Keywords
dental implants, gut microbiota, gut symbiosis, probiotics, prebiotics, gut microbiome, gut bone axis
This article is included in the Manipal Academy of Higher Education gateway.
The gut microbiota plays a pivotal role in systemic health, particularly in bone metabolism and periodontal integrity, through the gut–bone axis. Emerging evidence suggests that gut microbiota dysbiosis may indirectly contribute to dental implant failure by disrupting bone homeostasis and aggravating periodontal disease. Despite growing recognition of this relationship, comprehensive understanding of the underlying mechanisms and therapeutic strategies remains limited. This systematic review explores the impact of gut microbiota dysbiosis on implant-related tissues, its contribution to implant failure, and potential non-invasive interventions to improve outcomes.
A systematic search of PubMed, Scopus, Embase, and related databases was performed following PRISMA guidelines. Studies addressing the gut microbiota–bone health connection, gut microbiota–periodontium interactions, and their collective influence on implant success were included. Screening was based on predefined criteria, with data extraction focusing on mechanisms through which dysbiosis affects osseointegration and peri-implant health, as well as therapeutic approaches including probiotics, prebiotics, and dietary interventions.
The review revealed a strong association between gut dysbiosis and altered bone metabolism, impairing osseointegration and raising the risk of implant failure. Gut dysbiosis also exacerbated periodontal inflammation, predisposing to peri-implant mucositis and peri-implantitis. Non-invasive strategies such as probiotics, prebiotics, and targeted dietary modifications showed promise in restoring microbial balance, attenuating systemic inflammation, and supporting peri-implant tissue health. However, wide heterogeneity in study design and methodology restricted the strength and comparability of available evidence.
Gut microbiota dysbiosis is an emerging and underrecognized risk factor for implant failure. Modulating dysbiosis through non-invasive interventions may enhance osseointegration and peri-implant tissue integrity. Nevertheless, most current evidence arises from preclinical or small-scale human studies, highlighting the urgent need for robust, large-scale clinical trials.
dental implants, gut microbiota, gut symbiosis, probiotics, prebiotics, gut microbiome, gut bone axis
The human digestive tract hosts a complex and diverse community of microorganisms, collectively known as the gut microbiota, which significantly influences overall health and physiological functions.1 This growing interest has spurred a surge in research uncovering the gut microbiome’s intricate impact on overall health. In response, the food industry has introduced a wide array of fermented foods and probiotic products, mirroring the rising mainstream emphasis on gut health. However, despite this heightened focus, both consumers and healthcare professionals often grapple with confusion due to the still-evolving nature of the evidence.2
Gut microbes are integral for essential processes such as digestion, nutrient absorption, and synthesising vitamins, minerals, proteins etc.3 A balanced gut microbiota is closely linked to good health, while imbalances can lead to dysbiosis and various associated health problems.
The gut-brain axis—a two-way communication system between the gastrointestinal tract and the brain—highlights how gut microbes can impact mental health, cognitive processes, cardiovascular health, respiratory system and bone homeostasis through multiple biological pathways.4–6
Gut dysbiosis alters bone turnover by reducing osteocalcin and increasing C-terminal telopeptide of type I collagen (CTX) levels, indicating bone loss. It disrupts the Receptor Activator of Nuclear Factor Kappa-B Ligand/Osteoprotegerin (RANKL/OPG) balance, raises inflammatory cytokines, and impairs calcium, vitamin D, and Short Chain Fatty Acid (SCFA) production all of which hinder osseointegration and bone healing. These effects highlight the role of gut health in implant success.7
Despite their popularity, dental implants are not without challenges, and failures.8,9 The major problems associated with dental implant failures include poor bone regeneration and compromised soft tissue health, which are crucial for ensuring stability and longevity of implants.
Factors such as infection, peri-implantitis, inadequate osseointegration, and biomechanical overload are significant contributors to implant failure. Recent studies10–12 have highlighted the interlink between gut health, periodontium and bone health. However, to the best of our knowledge, no comprehensive systematic review currently addresses the relationship between gut microbiota dysbiosis and dental implant failures in totality.
Given the growing body of evidence supporting the gut-implant connection, this systematic review aims to explore the relationship between gut microbiota dysbiosis and dental implant failures by analysing its impact on bone homeostasis, immune response, and peri-implant tissue health.
Additionally, this review will examine non-invasive therapeutic options such as probiotics, prebiotics, and dietary interventions that can help restore microbial balance and enhance implant success rates.
By bridging the gap between oral and systemic health research, this study seeks to provide clinicians, researchers, and healthcare professionals a comprehensive understanding of an overlooked phenomena, gut microbiota dysbiosis and its effects on dental implant success.
Search strategy
A literature search was conducted using the following search terms “gut microbiota,” “dental implants,” “gut microbiome,” and “dysbiosis” on PubMed, Scopus, Embase and Google Scholar databases.
Inclusion and exclusion criteria
The inclusion criteria included articles which were published between 2014-2024 in the English language, involving both human and animal studies, Literature reviews, Scoping reviews, Meta-analyses, Systematic reviews, Clinical trials and Randomised Controlled Trials.
Non-peer-reviewed articles, Letters to the Editor, Book chapters, Case reports, articles in which full text was not available, articles published before 2014, and non-English language were excluded.
