Keywords
Montelukast, Reserpine, BDNF, CHRNA7, Forced swimming test, Elevated plus maze test, neuropsychotropic
This article is included in the Cell & Molecular Biology gateway.
Montelukast is a pharmaceutical agent predominantly used for the management of asthma and allergic rhinitis. It fulfils its role by antagonizing the cysteinyl leukotriene receptor type 1, hence inhibiting the effects of inflammatory leukotrienes such as LTD4.
Nonetheless, like to other pharmaceuticals, montelukast is associated with possible adverse effects. Prevalent symptoms include cephalalgia, abdominal discomfort, and influenza-like manifestations. In few instances, it has been linked to changes in mood, agitation, and sleep disruptions, particularly in youngsters.
to assess the potential psychotropic effects of montelukast by measuring changes in cortisol levels, examining genes involved in neurotransmission and neuroplasticity, and observing behavioral tests in treated animals.
Thirty-five albino male rats weighing 150-200 milligrams the rats were categorized into five groups, with seven individuals in each group. Group II received reserpine as the positive control, while montelukast was administered at doses of 5 mg/kg for group III, 10 mg/kg for group IV, and 20 mg/kg for group V. The negative control group I was treated with a solvent combination of DMSO and acetic acid.
reduction in cortisol levels, downregulation of BDNF and chrna7 genes, and significant alterations in behavioral assessments in the reserpine and montelukast groups in a dose-dependent manner.
Animals treated with montelukast exhibit significant alterations in behavior and biomarker levels, suggesting potential psychological changes induced by the medication.
Montelukast, Reserpine, BDNF, CHRNA7, Forced swimming test, Elevated plus maze test, neuropsychotropic
The medicines that may induce depression or contribute to depressive symptoms are prevalent. Annually, there is a monetary report about substances that induce effects comparable to depression.1 These medicines may directly influence neurotransmitter concentration in the central nervous system or may induce adverse effects such as drowsiness, appetite suppression, pain, exhaustion, and other effects that might lead to the onset of depression. The primary sickness may also increase these consequences.2 Reserpine, used as a standard pharmacological agent to produce a depression model, is a pharmaceutical rarely employed in current hypertension therapy and is recognized as possibly inducing psychological issues.3 Montelukast, which functions by inhibiting the activity of leukotriene D4 in the lungs,4 is used in the treatment of millions of patients globally.5 In 2008, the Food and Drug Administration warned of a probable link between leukotriene-modifying medicines and suicidality.6 According to a study that focusing on the mechanism that lead to neuropsychiatric events of montelukast by studying the metabolic pathway of montelukast in mice they found, that montelukast can cause alteration in the biosynthesis of steroid and neurotransmitter in the prefrontal cortex of the brain as well as The routes of branched-chain amino acids (leucine, isoleucine, and valine) which are crucial for the synthesis of neurotransmitters such as dopamine, adrenaline, noradrenaline, histamine, and serotonin (5-hydroxytryptamine, 5-HT),7also Corticosterone, the predominant glucocorticoid in mice, similar to cortisol in humans,8 was observed to be upregulated in the brain of mice which is similar to that found in the human. Elevated cortisol levels have been noted in patients with depression, and the elevated corticosterone levels in treated mice indicate that those exposed to montelukast are more susceptible to exhibiting depression-like symptoms. Moreover, hyper-activation of the HPA axis elevates levels of corticosterone, serotonin, and histamine.9
BDNF is the member of the nerve growth factor (NGF) family that is expressed the most. Other members of this family include NGF, neurotrophin-3, and neurotrophin-4,10 Postmortem studies indicate that BDNF levels are diminished in the cerebral cortex of individuals with depression and those who have committed suicide’s,11 The upregulation or downregulation of the BDND gene indicates brain injury and causes alterations in mental state.
Cholinergic Receptor Nicotinic Alpha 7 Subunit CHRNA7 gene encode A7 nAChR,12 Interestingly, it has been demonstrated that the release of several neurotransmitters, including the release of noradrenaline, serotonin, GABA, glutamate, and dopamine, is promoted by the activated a-7 nicotinic acetylcholine receptor (a7 nAChR) via the increased permeability to cations, particularly Ca(2+),13 Research indicates that nAChRs exhibit aberrant functionality in mental disorders.12
All animal operations adhered to the ARRIVE criteria and the preclinical ARRIVE Essential 10 checklist14 and received approval from the Animal Ethics Committee of Mustansiriyah University, Baghdad, Iraq (Approval No: [54]). Rats were anesthetized by intraperitoneal administration of ketamine (100 mg/kg, 10%, Alfasan, Holland) and xylazine (10 mg/kg, 20 mg/ml, Kepro, Holland). Euthanasia was conducted at the conclusion of the experiment by an overdose of ketamine/xylazine,15 in accordance with the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals (2020). All measures were taken to reduce animal suffering.
