Keywords
microneedling, gingival depigmentation, triphala curna, vitamin c, diode laser 445 nm, herbal therapy, periodontal esthetics, minimally invasive periodontics, randomized clinical trial
This article is included in the Manipal Academy of Higher Education gateway.
Physiologic gingival pigmentation may negatively impact smile esthetics, particularly in individuals with high smile lines. This randomized clinical trial aimed to compare the clinical effectiveness of three minimally invasive depigmentation techniques: microneedling with Triphala Curna, microneedling with vitamin C, and 445-nm diode laser ablation.
Eighteen systemically healthy patients with physiologic gingival pigmentation were enrolled and allocated into three groups (n = 6 each) via block randomization. Group 1 received microneedling followed by topical Triphala Curna application; Group 2 received microneedling with vitamin C; and Group 3 underwent diode laser depigmentation at a wavelength of 445 nm. In Groups 1 and 2, the procedure was repeated after two weeks. Clinical assessments were performed at baseline, 3 months, and 6 months for gingival pigmentation (Peeran Index), gingival thickness, and patient satisfaction (VAS scores). Statistical analysis included paired and independent t tests, chi-square tests, and descriptive statistics, with significance set at p < 0.05.
All the groups showed progressive clinical improvement. Complete depigmentation (grade 0) at 6 months was observed in 66.7% of patients in the Triphala group, 50.0% in the vitamin C group, and 33.3% in the laser group. Gingival thickness increased significantly in all groups, with the greatest increase observed in the Triphala group (from 1.10 ± 0.36 mm to 1.95 ± 0.41 mm, p < 0.001). Patient satisfaction also improved across all groups, with Triphala receiving the highest mean VAS score. No adverse events or pigmentation relapses were reported during study period.
Microneedling-assisted gingival depigmentation using Triphala Curna demonstrated the most favourable clinical and esthetic outcomes among the tested modalities. Results of vitamin C treatment were comparable to those of diode laser therapy, suggesting that herbal therapies combined with microneedling can serve as viable, biologically compatible alternatives to conventional laser techniques. Trial registration: CTRI/2025/09/095158.
microneedling, gingival depigmentation, triphala curna, vitamin c, diode laser 445 nm, herbal therapy, periodontal esthetics, minimally invasive periodontics, randomized clinical trial
The esthetic appeal of a smile is determined by the delicate balance between tooth form, alignment, color, and the surrounding gingival framework. While restorative and prosthetic components often receive clinical attention, gingival characteristics—particularly color, symmetry, and visibility—play an equally pivotal role in defining smile harmony.1 One of the most frequent esthetic concerns in this context is gingival hyperpigmentation, a benign yet conspicuous condition resulting from excessive melanin deposition in the gingival epithelium. Although physiological in origin, gingival pigmentation can be perceived as cosmetically undesirable, especially among individuals with high smile lines or darker skin tones, which impacts psychosocial well-being and self-confidence.2
Melanin, which is synthesized by melanocytes in the basal and suprabasal epithelial layers, is regulated through a cascade of genetic, ethnic, hormonal, and environmental influences. Melanogenesis is chiefly driven by the enzyme tyrosinase, which oxidizes tyrosine into dopaquinone, culminating in melanin formation. The severity and pattern of pigmentation can be clinically assessed using indices such as the Dummett Oral Pigmentation Index (DOPI), the Hedin Melanin Index (HMI), or the more recent Peeran classification.3
Over the years, various gingival depigmentation techniques have been proposed, including scalpel surgery, bur abrasion, cryotherapy, electrocautery, and free gingival grafting. While effective, these methods are often invasive and are associated with postoperative discomfort, delayed healing, and potential recurrence of pigmentation due to residual melanocytes or migration from adjacent untreated areas.4 Lasers, particularly diode lasers, have gained popularity because of their precision, minimal bleeding, and patient acceptance. Diode lasers operating at wavelengths such as 445 nm selectively target melanin chromophores, enabling efficient ablation with limited thermal damage. However, the high cost of laser equipment, the need for technical expertise, and occasional reports of relapse have prompted the search for more accessible alternatives.5
Microneedling (MN), which was originally developed for dermatological applications such as melasma and postinflammatory hyperpigmentation, is a minimally invasive technique that induces controlled microtrauma through fine needles. This mechanical stimulation triggers a cascade of biological responses—including the release of platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), and vascular endothelial growth factor (VEGF)—which collectively increase fibroblast activity, neocollagenesis, and angiogenesis. Additionally, MN creates microchannels that significantly increase the permeability of topically applied agents, making them effective drug delivery systems.6 Although its use in dermatology is well established, the application of MN in periodontal esthetics—particularly for gingival depigmentation—remains novel and underexplored.
