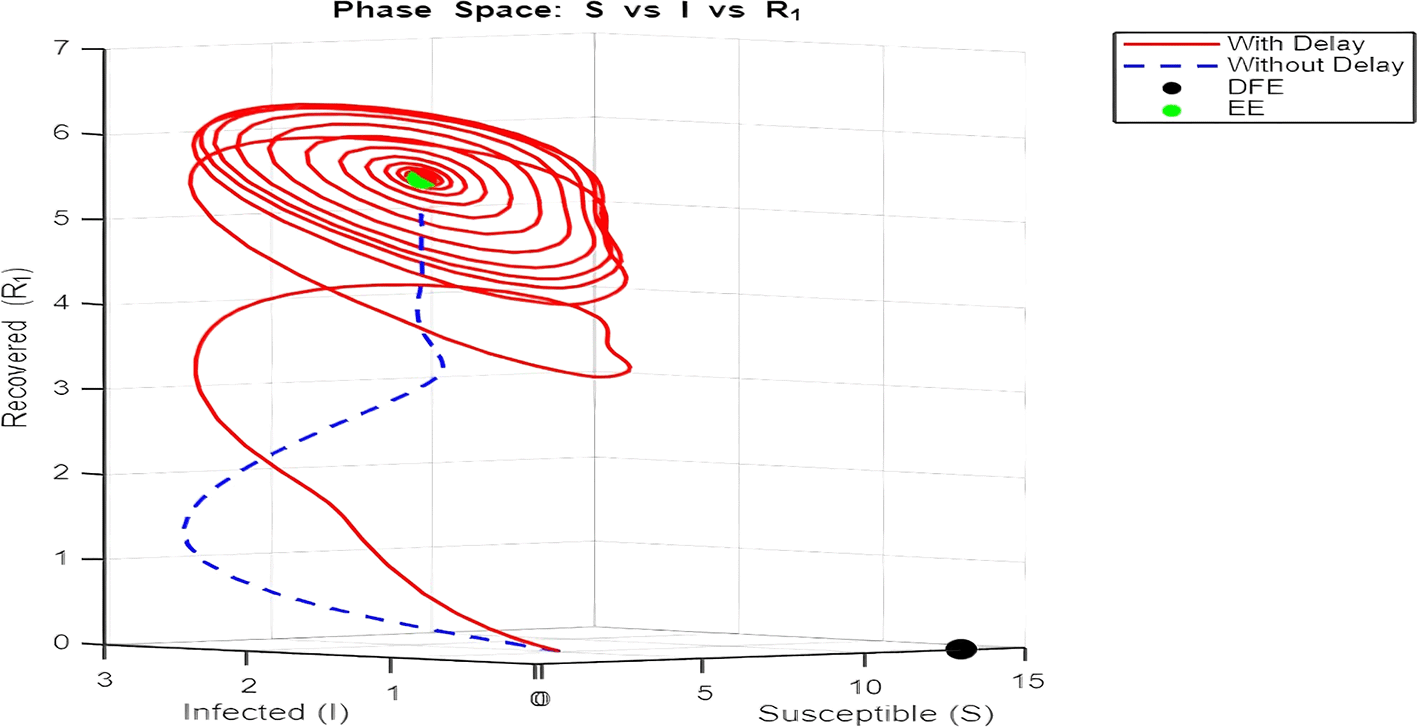
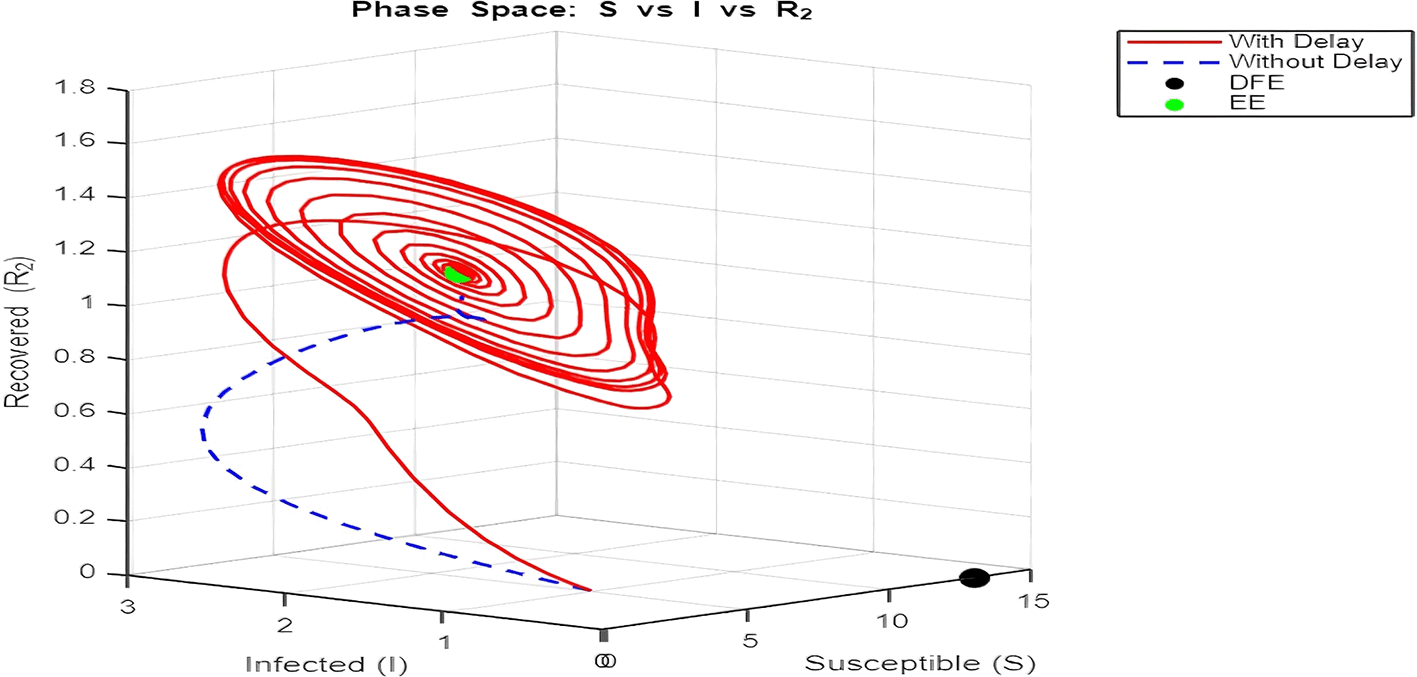
2. Figure 12 and Figures 13–Figure 15: Phase Space (Trajectories in and )
The phase space plot provides insights into the long-term behavior of the model with and without delay.
• Disease-Free Equilibrium (DFE):
The DFE point (marked as black in the plot) corresponds to the scenario where the infection dies out completely ( , ). This equilibrium is stable for small transmission rates ( ) or when the basic reproduction number ( ) is less than 1.
• Endemic Equilibrium (EE):
The endemic equilibrium (marked as green) represents a state where the infection persists in the population. This occurs when , allowing for a sustained infected population. For the delayed system, trajectories often show oscillations before converging to the EE, reflecting periodic outbreaks.
The trajectory with delay exhibits more complex behavior (such as spiraling orbits) before stabilization, highlighting the destabilizing impact of delayed recovery on the population dynamics.