Materials used in this study include Reserpine (Sigma/Germany, cas.no 50-55-5), and Montelukast powder from (Sigma/Germany, cas.no158966-92-8), solvent Dimethyl sulfoxide and acetic acid from (Mumbai–India), and corn oil from (Sigma Aldrich-Germany, Cat. No. C8267), distilled water from (pioneer, Iraq), xylazine vial (20 mg/ml) from (Kepro, Holland), Ketamine vial (10%) from (Alfasan, Holland, cat.no, V/NRP/97/0547) hematoxylin and eosin from (Sigma, Germany.
For this experimental animal study, thirty-five albino male rats weighting (150-200mg), rats were housed in spacious, cozy cages after being acquired from the animal house Iraqi Centre for Cancer and Medical Genetics Research (ICCMGR)/of Mustansiriyah University. They were permitted to acclimatize for 21 days in a regulated environment. there was a light schedule with 12 hours of light and 12 hours of darkness, the temperature was kept at 25 ± 1°C, and the humidity was kept between 40 and 50%. They have unlimited access to food and drink ad libitum.
Rats were then divided into five groups at randomly. Each group contain seven rats.
I. Group 1 (n = 7): Negative control group, rats received a mixture of distilled water, 5% glacial acetic acid, corn oil, and 5% Dimethyl sulfoxide (DMSO) was given orally for 14 days.
II. Group 2 (n = 7): The positive control, rats were administered a dose of reserpine at a concentration of 0.2 mg/kg of body weight intraperitoneally once daily for 14 days.16
III. Group 3(n = 7): rats orally administered dose of montelukast at a concentration of 5 mg/kg for 14 days.
IV. Group 4(n = 7): rats orally administered dose of montelukast at a concentration of 10 mg/kg for 14 days.
V. Group 5(n = 7): rats orally administered dose of montelukast at a concentration of 20 mg/kg for 14 days.
For preparing 0.2 mg/kg reserpine we first prepared a working solution of 1mg reserpine that dissolved in 5 ml of diluted glacial acetic acid, the glacial acetic acid in 5% concentration, the procedure done in a glass tube and mixed thoroughly by vortex until completely dissolved to get a final concentration of 0.2 mg/1ml of reserpine concentration from which about (0.2 ml to 0.175) was injected intraperitonially.17
To obtain a 5% DMSO concentration, five milligrams of montelukast were added to a laboratory glass. A small amount of DMSO was then added, and the mixture was stirred until the montelukast was completely dissolved. Corn oil was then used as a diluent and a vehicle of administration, and 5 mg/ml of montelukast solution was administered orally.18 The dosage was divided based on the weighting of the animals, and each group is represented in Table 1.
At the day 14, rats were permitted to fast for 12 hours, and under anesthesia with ketamine and xylazine injections intraperitoneal at 100 and 10 mg/kg, respectively, At the day 14 blood samples were extracted from the apex of the heart (left ventricle) using a 5 ml syringe, gauge 23, placed in gel tubes, and allowed to clot for 15 minutes at room temperature. Subsequently, they were subjected to centrifugation for 15 minutes at 3000 RPM. The obtained serum was aliquoted into Eppendorf tubes (1.5 ml). They were preserved at −20°C to quantify blood levels of cortisol.
Upon completion of the experiment (on day 14), following euthanasia, the skull was dissected, and the brains of seven rats from each group were extracted and rinsed with cold phosphate-buffered saline (PBS, pH 7.4) to eliminate residual blood and debris. Subsequently, the tissue was dried using filter paper, divided into two segments for histopathological examination and PCR analysis, and weighed using a sensitive balance. For histological examination, samples were kept in 10% neutral buffered formalin (Euroclone-Italy) to safeguard the tissue architecture against autolysis. For PCR analysis, 50-100 mg of brain tissue was combined with 1 ml of TRIzol (TransGen Biotech, cat. no. ER501-01) solution in an Eppendorf tube and then frozen for future use.
RNA was isolated from a tissue sample using the RNA extraction kit methodology. 50-100 mg of hippocampal brain tissue was promptly added to 1ml of TRIzol solution, then kept in the deepfreeze tell the day of examination.