Recent studies have evaluated MN in combination with topical agents such as vitamin C (ascorbic acid), a known antioxidant and tyrosinase inhibitor. In a prospective case series, Mostafa et al. (2023) reported significant reductions in pigmentation scores following microneedling with vitamin C, highlighting its clinical feasibility and patient acceptability.7 Similarly, Triphala Curna—a traditional Ayurvedic polyherbal blend comprising Emblica officinalis, Terminalia chebula, and Terminalia bellirica—has emerged as a promising natural agent. Like Triphala, which is rich in tannins, flavonoids, and gallic acid, Triphala has antioxidant, antimicrobial, anti-inflammatory, and melanin-inhibitory properties8 in vitro studies have demonstrated its ability to suppress tyrosinase activity, but its safety and effectiveness in oral applications, including plaque control and periodontal healing, have been widely documented.9 Despite its broad pharmacological potential, the use of Triphala Curna for gingival depigmentation has not yet been evaluated in a structured clinical trial.
To date, no randomized study has compared microneedling with Triphala Curna, microneedling with vitamin C, or diode laser therapy for gingival depigmentation in a single trial design. Given the increasing demand for biologically compatible, minimally invasive, and economically feasible esthetic treatments, such a comparative evaluation is both timely and clinically relevant.
The present randomized clinical trial was therefore undertaken to evaluate and compare the clinical effectiveness, esthetic outcomes, patient satisfaction, and safety of microneedling-assisted depigmentation using Triphala Curna, vitamin C, and a 445-nm diode laser over a 6-month follow-up period. By bridging herbal therapeutics, regenerative dentistry, and modern esthetic protocols, this study aims to inform and expand the scope of evidence-based periodontal esthetics.
1. Microneedling with Triphala Curna, vitamin C, or diode laser therapy resulted in significant reductions in gingival pigmentation scores over a 6-month period, with Triphala Curna resulting in the most favourable outcomes.
2. Each intervention led to a measurable increase in gingival thickness from baseline to 6 months, with microneedling-assisted therapies enhancing the biotype more effectively than diode laser alone.
3. All three treatment modalities are associated with high patient satisfaction and minimal postoperative discomfort, with microneedling combined with Triphala Curna demonstrating the highest patient-reported acceptability.
This study was designed as a prospective, single-center, randomized, parallel-arm clinical trial with a 6-month follow-up conducted in the Department of Periodontology, Manipal College of Dental Sciences, Mangalore. The aim of this study was to compare the clinical effectiveness, esthetic outcomes, and patient-reported satisfaction of three gingival depigmentation modalities: microneedling with Triphala Curna, microneedling with vitamin C, and 445-nm diode laser therapy.
The study was conducted in accordance with the principles outlined in the Declaration of Helsinki for research involving human subjects. The study adhered to the Consolidated Standards of Reporting Trials (CONSORT) 2025 guidelines for randomized clinical trials ( Figure 1). Ethical approval was obtained from the Institutional Ethics Committee of Manipal College of Dental Sciences Mangalore, Manipal Academy of Higher Education, Manipal (Protocol reference number: 24076) and the trial was prospectively registered with the Clinical Trials Registry of India (CTRI/2025/09/095158).
