Keywords
cell lines, methylation
cell lines, methylation
The epigenetic regulation of gene expression1 has recently attracted the interests of many researchers. The mechanism is known to regulate gene expression without modifying DNA sequences. Some examples of epigenetic regulation include promoter methylation2, histone modification3, the binding of transcription factors to gene promoter regions4, and miRNA regulation of target genes5.
Among them, the promoter methylation and the miRNA regulation of target genes are particularly important in the epigenetic regulation of gene expression. Promoter methylation is relatively stable, long term, and in some cases, heritable. It is generally believed that genes with hypermethylated promoters are repressed. In addition, there is mounting evidence that DNA methylation is involved in the development and progression of certain disease states. For example, aberrant methylation in cancer is frequently observed6 and the distinct patterns of promoter methylation between monozygotic (MZ) twin pairs have also been found to result in different health conditions7. In contrast to DNA methylation, miRNA regulation of target genes is more flexible and rapidly changing. miRNA expression can change even during cellular differentiation8. miRNA expression is often tissue-specific, and similar to DNA methylation, miRNA expression has been linked to human disease9. Thus, although miRNA-directed gene regulation is thought to result in subtle changes, it is generally believed that miRNAs are involved in many important biological processes ranging from cell division to aging.
Although DNA methylation of miRNA promoters has been studied extensively (e.g., in accordance with tumor formation10), the relationship between promoter methylation and miRNA regulation of target genes has not been thoroughly investigated. One likely reason for this is that the regulation of gene expression by promoter methylation is a form of pre-transcriptional control, whereas miRNA regulation of target genes is a form of post-transcriptional control, with the former taking place inside the nucleus and the latter outside the nucleus (cytoplasm). Thus, these two mechanisms are separated by both time and space, and as a result, to date, there have not been plausible biological reasons to suspect that promoter methylation and miRNA-mediated gene regulation operate in concert.
However, Su et al.11 recently found that miRNAs have a tendency to target genes with hypomethylated promoters. To my knowledge, their study was the first report suggesting cooperative regulation of gene expression by promoter methylation and miRNAs. In addition, Sinha et al. reported that genes promoters with high CpG content were more often targeted by miRNAs12. Saito and Sætrom also dicussed the relationship between miRNA-mediated gene regulation and various features of target genes, but they did not consider the methylation status of target genes promoters13. Although the study of Su et al represents the first evidence of a direct link between promoter methylation and miRNA regulation, the biological significance of their findings is not clear. In this study, I report that promoter methylation is miRNA-targeting-specific; that is, the amount of methylation observed at a given gene promoter is dependent on whether that gene is also a target of miRNA regulation. Furthermore, the miRNA-targeting-specific promoter methylation is also related to how miRNAs regulate target gene expression. Especially, I find that miR-548 miRNAs target genes with highly hypomethylated promoters.
In this study, I used publically available promoter methylation profiles from various resources, obtained from GEO ID: GSE3065314. This included 283 human promoter methylation profiles for distinct cell lines, ranging from hESC to various somatic samples, measured by HumanMethylation27 BeadChip (Illumina), which provides an efficient approach for surveying genome-wide DNA methylation profiles. The HumanMethylation27 panel targets CpG sites located within the proximal promoter regions of transcription start sites (TSS). Thus, it was suitable for our purpose. Promoter methylation profiles (GEO ID: GSE30653) also included data from both IMR90 and MRC5 cell lines, which were used to investigate relationships between promoter methylation and previously reported miRNA regulation and miRNA expression profile data15,16. Promoter methylation profiles in both BG02 and BG03 were also included in this study, and were compared to miRNA regulation and miRNA expression profile data (see below).
Additional promoter methylation profiles in IMR90 cell lines were obtained from GEO ID: GSM86800814, GEO ID: GSM73994017, and GEO ID: GSM37544218. They were compared to IMR90 promoter methylation profiles, GEO ID: GSM760387 within GEO ID: GSE30653. I also used promoter methylation profiles for IMR90 from GEO ID: GSE31848, which includes GSM868008; these data were generated using the Illumina HumanMethylation450 BeadChip. This BeadChip allowed us to interrogate > 485000 methylation sites per sample at single-nucleotide resolution. In addition, because this array also includes CpG sites outside of promoter regions, I restricted probes to a subset labeled as either TSS200 or TSS1500. Data from GSM739940 includes IMR90 promoter methylation profiles measured by the Illumina HumanMethylation27 BeadChip. However, because these data were generated by a different research group than that of GSE30653, I tested profiles from this dataset to confirm that obtained results were not research group dependent. Finally, I also used methylation profile data from GSM375442, which was generated using next generation sequencing (NGS). CpG methylation profiles from promoter regions were extracted using Bismark Software (Ver. 0.7.4)19 (see below); promoter regions were defined as nucleotide positions between -200 and +1200 basepairs (bp) from the transcription start site (TSS).