To quantify the expression of BDNF PRM (F: TGGCTGACACTTTTGAGCAC, R: CAAAGGCACTTGACTGCTGA) and chrna7 PRM (F: TGCAGTGAATGGAAGTTTGC, R: GGACACAGCCTCCACAAAGT) genes via the real-time quantitative polymerase chain reaction (RT-PCR) technique. This procedure included the separation of brain tissue, total RNA extraction by TRIzol and subsequent Complementary DNA (cDNA) synthesis, along with genomic DNA removal, was performed using TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China, cat. no. AT311-02).19 A total of 20 μL of cDNA was amplified using real-time PCR, including 10 μL of Perfect Start Green qPCR Super Mix (TransGen Biotech, Beijing, China; cat. no. AQ601),20 1 μL of primers derived from forward and reverse solutions, 4 μL of cDNA, and 4 μL of nuclease-free water.21
The finished solution was subjected to heat reaction in the RT-PCR apparatus. Table 2 and Table 3 presents the thermal cycling methodology along with the appropriate melting point for each primer as specified by the manufacturer’s guidelines
| Temperature | Time/Sec. | Cycle | ||
|---|---|---|---|---|
| Stage 1 | Denaturation | 94 | 55 | 1 |
| Stage 2 | Denaturation | 94 | 10 | 42 |
| Annealing | 58 | 15 | ||
| Extension* | 72 | 20 | ||
| Stage 3 | Dissociation | 55-95 | 1 | 1 |
| Temperature | Time/Sec. | Cycle | ||
|---|---|---|---|---|
| Stage 1 | Denaturation | 94 | 55 | 1 |
| Stage 2 | Denaturation | 94 | 11 | 45 |
| Annealing | 56 | 20 | ||
| Extension* | 72 | 20 | ||
| Stage 3 | Dissociation | 55-95 | 1 | 1 |
CALCULATION: The cycle threshold (CT) of the target genes was standardized against that of the internal control gene. The disparity in cycle threshold (Ct) values between the β-actin (internal control gene) and the target genes, BDNF and CHRNA7, was determined using the following equation 28:
The calibrator was selected from the control samples, and the DCT of the calibrator was computed using the following equation:
The DCT of the test samples was normalized to the DCT of the calibrator:DD CT was calculated according to the following equation
Finally, the expression ratio was calculated according to the formula:22
Elevated plus maze test (EPM): The maze has four arms, two non-consecutive open arms measuring 30 cm in length and 5 cm in width, and two closed arms that form a central zone measuring 5 cm by 5 cm. The EPM was positioned 60 cm above the floor in the center of the room. The mouse was positioned in one of the open arms, orientated away from the center, and allowed to roam freely for five minutes. All sessions were documented with a camera positioned above the EPM, and the duration spent in the arms and center of the maze was quantified. Open arms and central regions are classified as anxiety zones.23
Force swimming test: The animal finds itself in a cylindrical water container from which it cannot escape. Most animals will try to flee by means of vigorous swimming. The animal is said to have given up when it stops swimming and floats on the water’s surface. An animal that quits up quite fast is said to be exhibiting traits related to human despair. Animals are maintained in water for five minutes for each during which we determine their movement and stop times.24