The study was applied for CTRI registration prior to its commencement, and a CTRI reference number was generated; however, due to a technical glitch, the registration could not be completed and an official CTRI number was not issued at that time. Meanwhile, Institutional Ethics Committee approval was obtained, and the study was initiated and conducted prospectively in strict accordance with the approved protocol. Towards the end of the study, the CTRI registration process was re-initiated and successfully completed, resulting in allocation of the final CTRI number; consequently, the registry reflects a retrospective classification based on the administrative registration date. Nevertheless, all study procedures, including participant recruitment, interventions, data collection, and analysis, were carried out prospectively with no deviations from the original methodology.
The sample size was calculated based on the findings of Mostafa et al. (2023)8 published in Cureus. Using two proportions (94% vs. 0%), with an alpha error of 5% and a power of 90%, the calculated sample size was 18 participants in total, i.e., 6 per group. This sample size was considered appropriate as no prior randomized trial directly comparing these three interventions was available, and it also aligns with the feasibility of conducting a single-center study.
A total of 18 systemically healthy participants with physiologic gingival pigmentation were recruited and equally divided into three groups (n = 6 per group) using computer-based block randomization. Allocation was concealed using sealed envelopes prepared prior to the surgical appointments. Participants were enrolled after providing written informed consent and receiving a comprehensive explanation of the procedures, benefits, and potential risks.
Blinding was implemented at the level of the outcome assessment. The examiner responsible for recording pigmentation scores, gingival thickness measurements, and patient-reported outcome measures (PROMs) was blinded to group allocation.
Each participant underwent a standardized protocol depending on group assignment:
• Group 1: Microneedling followed by topical application of Triphala Curna
• Group 2: Microneedling followed by topical application of vitamin C
• Group 3: Gingival depigmentation using a 445 nm diode laser
The microneedling-based procedures in Groups 1 and 2 were repeated after two weeks. All patients were followed up and evaluated at baseline, 3 months, and 6 months. The inclusion and exclusion criteria were defined a priori and are listed in Table 1.
All the statistical analyses were performed using SPSS version 25.0 (IBM Corp., Chicago, IL, USA). Descriptive statistics (means ± standard deviations) were calculated for continuous variables such as gingival thickness and VAS scores.
The normality of the data distribution was assessed using the Shapiro–Wilk test.
• Paired t tests were used for intragroup comparisons of gingival thickness and patient-reported outcomes between timepoints (baseline, 3 months, and 6 months).
• Independent t tests were used to compare differences between two groups for continuous variables when appropriate.
• The chi-square test was used to compare categorical variables, including pigmentation grades and satisfaction responses, among the three groups.
A p value < 0.05 was considered statistically significant throughout the analysis. No missing data were recorded during the study. All randomized participants completed the study protocol and were included in the final analysis.