Data extraction
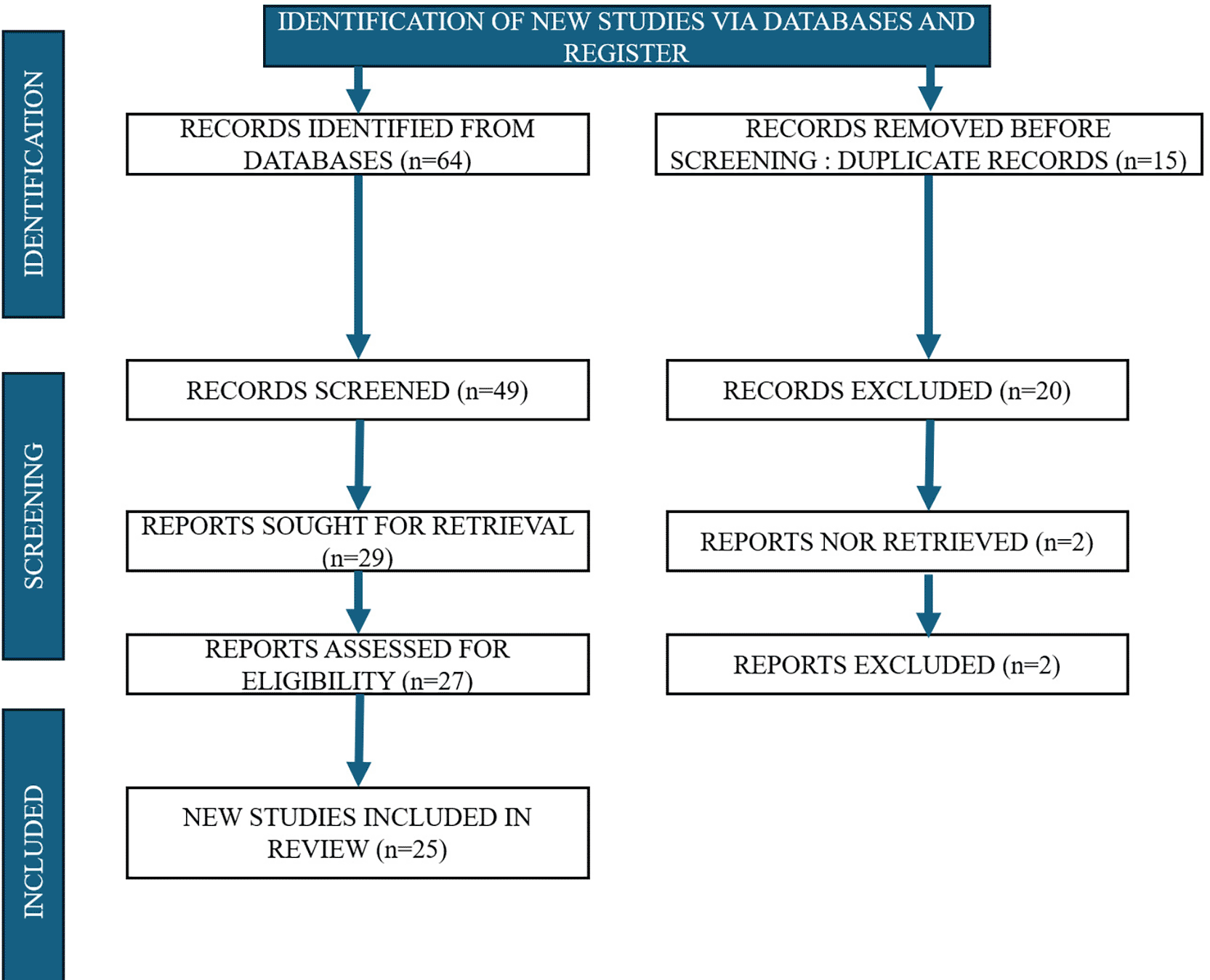
A total of 64 articles were initially identified from the database search. After removal of 15 duplicate articles, the remaining 49 articles were screened based on title and abstract against the predefined inclusion and exclusion criteria. Following this screening, 25 articles were included for full-text review and qualitative synthesis.
To ensure accuracy and reliability, two independent examiners screened and extracted the data. Discrepancies between the two reviewers were resolved through discussion, and in cases of disagreement, a third examiner was consulted. To assess inter-examiner reliability during the study selection process, Cohen’s Kappa statistic was calculated. The resulting Kappa value was 0.55, indicating a moderate level of agreement between the two independent reviewer.
This review was conducted in accordance with the PRISMA 2020 guidelines. A PRISMA flow diagram has been included in the manuscript [Figure 1], and the completed PRISMA 2020 checklist and A PRISMA flow diagram is also included in link https://doi.org/10.6084/m9.figshare.30076018.v1.
Bone metabolism is a dynamic and continuous process involving resorption and remodelling, both essential for maintaining bone strength and structure. This remodelling and resorption process can be driven by two types of cells which are: osteoclasts and osteoblasts respectively.13
Osteoclasts are the cells which are mainly responsible for breaking down and resorbing old bone cells. On the other hand, osteoblast cells are derived from bone marrow mesenchymal stem cells (BMSCs) and are very important for the formation of new bone tissue.
In a 2016 study by Hernandez et al.12 reported that gut microbiota dysbiosis can cause elevated intestinal permeability, promoting systemic inflammation that negatively affects the bone metabolism system. Changes to the gut microbiome can lead to impaired bone strength and reduced bone tissue material properties, potentially increasing fracture risk.14 These findings suggest a connection between gut health and bone homeostasis, indicating that the state of the gut microbiome may influence the success or failure of dental implants.14
The term “osteomicrobiology” describes the relationship between bone health and gut microbiota, highlighting how gut microorganisms can influence bone development, ageing, and pathological conditions related to bone tissue.15
Gut microbiota colonisation begins during childbirth and constantly evolves till old age.15 Research involving germ-free (GF) mice has provided valuable insights into the role of gut microbiota in bone metabolism. These studies have proved that GF mice will have a greater bone mass compared to mice which are conventionally raised. This study suggests that gut microbiota dysbiosis may decrease bone mass by promoting bone resorption through osteoclastic cell activity, mainly due to the immune responses they trigger in the gut.16 However, the relationship between gut microbiota on bone health is complex and not fully understood.