Effect of montelukast on gene expression depending on real time reverse transcriptase poly chain reaction (QRT-PCR): Gene expression analysis revealed differential regulation of CHRNA7 and BDNF genes across treatment groups details are presented in Table 4. The CHRNA7 gene showed variable expression patterns, with the control group exhibiting a mean fold change of 4.85 ± 3.42 as shown in Figure 1. Montelukast treatment at 20 mg/kg resulted in the lowest CHRNA7 expression (1.56 ± 1.05fold change). BDNF gene expression was relatively stable across groups, with slight variations observed
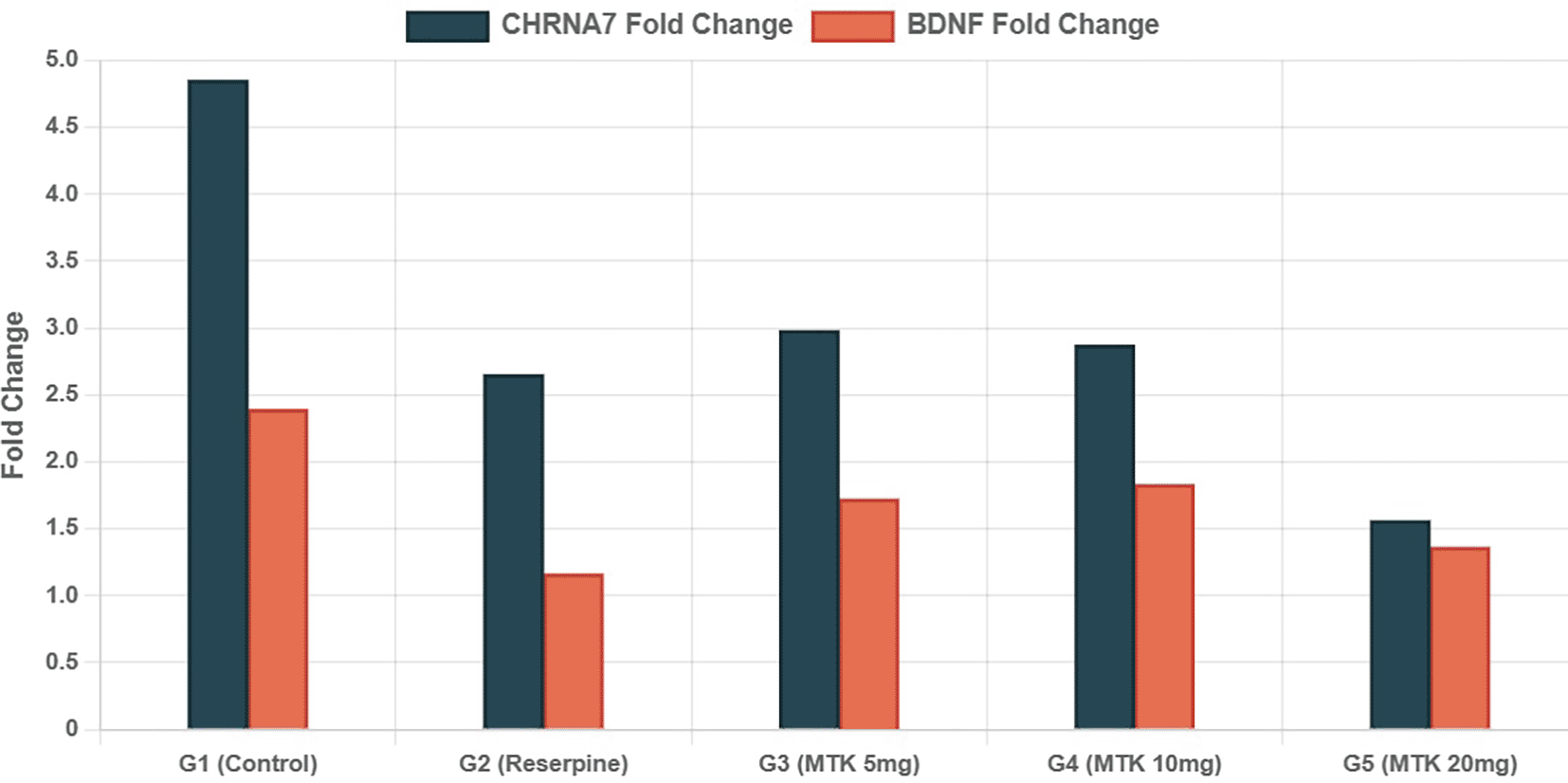
Data presented as fold change relative to control group (mean). n = 7 per group.
This section provides a detailed characterization of the behavioral profiles observed in each experimental group. The parameters assessed, indicative of general activity, despair-like behavior (in a forced swim test context), and anxiety-related responses (an elevated plus maze), are sourced from thorough examination of Swimming Time, Immobility Time, Open Arms Time, and Closed Arms Time allows for a comprehensive understanding of the behavioral state of each group details are presented in Table 8.
Forced Swimming Test: Montelukast treatment showed dose-dependent effects, with the highest dose (20 mg/kg) producing the most pronounced reduction in swimming time (187.00 ± 8.51 s) details are presented in Figure 2, Table 5 and Table 6.
Montelukast treatment showed a dose-dependent increase in closed time, with the 20 mg/kg dose group spending 197.14 ± 14.88 s in open arms details are presented in Table 7, Figure 3 and Figure 4.
Cortisol levels were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Cloud-Clone Corp., cat. no. CEA462Ge). Cortisol levels were markedly elevated in both reserpine (62.91 ± 9.27 ng/ml) and high-dose montelukast groups (91.12 ± 3.12 ng/ml) compared to control (11.57 ± 0.26 ng/ml) details are presented in Table 9 and Figure 5.
| Parameter | G1 (Control) | G2 (Reserpine) | G3 (MTK 5 mg/kg) | G4 (MTK 10 mg/kg) | G5 (MTK 20 mg/kg) | F-statistic | p-value |
|---|---|---|---|---|---|---|---|
| Cortisol (ng/ml) | 11.57 ± 0.26 | 62.91 ± 9.27 | 16.19 ± 1.95 | 45.32 ± 5.14 | 91.12 ± 3.12 | 389.42 | <0.001 |
The control group exhibited a normal morphology of neurons and glial cells across the molecular layer, external granular layer, external pyramidal layer, inner granular layer, inner pyramidal layer, ganglionic layer, and multiform layer (Figure 6). In contrast, the Reserpine-treated group displayed numerous vacuoles of varying sizes (indicative of demyelination), accompanied by degeneration and necrosis of glial cells, while the neurons maintained a normal appearance of pyramidal neuron types. The montelukast-treated groups at dosages of 5 mg/kg and 10mg/kg demonstrated normal meningeal diameter and normal morphology of neurons and glial cells throughout all cerebral cortical layers. However, the group treated with 20 mg/kg exhibited mild congestion of the meningeal blood vessels, along with congestion and perivascular edema of cerebral cortical blood vessels, while the glial cells and neurons across all cortical layers retained a normal appearance. details are presented in Figures 6–10.