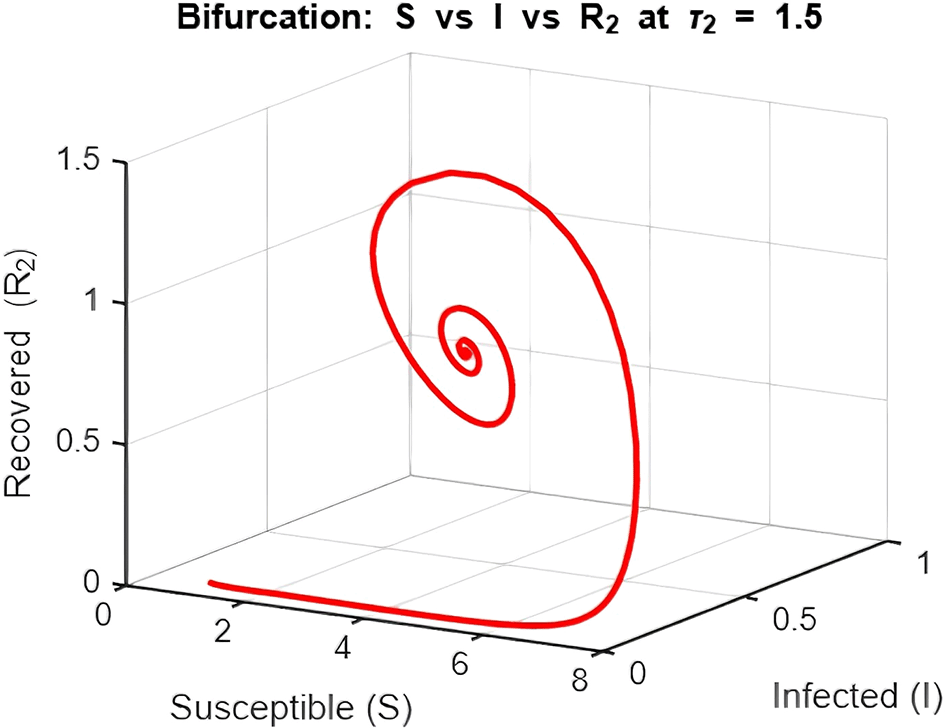
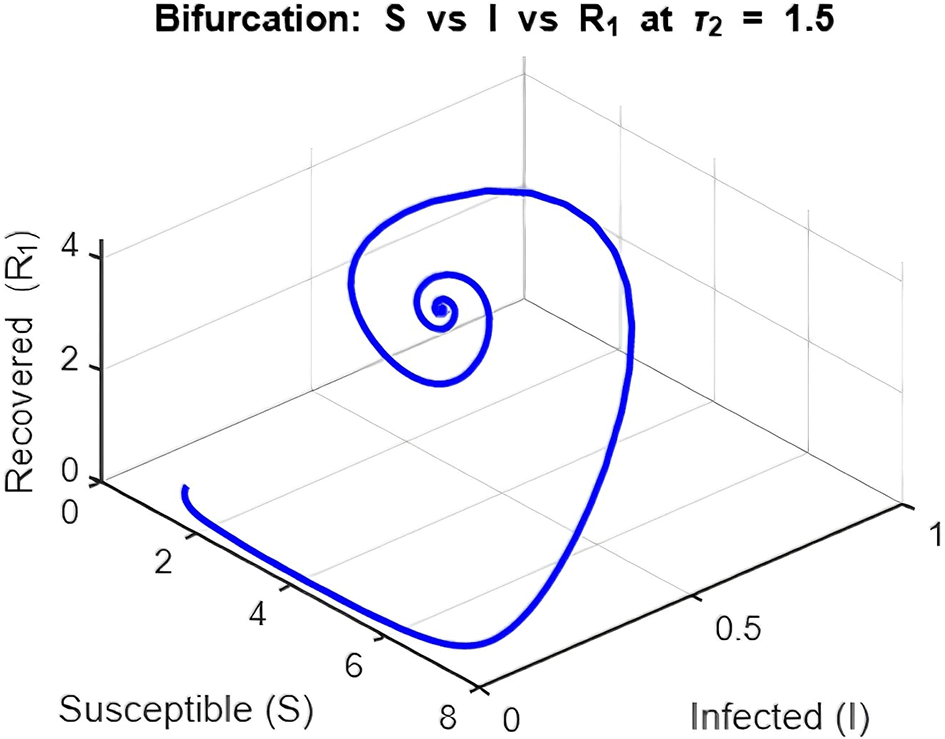
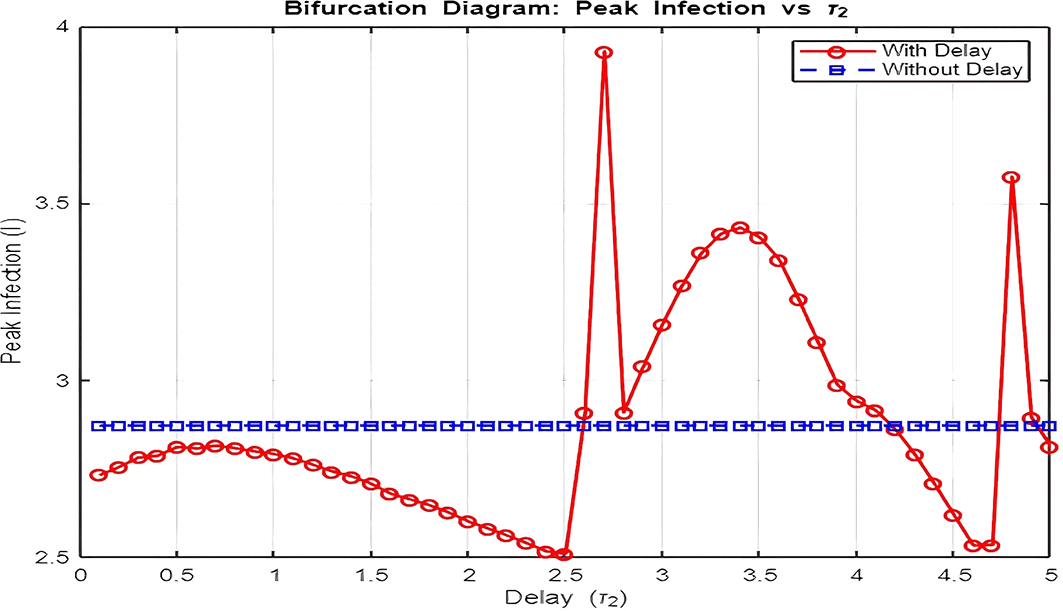
3. Figure 16: Bifurcation Analysis (Peak Infection vs. Delay )
The bifurcation diagram investigates how varying the delay ( ) influences peak infection levels.
• Without Delay ( ):
Peak infection levels remain relatively constant as the system lacks the feedback loop induced by delay. The dynamics stabilize quickly at the endemic equilibrium.
• With Delay ( ):
Introducing delay leads to increasing peak infection levels as grows. This is a direct consequence of the prolonged infectious period caused by delays in transitions to recovery ( and ). Moreover, as increases beyond a critical threshold, the system exhibits oscillatory outbreaks, as seen in the dynamics plot.
The bifurcation plot effectively distinguishes between the disease-free region ( ) and the endemic region ( ) by showing when peak infection levels transition from zero to non-zero values. The critical value of at which this transition occurs aligns with thresholds derived from the model’s reproduction number.