Some studies have shown that gut microorganisms can have both pro-anabolic effects (promoting bone formation) and anti-anabolic effects (inhibiting bone formation). For instance, gut bacteria might inhibit bone formation by disrupting insulin-like growth factor 1 (IGF-1) signalling, while simultaneously promoting bone resorption by enhancing Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL) signalling, which stimulates osteoclast activity.17 Gut microbiota plays a significant role in the regulation of bone metabolism through its interaction with the immune and endocrine systems, extracellular vesicles and gut microbiota metabolites as well [Figure 2].
In this section, we will explore how gut microbiota dysbiosis impacts bone health through various systems, including the immune, endocrine, and reproductive systems. Additionally, we will discuss how gut microbiota dysbiosis influences bone health through its own metabolites and extracellular vesicles.
Influence of gut-microbiota on bone health through the immune system
The gut microbiota (GM) influences bone health through its complex interactions with the immune system. The intestine, which harbours microorganisms, plays a vital role in maintaining immune function, and homeostasis, and in regulating inflammatory responses.18 When the gut microbiota becomes “dysbiosis,” it leads to increased intestinal permeability. This condition results in reduced expression of tight junction proteins that normally prevent harmful substances from passing into the bloodstream. As a result, bacteria and toxins can enter the circulation, leading to chronic inflammation and migration of inflammatory cells throughout the body. Such persistent inflammation is a contributing factor in several chronic inflammatory diseases, like inflammatory bowel disease (IBD) or Crohn’s disease, which are associated with bone loss.19
The gut microbiota affects bone health by interacting with the immune system, specifically two types of immune cells: T helper 17 (Th17) cells and regulatory T (Treg) cells. Th17 cells promote bone resorption by increasing the production of inflammatory molecules, such as Interleukin-17 (IL-17), Tumour Necrosis Factor-alpha (TNF-α), and the RANKL.20 RANKL is a crucial molecule that links the immune and skeletal systems by stimulating the formation and activity of osteoclasts.
Certain gut bacteria, like segmented filamentous bacteria (SFB) and Bifidobacterium adolescentis, normally present in the gut, play a pivotal role in immune regulation. Under healthy conditions, they are balanced by other gut microbes and do not cause harm. However, during gut dysbiosis, their activity can increase, leading to excessive expansion of Th17 immune cells, which release inflammatory signals that activate bone-resorbing cells, ultimately causing bone loss.21
On the other hand, Treg cells, which have immunosuppressive properties, play a protective role in bone health. These cells help to counteract the effects of Th17 cells by secreting anti-inflammatory cytokines such as Interleukin-10 (IL-10) and Transforming Growth Factor-beta (TGF-β).22 These cytokines inhibit the production of RANKL and other factors that promote osteoclast formation, while also enhancing osteoblast activity, essential for bone formation. The presence of certain beneficial microbes, such as Lactobacillus and Bifidobacterium species, can increase Treg cell populations and promote bone health by shifting the balance away from Th17 cells.23
Figure 3 describes the role of Th17 and Treg cells in bone homeostasis.
Influence of gut-microbiota on bone health through Endocrine system
Parathyroid hormone PTH is crucial for calcium balance and bone health, influencing bone remodelling.24 In primary hyperparathyroidism, gut microbiota, particularly SFB, facilitates bone catabolism by promoting the migration of TNF+ T cells and Th17 cells from the gut to the bone marrow. This migration leads to increased bone resorption.25,26
Conversely, intermittent PTH administration, which stimulates bone formation, requires a healthy gut microbiota to be effective. The microbiota-derived metabolite ‘Butyrate’ plays a key role in this process by enhancing the anabolic effects of intermittent PTH. Butyrate stimulates the differentiation of Treg cells, which in turn activate Wnt signalling pathways in bone marrow cells, promoting bone formation.26
A recent study demonstrated that moderate levels of Butyrate produced by the gut microbiota are crucial for the formation of intermittent parathyroid hormone (iPTH) to stimulate bone genesis in mice. In the absence of gut microbiota, the anabolic effects of iPTH and antibiotic treatment did not lead to an increase in Treg cells in the gut or bone marrow (BM). However, when butyrate was administered, it restored the bone-building activity of iPTH and elevated Treg cell numbers. Butyrate promotes Treg cell differentiation by binding to G-protein-coupled receptor 43 (GPR43) on dendritic cells, which then induces the expression of the Wnt ligand Wnt10b in BM CD8+ T cells and activates Wnt-dependent pathways for bone formation.27
Another vital hormone for osteogenesis and osteoclastogenesis is Estrogen.28 The estrogen receptor α (ERα) mediates estrogen’s effects, leading to bone formation.29 Estrogen also helps protect bone mass by downregulating immune responses and maintaining a balance between osteoblasts and osteoclasts. It suppresses the expression of RANKL in immune cells and boosts the production of osteoprotegerin (OPG), which prevents bone loss.30 Estrogen deficiency, often associated with conditions like menopause, results in increased inflammation and bone resorption due to a rise in inflammatory cytokines like TNF-α. The gut microbiota directly affects estrogen levels and metabolism by encoding enzymes such as β-glucuronidases, β-glucosidases, hydroxysteroid hydrolases, and sulfatases, which modify estrogen molecules to enhance their reabsorption in the intestine. This regulation affects both local and systemic estrogen levels.31,32
Li et al.33 demonstrated that bone loss related to a deficiency in sex hormones relies on the quality of gut microbiota (GM). When estrogen levels were reduced using leuprolide (a gonadotrophin-releasing hormone agonist) in GF mice, it did not lead to increased bone breakdown or trabecular bone (Tb) loss. The underlying mechanism showed that a lack of estrogen caused a decrease in the expression of proteins that maintain tight junctions in the intestines, resulting in higher intestinal permeability and increased levels of endotoxins in the blood; however, these effects were not observed in GF mice. Only mice raised under normal conditions showed higher levels of osteoclast-promoting cytokines in both the bone marrow and small intestine after estrogen depletion, suggesting that the bone loss linked to low estrogen levels is associated with inflammation driven by GM.33
The role of sex hormones in gut microbiota dysbiosis and its influence on bone health
The gut microbiota which comprises approximately 1013 to 1014 microorganisms34 is involved in a gamut of biological processes and its imbalance can be an aetiology for various diseases, including but not limited to neoplasms, autoimmune conditions, and cardiovascular issues.35 Notably, the bacteria-to-human cell ratio varies between genders, with women having a higher ratio (2.2) compared to men (1.3).36
Estrogen, which are primarily produced in the ovaries, adrenal glands, and adipose tissue, impact bone health and influences the composition and activity of the gut microbiota as stated earlier. Estrogen is metabolized by gut microbes through processes like β-glucuronidation, which affects their systemic levels and biological activity.31 The resultant metabolites of estrogen can modulate bone metabolism directly by interacting with bone cells or indirectly through changes in the gut microbiota.