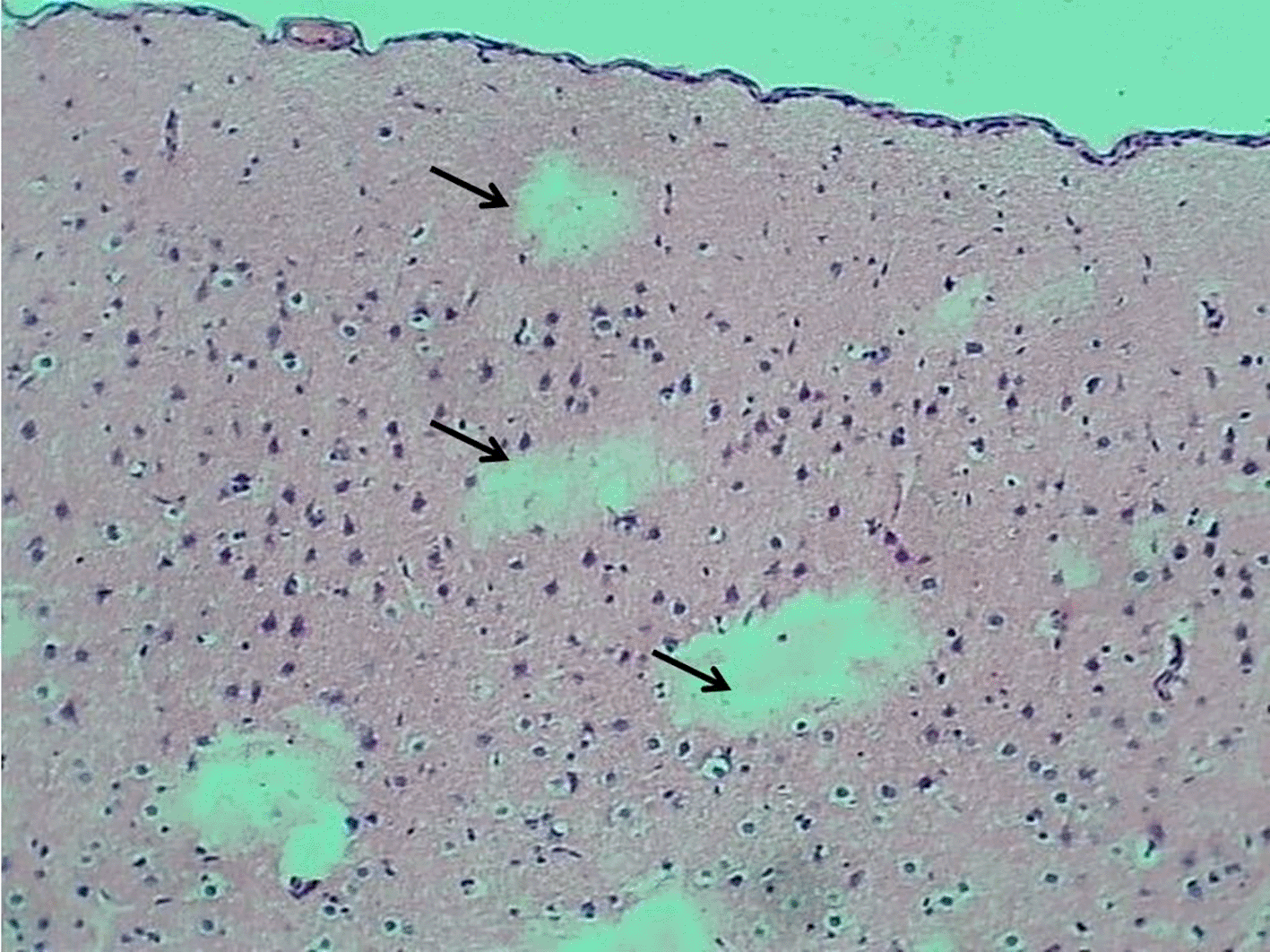
H&E stain 100x.