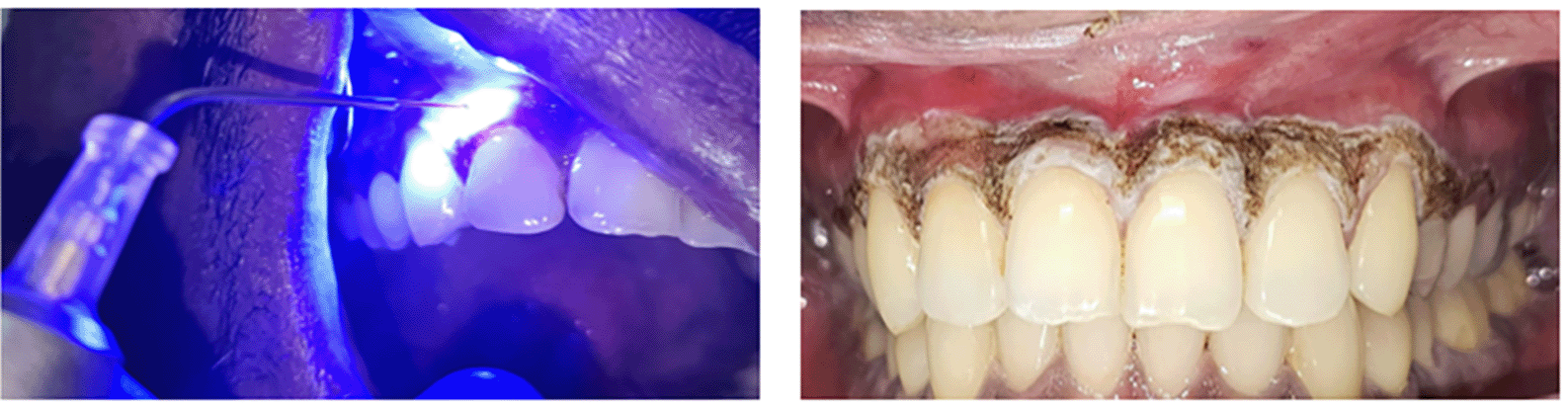
All enrolled participants underwent full-mouth scaling and received individualized oral hygiene instructions one week prior to the intervention. To rule out hypersensitivity reactions to Triphala Curna, a standardized forearm patch test was performed on all the subjects allocated to the Triphala group (Group 1). On the day of the procedure, local anaesthesia was administered using 2% lidocaine with 1:100,000 epinephrine in the anterior maxillary and mandibular regions ( Figure 1).
In both the Triphala and vitamin C groups, microneedling was performed via sterile 1.5 mm lancet needles. The procedure was carried out across the pigmented gingiva extending from the right to the left first premolar in both arches. Controlled, uniform micro perforations were made until pinpoint bleeding was observed, ensuring consistent penetration while minimizing trauma to adjacent tissues ( Figures 2 and 3).
• Group 1 (Microneedling + Triphala Curna):
Immediately following microneedling, Triphala Curna powder (1000 mg/mL; Ketzeal Overseas, New Delhi, India) was reconstituted with sterile saline to form a smooth paste. The paste was applied topically over the treated gingiva and left undisturbed for 10 minutes to facilitate transepithelial absorption ( Figure 4).
• Group 2 (Microneedling + Vitamin C):
A sterile ascorbic acid (vitamin C) powder (Purenso® Select, India) was similarly mixed with sterile saline to achieve a paste-like consistency and applied to the microneedled regions. The paste remained in contact with the gingiva for 10 minutes to optimize the delivery of the antioxidant and depigmentation effects ( Figure 5).
Both groups underwent a second identical application of the assigned biomaterial after 2 weeks, following the same protocol. No dressing was placed postprocedure, and patients were instructed to avoid brushing the treated areas for 24 hours and to refrain from using any medicated or abrasive mouthwashes during the initial healing period.
• Group 3 (Laser Depigmentation – 445 nm Diode Laser)
In Group 3, gingival depigmentation was performed using a 445 nm diode laser (Sirona®, Germany). Following adequate local anaesthesia, the pigmented gingiva was carefully ablated using the fibre tip in contact mode, employing slow, sweeping motions across the pigmented epithelium. Special care was taken to avoid overtissue carbonization and to preserve adjacent nonpigmented areas. The entire procedure was completed in a single session, and no topical agents were applied postoperatively ( Figure 6).
Patients in all three groups were monitored postoperatively and given uniform postsurgical instructions. Clinical follow-up visits were conducted at 3 months and 6 months following the initial procedure. At each visit, standardized clinical photographs were obtained, and assessments were performed for pigmentation grade, gingival thickness, and patient-reported outcome measures ( Figures 7, 8, 9 and 10).
Eighteen systemically healthy individuals (14 females and 4 males), aged 19–44 years (mean age: 32.78 ± 13.90 years), presenting with physiologic melanin pigmentation of the anterior labial gingiva were included. Allocation into three groups of six patients each (n = 6) was carried out through block randomization, which was performed immediately before the surgical procedure using sealed envelope software. All patients exhibited Grade 2 pigmentation on the Gingival Pigmentation Index (GPI) and had Class I or II smile lines.