Increased levels of estrogen, such as those observed in premenopausal women, are associated with a more diverse and balanced gut microbiota. This microbial diversity has been linked to better bone density and a lower risk of osteoporosis.32 In contrast, the reduction in estrogen levels during menopause leads to a decrease in microbial diversity, which is correlated with bone loss and increased osteoporosis risk.32
Testosterone, the predominant androgen in males, also influences gut microbiota and bone health. Testosterone affects gut microbiota by modulating microbial diversity and composition. For example, an animal study that measured unconjugated and glucuronidated androgen levels found that unconjugated dihydrotestosterone (DHT) levels in the faeces of young adult males were 70 times higher than in their serum. In germ-free mice, the distal intestine showed high levels of glucuronidated testosterone and DHT but very low levels of free DHT, suggesting that the gut microbiome influences the metabolism and deglucuronidation of androgens.37
Influence of gut-microbiota metabolites on bone health
The GM affects bone physiology through the production of various metabolites, referred to as “postbiotics.” These various secondary metabolites like short-chain fatty acids, polyamines, and hydrogen sulfides regulate the health and function of organs from the gut itself.38
Short-Chain Fatty Acids (SCFAs): The gut microbiota produces different types of SCFAs, which include acetate, propionate, and butyrate, by fermenting dietary fibres.39 Each of these SCFAs contributes to bone health in different ways. Acetate, produced by various bacterial species, helps increase bone mass by inhibiting osteoclasts, which cause bone resorption.40 Propionate and butyrate, which are produced by specific bacterial strains such as Akkermansia muciniphila, Faecalibacterium prausnitzii, and others, have been shown to prevent bone loss by reducing the number of osteoclasts and their differentiation.41,42
Polyamines : Polyamines, the organic compounds derived from amino acids, are mainly produced by gut microbes and play a vital role in regulating bone formation and resorption.43 These polyamines, such as spermine and spermidine, are involved in essential biological processes like cell growth, differentiation, and Apoptosis. Polyamines promote the differentiation of mesenchymal stem cells into bone-forming cells and reduce fat accumulation.44 They also act as inhibitors of osteoclastogenesis by decreasing the number of bone-resorbing cells. However, a lack of polyamine production can limit these beneficial effects.45 Moreover, excessive concentrations of polyamines like spermidine have been linked to an increased risk of bone fractures.46
Hydrogen Sulphide: Hydrogen sulphide (H2S), another metabolite produced by gut microbes, plays a significant role in bone formation and skeletal development. It is mainly generated by certain gut bacteria, such as Desulfovibrio, through the breakdown of amino acids like cysteine.47 H2S supports bone health by promoting the self-renewal and osteogenic differentiation of BMSCs through the Wnt/β-catenin signalling pathway.48
Influence of gut-microbiota on bone health through extracellular vesicles
Extracellular vesicles (EVs) are small, spherical nanostructures ranging between 10 to 400 nm in diameter that are released by bacteria as a form of interspecies communication. These vesicles consist of bioactive proteins, lipids, nucleic acids, and other molecules, allowing them to transport their contents over long distances within the body in a targeted and protected manner.49 The characteristics of EVs, such as their structure and composition, vary depending on the originating microbe and the environmental conditions.50
Recent research has demonstrated that extracellular vesicles (EVs) play important roles in several health conditions. For instance, EVs from Lactobacillus rhamnosus GG (LGG) and Lactobacillus reuteri have been found to alleviate inflammation in colitis and enhance gut health by modifying the composition of the gut microbiota.51 Additionally, EVs from Lactobacillus sakei and Akkermansia muciniphila have demonstrated anti-inflammatory effects, enhanced immune response, and protection against diet-induced obesity.52,53 A. muciniphila derived EVs have been found to accumulate in bone tissues, where they inhibit osteoclast formation and promote osteoblasts.54
Impact of dysbiosis on systemic bone turnover markers and Osseointegration
Gut dysbiosis significantly impacts systemic bone turnover markers and impairs osseointegration, both of which are critical for the long-term success of dental implants. A healthy gut microbiota contributes to bone homeostasis by regulating nutrient absorption, immune responses, and the production of bioactive metabolites.7,55 However, in a dysbiotic state, these functions are disrupted, leading to systemic consequences. One key effect is the alteration of bone turnover markers. Dysbiosis has been associated with decreased levels of osteocalcin, a marker of bone formation, indicating reduced osteoblastic activity. At the same time, levels of C-terminal telopeptide of type I collagen (CTX), a marker of bone resorption, tend to rise, reflecting increased osteoclastic activity and net bone loss.7 Additionally, dysbiosis disturbs the RANKL/OPG balance—upregulating RANKL and downregulating osteoprotegerin—thus promoting osteoclastogenesis and resorption over bone formation.56
These systemic imbalances translate into compromised osseointegration, the process by which the dental implant fuses with surrounding bone. Elevated levels of inflammatory cytokines such as TNF-α and IL-6, commonly seen in dysbiosis, inhibit osteoblast differentiation and delay bone healing.55 Furthermore, dysbiosis can impair the intestinal absorption of calcium and vitamin D—nutrients essential for bone mineralization. Deficiencies in these nutrients can weaken peri-implant bone quality and slow the healing process.40 A healthy gut microbiota also produces short-chain fatty acids (SCFAs) like butyrate, which are known to enhance bone formation and modulate immune responses.40 In dysbiosis, the reduced production of SCFAs deprives the bone microenvironment of these protective effects. Moreover, dysbiosis-induced immune dysregulation shifts the host toward a chronic pro-inflammatory state, which has been implicated in peri-implant bone loss and implant failure.40 Altogether, these mechanisms underscore the systemic influence of gut health on local bone metabolism and highlight the need to consider gut microbial balance as part of peri-implant disease prevention and management strategies.