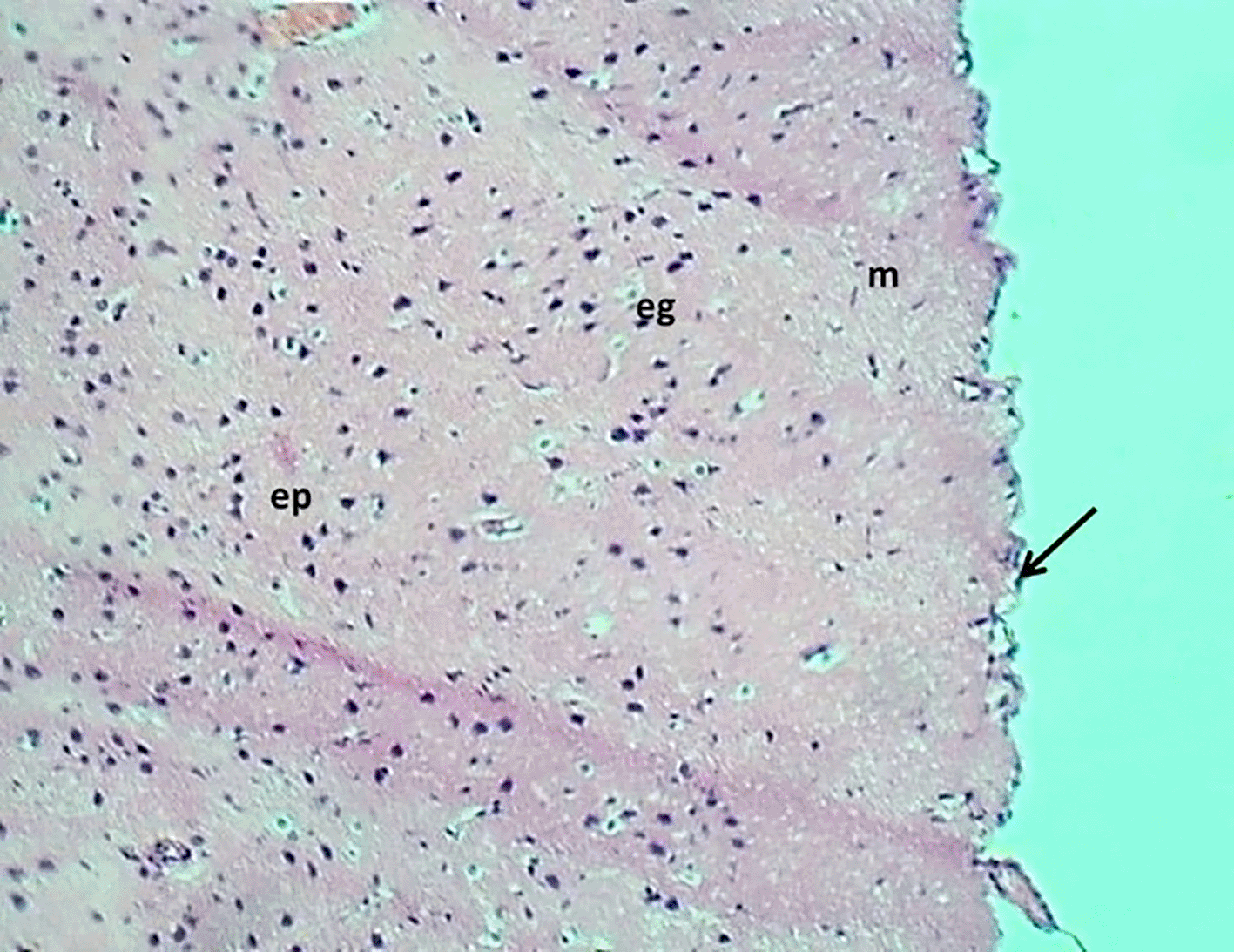
H&E stain 100x.