Patients were assigned to the following intervention groups:
• Group 1: Microneedling followed by Triphala Curna application
• Group 2: Microneedling followed by vitamin C application
• Group 3: Diode laser ablation (445 nm)
All patients completed the study, with assessments conducted at baseline, 3 months, and 6 months postoperatively. No patient was lost to follow-up.
All 18 participants completed the 6-month follow-up period, and no missing data were reported.
At baseline, all patients presented with Grade 2 pigmentation, indicating moderate to heavy physiologic melanin deposition. By the 3-month follow-up, the pigmentation scores in all the groups had decreased to Grade 1, reflecting partial clinical improvement. At 6 months, complete clinical depigmentation (Grade 0) was achieved in 66.7% of patients in Group 1, 50.0% in Group 2, and 33.3% in Group 3, whereas the remaining patients in each group retained Grade 1 pigmentation. Although the intergroup difference was not statistically significant (chi-square test, p = 0.513), the clinical outcomes clearly favoured Group 1. Importantly, no cases of repigmentation were observed in any group during the 6-month follow-up.
The mean gingival thickness (GT) at baseline was 1.10 ± 0.36 mm in Group 1 and 0.95 ± 0.36 mm in both Group 2 and Group 3, indicating a uniformly thin gingival biotype across the study cohort. At 3 months, the mean GT increased to 1.42 ± 0.44 mm in Group 1, 1.20 ± 0.30 mm in Group 2, and 1.23 ± 0.36 mm in Group 3. By the 6-month follow-up, the GT had further improved, reaching 1.95 ± 0.41 mm in Group 1, 1.53 ± 0.25 mm in Group 2, and 1.60 ± 0.28 mm in Group 3.
Paired t tests confirmed statistically significant intragroup increases in GT at both 3 and 6 months (p < 0.001 for all groups). The greatest mean gain from baseline to 6 months was observed in Group 1 (+0.85 ± 0.16 mm), followed by Group 3 (+0.65 ± 0.08 mm) and Group 2 (+0.58 ± 0.15 mm). Independent t tests revealed a statistically significant difference between Group 1 and Group 2 (p = 0.011), whereas no significant difference was detected between Group 2 and Group 3, indicating that the results of vitamin C were comparable to those of the diode laser.
At 3 months, esthetic satisfaction scores were high across all groups, with Group 2 showing the highest early response (8.83 ± 1.17). By 6 months, Group 1 achieved the highest satisfaction score (9.83 ± 0.41), marginally outperforming Group 3 (9.80 ± 0.41) and Group 2 (9.67 ± 0.52).
Pain scores, recorded on a 10-point VAS (a lower score indicating less pain), were lowest in Group 1 at both 3 and 6 months. Although the intergroup differences in VAS scores were not statistically significant, the data consistently indicated superior comfort in patients treated with microneedling and Triphala.
Additionally, all participants across the three groups reported improved smile confidence, natural gingival appearance, and a willingness to recommend the procedure to others by the end of the study period.
Throughout the course of the study, no adverse effects were reported in any of the 18 patients. There were no incidents of postoperative infection, allergic reactions, excessive bleeding, delayed healing, or tissue trauma. All patients demonstrated normal healing responses and a healthy soft tissue appearance during follow-up. Importantly, no rebound pigmentation or aesthetic relapse was observed in any patient.
The clinical findings from this randomized trial suggest that all three treatment modalities—microneedling with Triphala Curna, microneedling with vitamin C, and diode lasers—are safe and effective options for gingival depigmentation. However, microneedling combined with Triphala Curna (Group 1) emerged as the most effective technique, producing the greatest improvement in gingival thickness, highest rate of complete depigmentation, lowest discomfort, and highest overall patient satisfaction. While Group 2 (vitamin C) did not outperform Triphala, it delivered comparable results to the diode laser group, suggesting that it may serve as a viable, minimally invasive, and herbal alternative to conventional laser-based depigmentation approaches.