Periodontitis is a chronic inflammatory disease caused by dental plaque.57 It leads to gum inflammation, tooth loss, and can exacerbate systemic chronic diseases like rheumatoid arthritis, IBD, and diabetes.58
Chronic periodontitis, which is a long-term inflammatory condition of the gingiva, is linked to a reduction in the alpha diversity of the gut microbiota. Alpha diversity refers to the variety and abundance of different species within the gut microbiota. A decline in this diversity suggests that periodontitis can result in a disruption or dysbiosis in the gut microbiota. This suggests that the severity of periodontal disease may impact the degree of disruption in the gut microbiota.59,60
Clinical studies have revealed notable differences in the gut microbiota composition of individuals with severe periodontitis (stage III/IV) compared to healthy controls. Patients with periodontitis exhibited elevated levels of bacteria such as Bacteroides, Faecalibacterium, Fusobacterium, and Lachnospiraceae.11
Other studies found increased levels of Firmicutes, Proteobacteria, Verrucomicrobia, and Euryarchaeota, while Bacteroidetes were less abundant.10Additionally, lower diversity and higher amounts of specific bacteria like Fusobacterium nucleatum ss vincentii, Campylobacter sp. HMTG43, and Treponema sp. HMTG77-like were observed in patients with deep periodontal pockets and tissue loss.61 These findings suggest that swallowing high amounts of periodontal pathogens over time can disrupt the gut microbiota, creating an “inflamed” microbial environment. However, the gut microbiota is generally less affected by periodontitis than the salivary microbiota.62
Two Mendelian randomization studies identified certain gut bacteria, such as Prevotella 7, Lachnospiraceae UCG-008, and Enterobacteriales, that could increase the risk of periodontitis, while others, like Butyricicoccus and Ruminiclostridium 6, might decrease the risk.63
Oxidative Stress in Periodontitis and Its Relevance to Implant Failure
Oxidative stress plays a pivotal role in the pathogenesis of periodontitis and may contribute to the failure of dental implants through its effects on both the local periodontal environment and systemic bone metabolism. Periodontitis is characterized by an imbalance between reactive oxygen species (ROS) production and the body’s antioxidant defense mechanisms, leading to oxidative damage of cellular components such as lipids, proteins, and DNA. This oxidative imbalance exacerbates inflammatory responses, enhances osteoclastogenesis, and promotes alveolar bone loss.64
Elevated levels of oxidative stress markers in the gingival crevicular fluid, saliva, and serum of patients with chronic periodontitis, including malondialdehyde (MDA), 8-hydroxydeoxyguanosine (8-OHdG), and nitric oxide (NO).64 These markers correlate with disease severity and are indicative of heightened tissue destruction. In the context of implant dentistry, similar oxidative processes are believed to impair peri-implant healing and osseointegration. Excessive ROS not only inhibits osteoblastic differentiation but also induces apoptosis in osteoblasts, which are crucial for forming the bone-to-implant interface.65
Moreover, oxidative stress interplays with gut dysbiosis to amplify systemic inflammation. Gut microbiota imbalance leads to increased intestinal permeability and the translocation of bacterial endotoxins like lipopolysaccharides (LPS) into circulation, triggering widespread oxidative responses. This systemic oxidative stress exacerbates the local redox imbalance in peri-implant tissues, creating a hostile environment for bone remodeling and immune regulation.65
This section will explore the different mechanisms through which gut microbiota dysbiosis increases the chances of periodontitis and potentially contributes to dental implant failures [Figure 4].