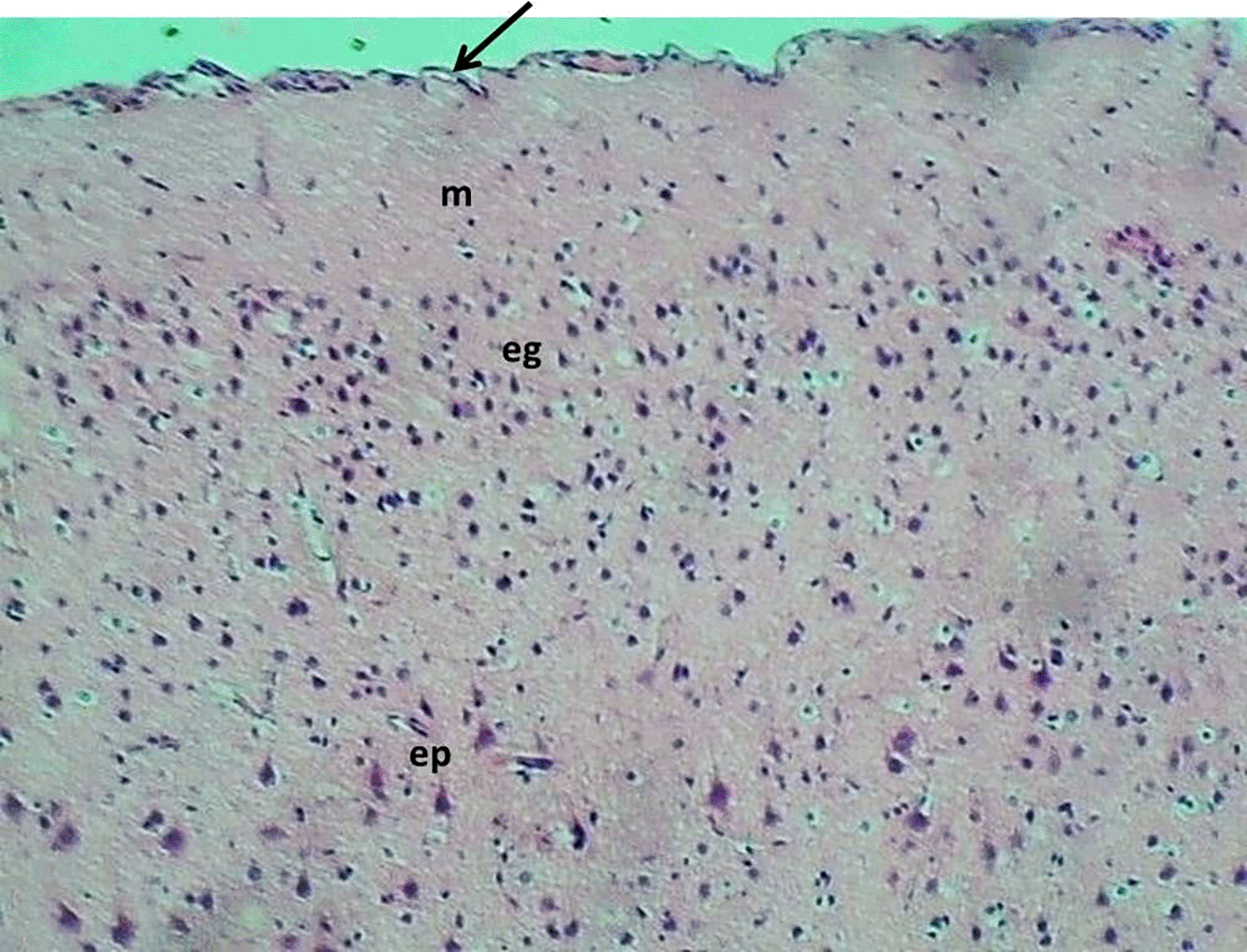
H&E stain 100x.
Analyses were performed in SPSS v26 and GraphPad Prism v9 (Excel 2021 for data verification/graphics). Data are reported as mean ± SD in tables and mean ± SEM in figures. Two-tailed tests were used with α = 0.05; exact p values are given where possible, and figures use p < 0.05, p < 0.01, p < 0.001. Assumptions were examined by Shapiro–Wilk tests and Q–Q plots (normality) and Levene’s test (homogeneity).
Group differences in behavioral outcomes (Forced Swimming Test; Elevated Plus Maze) and biochemical measures (dopamine, serotonin, norepinephrine, BDNF, cortisol) were tested by one-way ANOVA with Tukey HSD (or Tukey–Kramer) for pairwise comparisons. When comparisons were planned only versus the negative control, Dunnett’s test was applied. If variances were unequal, Welch’s ANOVA with Games–Howell post-hoc tests was used; for non-normal data, Kruskal–Wallis with Dunn’s tests (Bonferroni-adjusted) replaced parametric methods.
Gene expression was analyzed on ΔCq values (normalized to β-actin) using one-way ANOVA or the above non-parametric/heteroscedastic alternatives as required. For presentation, fold changes relative to control were computed by the 2^–ΔΔCq method; inference relied on ΔCq, with 95% confidence intervals provided.
Dose–response across montelukast groups (5, 10, 20 mg/kg) was evaluated using linear orthogonal polynomial contrasts within ANOVA, reporting slope, 95% CI, and η2p; Associations between behavioral and biochemical variables were assessed with Pearson correlations.
Determinants of behavioral outcomes were examined using multiple linear regression with immobility time and Elevated Plus Maze indices as dependent variables and neurotransmitters, BDNF, cortisol, and treatment group/dose as predictors. All analyses were complete-case.