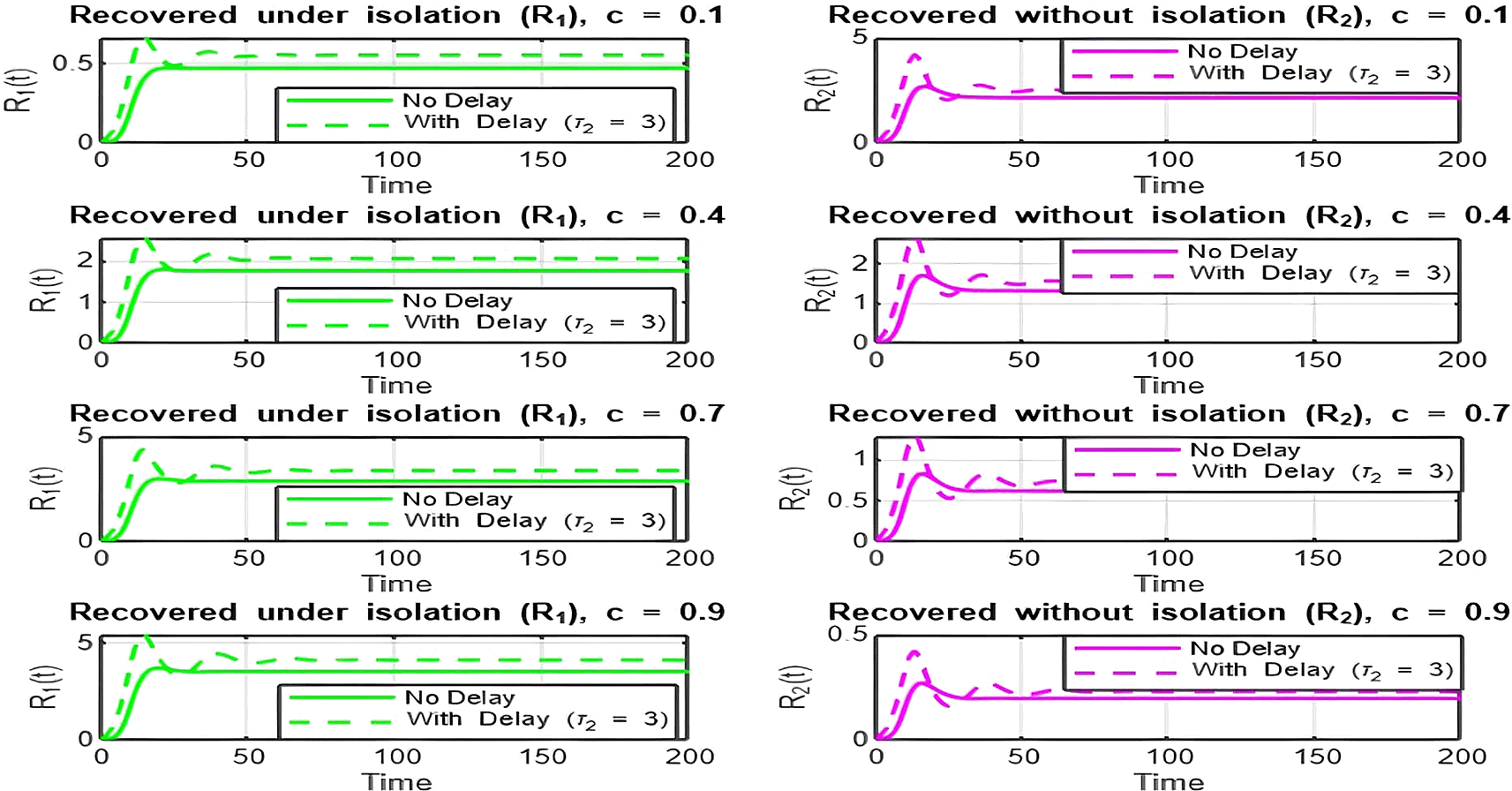
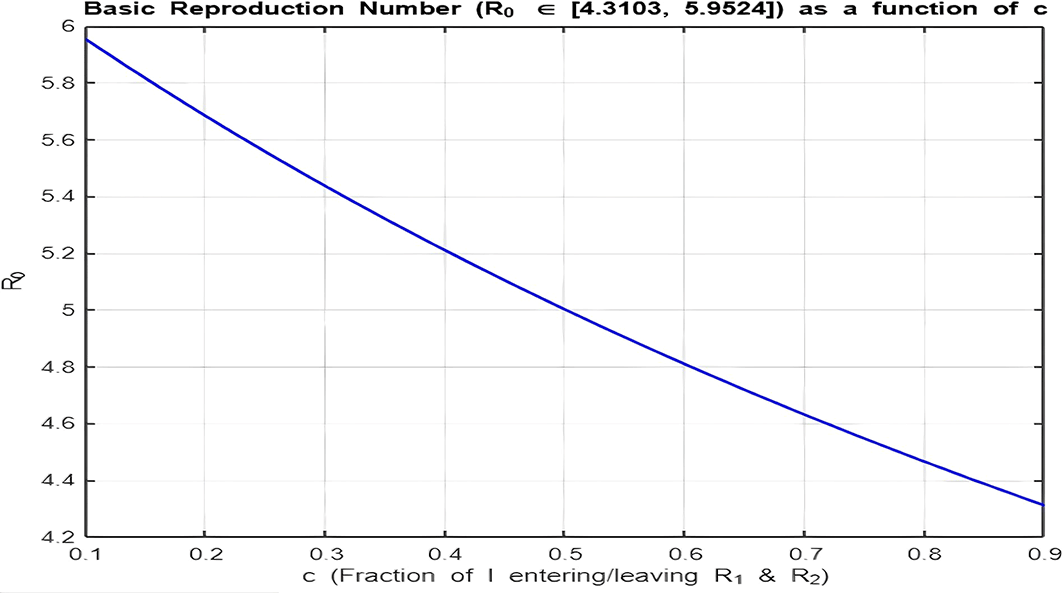
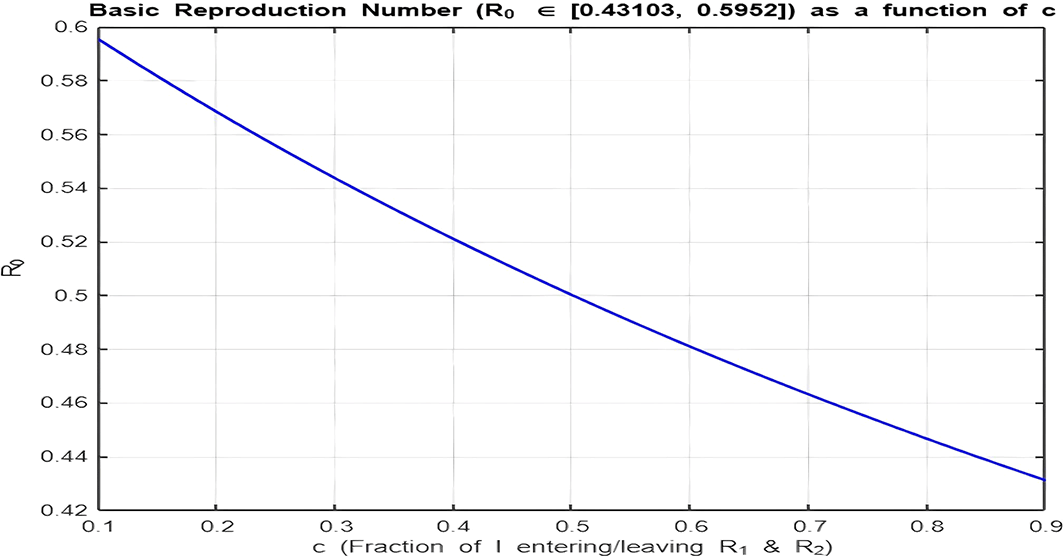
4. Isolation Parameter
The isolation parameter reflects the fraction of recovered individuals ( ) who effectively isolate themselves and avoid further transmission. The two plots ( Figure 9 and Figure 10) show how depends on , and the behavior is analyzed for and :
• Figure 9 ( ):
With the range , there is a decreasing relationship between and , indicating that as the isolation parameter increases, decreases. Increasing reduces the effective transmission by isolating recovered individuals, helping to lower .
• Figure 10. ( ):
With the range , there is a decreasing relationship between and , but values are already below 1. Increasing further accelerates the decline, making it harder for the infection to persist in the population. Here, lower isolation efforts (small ) are sufficient to maintain .
5. Figure 11: Sensitivity Analysis of