GM dysbiosis causes increased periodontal pathogen load leading to periodontitis
Gut dysbiosis, often resulting from prolonged use of antibiotics can significantly impact oral health by increasing the prevalence of periodontitis-related pathogens like Enterococcus and Dysgnomonas. This imbalance in the gut microbiota can aggravate periodontitis. In an animal model study performed by X Yuan et al., they highlighted that a faecal microbiota transplant (FMT) using normal mouse faeces helped restore gut microbiota balance, and reduced periodontitis-related pathogens thereby showcasing the dynamic interplay between intestinal and oral flora.66
Also, individuals with different metabolic phenotypes, or the same genetic background, diet, and associated with specific gut microbial profiles, can elevate the degree and risk of periodontal inflammation. For example, insulin-sensitive mice exhibit more severe alveolar bone loss when periodontitis is induced as compared to insulin-resistant and normal mice, with higher levels of bacteria like Porphyromonadaceae and Prevotellaceae in their oral flora.67 Similar patterns are seen in patients with active Crohn’s disease, where there is an enrichment of Capnocytophaga, Rothia, and TM7 flora associated with periodontal disease, however, its treatment can help restore the bacteria to healthy levels.68
GM dysbiosis results in an impaired oral mucosal barrier leading to periodontitis
IBD can cause oral problems like aphthous ulcers, cobble stellate mucosal structures, and non-caseating granulomatous inflammation in the mouth, indicating that IBD might weaken the protective barrier in the mouth.69 High levels of inflammatory molecules, like TNF-α, are found in IBD and can damage this barrier by breaking down key proteins (like E-cadherin and F-actin) or by disrupting tight cell connections. This damage might happen through MLCK/p-MLC, NF-kB, p38 MAPK, and ERK signalling pathways.70,71
Additionally, TNF-α and IL-1β, can harm epithelial cells by making them more permeable and less able to hold their structure. These molecules also trigger cell death in epithelial tissues and decrease the levels of proteins that hold cells together, contributing to periodontal disease.71 Research shows that people with Crohn’s disease have higher levels of inflammatory markers like TNF-α in their saliva and gums, which can worsen periodontitis.72 However, it’s unclear if gut-related inflammation directly increases TNF-α in the gums to make periodontitis worse, and further research is needed.
GM dysbiosis causes abnormal neutrophil function leading to periodontitis
The immune response, especially involving neutrophils, plays a vital role in the onset and progression of periodontitis. Neutrophils are a key part of the body’s defence system, but in periodontitis, their activity can become dysfunctional.73 Studies show that when gut dysbiosis occurs such as from antibiotic treatment that disrupts the balance of gut bacteria neutrophil production in the bone marrow decreases. This imbalance leads to reduced levels of important immune factors like IL17A and granulocyte colony-stimulating factor (G-CSF), which are necessary for producing neutrophils. Restoring gut flora has been shown to improve neutrophil levels.74
In patients with IBD, neutrophils may become overly active in the bloodstream but are less able to migrate to infection sites like the gingiva, which worsens inflammation. These neutrophils release harmful enzymes, such as matrix metalloproteinase-8 (MMP-8), which break down collagen fibres in the gingiva, further destroying periodontal tissues.75
Neutrophil extracellular traps (NETs), which help fight infections, are more frequently formed, but neutrophil movement (chemotaxis) is reduced, meaning fewer neutrophils reach the inflamed gingiva. This defect in neutrophil migration leads to uncontrolled inflammation, contributing to more severe periodontitis. This research underscores how gut microbiota imbalances can affect neutrophil function, thereby linking gut health to the progression of gum disease.76
GM dysbiosis causes abnormal T-cell recycling leading to periodontitis
Faecal transplantation has been shown to reduce the expression of cytokines related to Th17 cells and increase the expression of those linked to Treg cells in gingival tissues in mice with antibiotic-induced intestinal dysbiosis and periodontitis.66 This suggests that the balance between Th17 and Treg cells is important to regulate gut health and prevent periodontitis.66 Moreover, specific gut microbes that arise due to the movement of oral pathogens to the gut may play a role in controlling periodontitis. The migration of Th17 cells from the gut to the gingiva is believed to contribute to oral inflammation, such as periodontitis.77
The exact mechanism behind T-cell migration to periodontal tissues is not yet fully understood, but it resembles how T cells move to inflamed gut tissue in conditions like IBD. In IBD, T cells move to the gut by interacting with certain molecules like α4β7/α4β1 integrin and mucosal adhesion molecules (MAdCAM-1/VCAM-1).78 Similarly, molecules like intercellular adhesion molecule-1 (ICAM-1) and other adhesion-related proteins are involved in IBD and are also present in the development of periodontitis.79
T cells in patients with IBD become more responsive to these adhesion molecules, allowing them to migrate to other parts of the body, including the gums. Interestingly, genetic variants of ICAM-1 and VCAM-1, which promote T-cell adhesion, are more highly expressed in the tissues of periodontitis patients, and their expression correlates with the severity of the disease.80
Proinflammatory cytokines, such as IL-1β, TNF-α, and IFN-γ, as well as certain oral bacteria, can further increase the expression of these adhesion molecules in the gingival cells. This heightened expression helps T cells stick to and accumulate in the gums, contributing to excessive inflammation, tissue damage, and thus leading to the progression of periodontitis.81
Addressing gut dysbiosis is essential as it affects not only digestive health but also overall well-being, including immune function, mental health, and metabolic regulation. Treatment options include both invasive and non-invasive approaches. Invasive methods, like faecal microbiota transplantation (FMT), involve introducing a healthy bacterial community from a donor to directly modify the gut microbiota. While promising, these options are complex and require careful handling due to potential risks. In contrast, non-invasive methods have gained popularity for their ease of implementation and lower risk. These include dietary interventions, probiotics, prebiotics, and lifestyle modifications which can gradually and naturally support a healthier microbiome balance. While non-invasive strategies like diet, probiotics, and FMT show potential, their use in dental implantology must be substantiated through targeted research.
This section exclusively focuses on non-invasive modalities for treating gut dysbiosis, as exploring invasive options lies outside the purview of this article.