Montelukast is a widely used pharmaceutical; in 2022, it ranked as the seventeenth most frequently prescribed drug in the United States, with over 29 million prescriptions issued,25 Montelukast is used for a number of conditions including asthma, exercise induced bronchospasm, allergic rhinitis, and urticaria Montelukast Monograph,26 also the psychological safety of montelukast has raised concerns among scientists during the last two decades.27
In the current investigation, we seek to determine the association between montelukast and its potential psychological adverse effects by using several behavioral and physiological indicators, as well as assessing its impact on CHRNA7 and BDNF gene expression.
The result of forced swimming test demonstrates that montelukast administration produces dose-dependent psychotropic effects comparable to reserpine, we observed an increase in immobility time in the montelukast-treated group compared to the negative control group in a dose-dependent way, particularly evident in Group V (15 mg/kg). An increase in immobility time during the forced swimming test indicates depressive-like behavior in the animal model.28
The observed increase in immobility duration may be associated with diminished serotonergic neurotransmission, since serotonin (5-HT) depletion or synthesis inhibition results in heightened immobility.29 In contrast, conventional antidepressants that augment monoaminergic transmission reliably diminish immobility in the Forced Swim Test (FST).
The Elevated Plus Maze (EPM) test in this research demonstrated that Montelukast elicits anxiety-like behavior in a dose-dependent fashion. Rats administered Montelukast (5–20 mg/kg) exhibited reduced time in the open arms and increased time in the closed arms relative to the control group. The group administered 20 mg/kg exhibited behavioral patterns similar to those of the reserpine-treated positive control, indicating heightened anxiety. The results align with growing clinical data indicating a correlation between Montelukast and neuropsychiatric adverse effects, such as anxiety, agitation, sleep problems, and depression. A significant quantity of post-marketing studies has compelled regulatory bodies to issue warnings. In 2020, the U.S. FDA issued a boxed warning for Montelukast owing to the potential for severe mental health adverse effects, especially in children and adolescents.30 The anxiogenic effects identified in this research reinforce these concerns and underscore the need for care when administering Montelukast, particularly for mild diseases like allergic rhinitis. These findings emphasize the significance of behavioral safety profile in pharmacological repurposing and prolonged use contexts.
Gene expression analysis revealed differential regulation of CHRNA7 and BDNF genes across treatment groups. The CHRNA7 gene showed variable expression patterns. Montelukast treatment at 20 mg/kg resulted in the lowest CHRNA7 expression. BDNF gene expression was relatively stable across groups, with slight variations observed.
This work investigated the expression of CHRNA7 and BDNF to elucidate the molecular pathways associated with the possible neuropsychological adverse effects of montelukast. CHRNA7, which encodes the α7 nicotinic acetylcholine receptor, is crucial in regulating neuronal excitability, inflammation, and anxiety-associated behavior. Our findings indicate that CHRNA7 expression was markedly downregulated in the montelukast 20 mg/kg group, with expression levels (1.56 ± 1.05) lower than those seen in the reserpine-treated group. This indicates that high-dose montelukast may aggravate cholinergic dysfunction, a mechanism associated with mood and anxiety problems.31
Likewise, BDNF, an essential neurotrophins implicated in synaptic plasticity, learning, and mood regulation, was diminished in the reserpine cohort and exhibited partial recovery in the 5 and 10 mg/kg montelukast groups. Nonetheless, expression in the 20 mg/kg group persisted at a modest level (1.36 ± 0.91), indicating a suppression of neurotrophic signaling at higher dosages. Although the ANOVA for BDNF did not achieve significance (p = 0.105), the tendency indicates that low-dose montelukast may provide minor neuroprotective benefits, but larger dosages may inhibit plasticity-related genes, hence contributing to the observed anxiogenic behavior.
Cortisol levels were significantly elevated in montelukast-treated groups, particularly at the highest dose, exceedingly even the reserpine group, Initially, elevated cortisol levels induce euphoria; but, sustained exposure to high concentrations may lead to the emergence of other psychological symptoms, including irritation, emotional lability, and sadness. Cortisol shortage may induce agitation; nonetheless, in such instances, the majority of patients exhibit apathy and depression.29
The combined behavioral and gene expression results from this research emphasize the need for care in the chronic or off-label administration of montelukast, especially at elevated dosages, and reinforce the significance of assessing central nervous system safety during medication use.
Zenodo. Montelukast altered BDNF and CHRNA7 gene expression were associated with behavioral changes in rats, https://doi.org/10.5281/zenodo.16920011.32
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0).
Zenodo. The ARRIVE Essential 10: author checklist – Montelukast altered BDNF and CHRNA7 gene expression were associated with behavioral changes in rats, https://doi.org/10.5281/zenodo.17008722.33
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)