Figure 11 presents the sensitivity analysis of the basic reproduction number ( ) with respect to model parameters. The results reveal that the transmission rate ( ), recruitment rate ( ), and recovery rate into ( ) are the parameters that contribute most significantly to .
This study provides a thorough analysis of the Ebola transmission dynamics using a deterministic SIRR model that incorporates key features such as nonlinear incidence rates and delayed recovery. By examining the stability of disease equilibria and the role of the basic reproduction number ( ), we have highlighted critical factors that influence the onset and persistence of outbreaks. The sensitivity analysis and bifurcation analysis, along with numerical simulations, underscore the importance of timely interventions and targeted strategies to reduce transmission, limit susceptible populations, and improve recovery rates. These insights offer valuable guidance for public health policy, particularly in managing outbreaks by identifying threshold conditions and critical parameters that determine the trajectory of disease spread. Overall, our findings contribute to a deeper understanding of Ebola dynamics and demonstrate the importance of considering delays in disease models to capture the complexities of real-world transmission.
This study did not involve human participants or personal data; therefore, ethical approval and consent were not required.
As outlined in our manuscript, the present study is purely theoretical in scope, focusing on conceptual development and does not involve the generation or analysis of empirical data.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
References
1. https://pmc.ncbi.nlm.nih.gov/articles/PMC12441668/.Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious Disease Modelling
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biostatistics, Machine learning, epidemiology, Causal Inference, Measurement error, Bayesian, meta-analysis.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Scientific Computing, Biomathematics, Statistical Computing, Numerical Analysis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 02 Sep 25 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)