Diet
Diet significantly influences gut microbiota composition, which in turn affects systemic inflammation, immune function, and bone metabolism—factors crucial for peri-implant healing and osseointegration. Gut dysbiosis has been linked not only to gastrointestinal conditions but also to systemic alterations that may impair implant success by increasing inflammatory cytokines and compromising bone health.82,83
Among various dietary patterns, the Mediterranean Diet stands out for enhancing gut microbial diversity and immune modulation, with components like polyunsaturated fats, polyphenols, and omega-3 fatty acids promoting beneficial bacteria such as Faecalibacterium, Lactobacillus, and Bacteroides.84–86 These changes support anti-inflammatory responses and improved mineral absorption, aiding peri-implant bone regeneration.87
A gluten-free diet (GFD), while primarily used in celiac disease and IBS, has shown promising results in restoring gut microbial balance by reducing pro-inflammatory strains and enhancing gut barrier integrity, which may indirectly support systemic conditions favorable for implant success.88–92 This diet can also reduce gut permeability and inflammation, thus enhancing the integrity of the gut barrier for those sensitive to gluten.
The ketogenic diet has shown mixed effects on the gut microbiome. While it may reshape microbial composition and offer systemic health benefits such as reduced inflammation and improved metabolic markers, concerns about decreased microbial diversity and increased pro-inflammatory bacteria exist.93,94 Including prebiotics, probiotics, and fermented foods may help offset these effects.95
Dowis et al. highlight the broader health benefits of the ketogenic diet, though long-term adherence remains challenging due to its restrictive nature.96,97
Probiotics and prebiotics
Probiotics are live microorganisms that, when consumed in sufficient quantities, confer health benefits to the host. Prebiotics are non-digestible fibers that serve as food for the beneficial bacteria (probiotics) in your gut. They help stimulate the growth and activity of these good bacteria. By feeding probiotics, prebiotics indirectly support gut health.98 They typically consist of beneficial bacteria and sometimes yeast, available in the form of dietary supplements or found naturally in fermented foods like yogurt, kombucha, and sauerkraut.99 An imbalance in gut microbiota, often linked to the development of various gastrointestinal disorders, has made probiotics a key area of research for managing dysbiosis. The goal of probiotic supplementation is to help restore balance in the gut by introducing beneficial microbes.99
Numerous clinical studies, including meta-analyses of randomized controlled trials, have demonstrated that probiotic supplements can significantly reduce gastrointestinal symptoms such as discomfort and abdominal pain.100–103 Research also suggests that multistrain probiotics, which include multiple types of beneficial bacteria, may be more effective than single-strain probiotics in managing symptoms. Notably, the impact of probiotic supplementation tends to be more pronounced when taken over a period of 8 weeks or longer, highlighting the importance of sustained use for maximum benefit.99
Various probiotics, such as Lactobacillus rhamnosus GG (LGG), L. reuteri (LR), L. paracasei, and strains of Bifidobacteria, have been widely studied for their role in altering GM composition and function, enhancing the epithelial barrier, and modulating host immune responses.104–106 These probiotics have demonstrated potential to prevent bone deterioration by reducing systemic inflammation, an effect that is particularly significant in models of bone disease like osteoporosis and osteopenia and arabinoxylan-oligosaccharides (AH-HAS) have also shown promise in modulating the gut environment to favour short-chain fatty acid (SCFA) production, an essential factor in promoting bone health by lowering inflammation and supporting calcium absorption.14,107
Low-FODMAP diet
For individuals with IBS, a low-FODMAP diet is one of the most effective strategies for addressing gut dysbiosis. FODMAPs, or fermentable oligosaccharides, disaccharides, monosaccharides, and polyols, are short-chain carbohydrates that the small intestine struggles to absorb efficiently.108 When fermented by colonic bacteria, these carbohydrates can lead to gas production, bloating, diarrhoea, and other IBS-related symptoms.109 By temporarily reducing FODMAP intake, individuals can experience significant symptom relief.
There are studies which investigated the impact of a low-FODMAP diet combined with either fructo-oligosaccharides (FOS) or a placebo (maltodextrin) in patients with IBS-D or IBS-M. Results indicated that the low-FODMAP diet significantly reduced symptom severity, with 80% of patients who took the placebo reporting symptom improvement, compared to only 30% of those who received FOS. This suggests that, in the short term, reducing FODMAP intake can be more effective for alleviating IBS symptoms than prebiotic supplementation.109
However, the low-FODMAP diet led to a reduction in beneficial gut bacteria, such as Actinobacteria, Bifidobacterium, and Faecalibacterium prausnitzii, and decreased levels of proinflammatory markers (IL-6 and IL-8) as well as n-butyric acid, an important short-chain fatty acid. Although this dietary intervention alleviates IBS symptoms, it may negatively influence the composition of the gut microbiota over time.110
Although FODMAPs can provoke symptoms in individuals with IBS, they also serve as prebiotics, essential for nourishing beneficial gut bacteria. Consequently, prolonged adherence to a low-FODMAP diet might decrease the diversity and activity of the gut microbiota. The study underscores the importance of further research into the long-term impacts of this diet and advocates for the gradual reintroduction of tolerable FODMAPs to maintain a balanced microbiome.110
Soluble vs. insoluble fibers
The distinction between soluble and insoluble fibers is important in addressing dysbiosis.111 Soluble fibers, such as those found in oats, psyllium husk, and flaxseeds, dissolve in water and form a gel-like substance that can be fermented by gut bacteria, leading to SCFA production.112 These fibers help improve stool consistency and reduce gut inflammation. Insoluble fibers, on the other hand, add bulk to the stool but are not as easily fermented, and in some cases, can exacerbate symptoms like bloating and discomfort, especially in IBS patients.113 Therefore, a diet rich in soluble fibers is often recommended for individuals with gut dysbiosis to improve symptoms and support beneficial microbial activity.114
One of the most important by-products of fiber fermentation by gut bacteria is the production of short-chain fatty acids (SCFAs). SCFA production, especially butyrate, is essential for maintaining mucosal health and suppressing proinflammatory cytokines implicated in bone resorption, such as IL-1β, IL-6, and TNF-α.115 Altered SCFA profiles have been observed in individuals with gut dysbiosis and may reflect compromised regulatory immune function. Given that SCFAs influence systemic immune tone, enhancing their production through dietary means may be a non-invasive strategy to improve peri-implant tissue healing outcomes.116
Fecal microbiota transplantation (FMT)
Fecal microbiota transplantation (FMT) has emerged as a promising therapeutic approach for restoring gut microbial balance in patients with dysbiosis-related conditions. The procedure involves the introduction of stool from a healthy donor into the gastrointestinal tract of a recipient, aiming to re-establish a healthy and diverse microbiota composition.117 Given the increasing evidence linking gut microbiota with systemic inflammation and immune function, FMT is being explored not only for gastrointestinal conditions but also for its potential systemic effects that could impact peri-implant tissue healing and bone remodeling.