This randomized clinical trial aimed to evaluate the relative effectiveness of three gingival depigmentation techniques—microneedling with Triphala Curna, microneedling with vitamin C, and diode lasers—in terms of clinical pigmentation reduction, gingival biotype modification, and patient satisfaction. The findings of the study confirmed all three hypotheses: (1) that microneedling with Triphala Curna leads to significant depigmentation, (2) that gingival thickness improves with this intervention, and (3) that the overall patient experience is highly positive. The intervention demonstrated measurable improvements in not only objective clinical outcomes but also patient-reported satisfaction, reinforcing its potential for routine clinical application.
Melanin-induced gingival pigmentation, while physiologic, remains a major esthetic concern for individuals with high smile lines or excessive gingival display. Existing methods for depigmentation, including scalpel surgery, bur abrasion, electrocautery, and laser ablation, are all clinically effective but may be limited by cost, complexity, operator dependency, and occasional adverse effects or recurrence.10 In contrast, herbal and microneedling-assisted methods represent an emerging paradigm shift—offering biological compatibility, ease of use, affordability, and increased patient comfort.
In the present study, microneedling combined with Triphala Curna resulted in complete depigmentation in 66.7% of the patients, along with the greatest increase in gingival thickness over 6 months (from 1.10 ± 0.36 mm to 1.95 ± 0.41 mm). The pigmentation scores decreased significantly in all patients, with most progressing from Grade 2 at baseline to Grade 0 at 6 months. These findings are in agreement with those of Ozsagir et al., who demonstrated the regenerative potential of microneedling when combined with bioactive agents such as injectable PRF, resulting in significant gains in gingival thickness through neocollagenesis and neoangiogenesis.11
The use of Triphala Curna, a traditional polyherbal formulation consisting of Terminalia chebula, Terminalia bellirica, and Emblica officinalis, is novel in this context. Pharmacologically, Triphala is rich in flavonoids, gallic acid, and tannins, which possess antioxidant, anti-inflammatory, and antityrosinase activities.12 This biological profile offers a plausible mechanistic explanation for both the pigment inhibition and regenerative effects observed in this study. In addition to synthetic depigmenting agents, Triphala also improves tissue firmness and reduces the risk of microbial colonization, making it uniquely suited for intraoral use.
Importantly, the vitamin C group (Group 2) presented results comparable to those of the diode laser group, with statistically similar gingival thickness gains and pigmentation reductions at 6 months. This finding is consistent with the findings of Mostafa et al., who demonstrated that microneedling with topical ascorbic acid significantly reduced gingival pigmentation scores over a short follow-up period.13 Ascorbic acid exerts its effects by inhibiting tyrosinase, disrupting melanin synthesis pathways, and promoting collagen biosynthesis.7 However, unlike Triphala, it does not exhibit antimicrobial or anti-inflammatory activity, which may explain the relatively small improvements in gingival firmness and patient comfort noted in our study.
Laser therapy (Group 3) remains the gold standard for depigmentation, with established precision and predictable results. However, diode lasers require significant investment and operator training. In this study, while diode lasers demonstrated good clinical outcomes, their results did not surpass those achieved with either Triphala or vitamin C, and the reported pain scores were slightly higher, possibly because of tissue charring and longer healing times. This aligns with the clinical experience reported by Reddy et al., where a diode laser, although effective, was less favoured by patients due to thermal side effects.12
Interestingly, no repigmentation was observed in any group at 6 months, a significant outcome given that conventional techniques—especially scalpels and electrocautery—often lead to recurrence within 3–6 months due to residual melanocyte migration. The observed stability in pigmentation may be attributed to the melanocyte-inhibiting effects of Triphala, which has been shown to suppress tyrosinase activity and disrupt melanogenic pathways in vitro.14,15
Furthermore, improvements in the gingival biotype, as reflected by increases in gingival thickness, enhance not only esthetics but also tissue resilience to future trauma or surgical manipulation. Such an increase in biotype thickness has clinical relevance in periodontics, orthodontics, and implant dentistry, as thinner phenotypes are more prone to recession and surgical relapse.16
Patient-reported outcome measures (PROMs) constitute another layer of validation. The Triphala group reported the highest satisfaction scores, the least discomfort, and 100% willingness to recommend the procedure. The psychosocial impact of esthetic improvement cannot be overstated, and these PROMs reinforce the holistic benefit of this intervention.