A 2017 open-label trial conducted in Japan involving 10 IBS patients reported that FMT improved stool consistency and psychological well-being, with better outcomes observed in recipients who received microbiota from donors with higher levels of Bifidobacterium.118 Similarly, a systematic review by Halkjær et al. noted that 58% of patients across several small studies experienced symptomatic improvement following FMT, without significant adverse effects, although the limited number of trials calls for cautious interpretation.118
Recent randomized controlled trials (RCTs) have strengthened the case for FMT’s role in reshaping gut microbial ecology. For example, a placebo-controlled RCT by El-Salhy et al. demonstrated that FMT induced dose-dependent increases in microbial diversity and promoted the colonization of beneficial bacteria, supporting its potential as a precision-targeted intervention for microbial modulation.119 Although not all studies observed symptomatic relief, as seen in another RCT by Halkjær et al., a consistent increase in gut microbial diversity post-FMT was reported, reinforcing its capacity to restore a healthier microbial environment even in the absence of immediate clinical outcomes.120
While its direct application in dental implantology is yet to be explored, the systemic benefits of FMT such as reduced gut-derived inflammation, improved immune regulation, and enhanced microbial metabolite production could theoretically contribute to improved osseointegration and peri-implant health. Future research should investigate whether restoring gut eubiosis through FMT can translate into improved outcomes in implant dentistry by modulating host-microbiota immune interactions.
Although growing evidence suggests that systemic factors such as bone metabolism, immune-inflammatory pathways, and overall host-microbiome interactions support the notion that gut microbiota dysbiosis may influence dental implant outcomes, certain limitations must be acknowledged.
Many mechanistic insights into gut dysbiosis and its systemic effects, including peri-implant bone loss, are derived from animal models (e.g., mice, rats). While these studies offer controlled environments and allow for precise manipulation of variables (such as microbiota composition, diet, or antibiotic exposure), interspecies differences in immune function, microbiome diversity, and bone remodeling rates limit the direct clinical translation of their findings.
Moreover, the microbiome of animals differs significantly from that of humans, and osseointegration in animal models may not fully replicate the complex biological processes involved in human implant integration. These factors underscore the need for caution when extrapolating animal data to predict clinical outcomes in humans.
A considerable proportion of current evidence comes from retrospective observational studies, where historical patient data is analyzed to identify associations between gut dysbiosis and implant complications. While these studies are valuable for hypothesis generation and trend analysis, they are inherently limited in establishing causal relationships.
Confounding variables—such as patient age, systemic diseases, oral hygiene, and implant types—may not be consistently controlled for. This makes it challenging to isolate gut dysbiosis as the primary contributing factor to implant failure. Additionally, these studies often lack standardized methods for microbiota profiling or uniform criteria for defining peri-implant disease severity.
Most human studies assessing the gut-implant connection are cross-sectional or short-term, providing only a snapshot of microbial and inflammatory dynamics at a single time point. Such designs cannot capture the temporal evolution of dysbiosis or its long-term influence on peri-implant tissue healing and implant survival.
Longitudinal studies are essential to determine whether gut microbiota changes precede peri-implant bone loss or occur as a consequence. They also allow for the evaluation of progressive shifts in cytokine levels, systemic biomarkers, and microbiota profiles over time, which is critical for identifying patients at risk and implementing early interventions.
The gut microbiome is influenced by a wide array of host and environmental factors, many of which also affect implant outcomes. These include Dietary habits (e.g., fiber intake, fermented food consumption), Systemic conditions (e.g., diabetes, metabolic syndrome, inflammatory bowel disease), Medication use (e.g., antibiotics, proton pump inhibitors, immunosuppressants), Lifestyle behaviors (e.g., smoking, physical activity)
Future studies should focus on longitudinal clinical trials exploring how gut-targeted interventions influence peri-implant healing. Understanding the role of systemic markers, gut-derived metabolites, and inflammatory profiles may help establish a causal link. Interdisciplinary collaboration is essential for developing personalized therapeutic approaches.
Within the limitations of this review, the following conclusions can be drawn:
1. The gut microbiota plays a vital role in overall health and immune function, influencing various bodily systems, including oral health.
2. There may be a possible link between gut microbiota imbalances and bone health.
3. Gut dysbiosis leads to systemic inflammation, weakened immune responses, and impaired healing processes, all of which can negatively affect the stability and longevity of dental implants.
Therefore, gut microbiota health should be considered along with other systemic factors while evaluating patients for dental implant placement.
PRISMA checklist and flow chart for The gut-bone axis: gut microbiota dysbiosis and dental implant failures – Is there a link?: A Systematic review, https://doi.org/10.6084/m9.figshare.30076018.v1.121
Data are available under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Not applicable
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Human genetics,
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
No
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Human microbiome - oral and faecal. Oral health. Functional gastrointestinal disorders.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 16 Sep 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)