Gingival pigmentation, although physiological, can be esthetically displeasing. This study introduces and compares two microneedling-based approaches—using Triphala Curna and vitamin C—with 445-nm diode laser therapy. The findings support the clinical utility of herbal microneedling techniques, particularly Triphala Curna, as accessible, patient-friendly, and effective alternatives for managing gingival hyperpigmentation. Microneedling-assisted gingival depigmentation using Triphala Curna represents a cost-effective, minimally invasive alternative to diode laser therapy, with superior improvement in gingival thickness and patient satisfaction. This technique can be seamlessly integrated into routine periodontal esthetic care, especially for patients seeking biologically compatible, non-laser-based depigmentation solutions.
While this study yielded promising results, certain limitations should be noted. The small sample size and single-center design may restrict the broader applicability of the findings. The six-month follow-up period, although adequate for short-term assessment, may not fully reflect long-term outcomes such as recurrence. Additionally, the absence of histological analysis limits the confirmation of melanocyte reduction.
Future studies should involve larger, multicenter cohorts, incorporate split-mouth designs to reduce variability, and include histological assessments using techniques such as Masson Fontana staining. Extended follow-up periods beyond one year would further strengthen the clinical evidence.
This study provides compelling evidence that microneedling-assisted depigmentation using Triphala Curna offers a safe, effective, and biologically harmonious approach for managing physiologic gingival pigmentation. The intervention delivered consistently superior outcomes across all evaluated parameters, including extent of depigmentation, enhancement of gingival thickness, and patient-reported satisfaction—without a single adverse event or relapse over the six-month follow-up. Notably, the results achieved with Triphala not only surpassed those of other groups but also utilized an accessible, equipment-independent protocol rooted in herbal pharmacology.
While diode laser therapy remains a widely accepted gold standard and vitamin C has demonstrated comparable efficacy in many respects, the integration of microneedling with Triphala Curna clearly stands out for its clinical performance, patient comfort, and regenerative potential. These findings underscore the promise of biologically driven, minimally invasive therapies in contemporary esthetic periodontal care, positioning microneedling with Triphala as a leading candidate in the evolving landscape of gingival depigmentation protocols.
Ethical approval was obtained from the Institutional Ethics Committee of Manipal College of Dental Sciences Mangalore, Manipal Academy of Higher Education, Manipal (Protocol reference number: 24076), and the trial was prospectively registered with the Clinical Trials Registry of India (CTRI/2025/09/095158). Participants were enrolled after providing written informed consent and receiving a comprehensive explanation of the procedures, benefits, and potential risks.
Statistical analysis for this study was performed using IBM SPSS Statistics version 25.0 (IBM Corp., Chicago, IL, USA). No custom software or code was developed or used.
FIGSHARE: Microneedling-assisted gingival depigmentation clinical dataset.
This project contains the following underlying data: https://doi.org/10.6084/m9.figshare.29986912.v117
• Datasets.xlsx (Raw clinical data for pigmentation scores, gingival thickness, and VAS scores)
• Comparative evaluation of microneedling with triphala curna, microneedling with vitamin c, and 445 nm diode laser for gingival depigmentation: a randomized 6-month clinical trial consort checklist
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
This project contains the following extended data: https://doi.org/10.6084/m9.figshare.29986912.v117
• Informed Consent Form.pdf (Template used for obtaining participant consent)
• Patient Information Sheet.pdf (Information sheet provided to participants)
• Representative Clinical Images.zip (Baseline and follow-up clinical photographs for each group)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0),which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Figshare: CONSORT checklist for “comparative evaluation of microneedling with triphala curna, microneedling with vitamin c, and 445 nm diode laser for gingival depigmentation: a randomized 6-month clinical trial” https://doi.org/10.6084/m9.figshare.30958703.v118
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0),which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)