In order to compare miRNA-targeting-specific promoter methylation with target gene miRNA regulation and miRNA expression profile data from BG02 and BG03 cell lines, both miRNA and mRNA profiles were obtained from GEO ID: GSE1447320. Gene (mRNA) expression profiles of undifferentiated and differentiated BG02 and BG03 cell lines were obtained from the GEO IDs GSM551204 and GSM551206, and GSM551216 and GSM551218, respectively. Corresponding miRNA expression profiles of these two cell lines were obtained from the GEO IDs GSM361147 and GSM361271 (BG02) and GSM361288 and GSM361289 (BG03). Raw data files were downloaded from the site for further analysis and were normalized so as to have a mean of 0 and a variance of 1.
In order to infer miRNA-targeting-specific promoter methylation, I employed the MiRaGE method21 (see below). The MiRaGE method, which was implemented on a public domain MiRaGE server and Bioconductor MiRaGE package, was first used to infer miRNA regulation of target genes. This software was designed to infer the differential expression of miRNA target genes between two experimental conditions based on the expression profiles of the target genes in question; however, in this study, I used this method to infer miRNA-targeting-specific promoter methylation by substituting gene expression profiles with the promoter methylation profiles of each gene.
I first prepared a control dataset (pseudo) in which the expression level of all genes assigned a value of 1. Then, the amount of methylation at each gene was used as values of treatment data set. Although, usually, the ratio of number of methylated sites to the total number of methylated and non-methylated sites is used to describe promoter methylation levels, I employed a method in which total methylation values were used. I did this because I found that P-values computed when using methylation data were more strongly correlated to the P-values calculated from target gene miRNA regulation data (see below), which is likely due to the fact that the frequency of CpGs is also related to miRNA targeting12; i.e., genes with promoters that contain more CpGs were more often targeted by miRNAs as mentioned above. Using this procedure, I attributed two P-values to each miRNA, one expressing the degree of promoter hypermethylation, and the other expressing the degree of promoter hypomethylation. For this purpose, depending on the methodology and/or type of deposited data set, I computed P-values that describe promoter methylation as follows:
In this case, the promoter methylation profiles used to replace "gene expression" values within the MiRaGE method were
where M0i represents the scaled values of signal_B (intensity estimated of methylated DNA), which are expressed as the amount of promoter methylation of ith gene,
where N is the total number of genes considered and Mi is the raw value of signal_B. This signifies that the amount of promoter methylation is scaled so as to have a mean 〈M0i〉 of zero and standard deviation σM0i of 1. exp is applied in this instance because I want to consider the amount of methylation rather than the ratio of the amount of promoter methylation. Because P-values are computed after the pair of input values are transformed to a logarithmic ratio, by substituting 1 in the control dataset and an exponential value in the treatment dataset based on raw values results in the usage of raw values as differential expression/promoter methylation (see below).
In this case, the promoter methylation profiles used to replace "gene expression" values within the MiRaGE method were
where Ci takes 1 only when Mi = 0; otherwise, it takes on 0, so as to avoid infinite values after transformation to the logarithmic ratio.
In this case, the promoter methylation profiles used to replace "gene expression" values within the MiRaGE method were
where βi is the ratio of methylated sites to unmethylated sites,
where Ui is the signal from unmethylated sites (signal_A) and C is the regulation constant, which typically takes on the value of 100. Since only β values were deposited in the public datasets used, we could not avoid using them; however, the correlation with target gene miRNA regulation was substantially decreased. An explanation for this is noted above.
In this case, the promoter methylation profiles used to replace "gene expression" values within the MiRaGE method were
where max(Mi) is the maximum value of Mi and Mi is computed in this case as follows:
where yj, 0 ≤ yj ≤ 100 is the percentage of methylation at site j, which was computed by the Bismark Software19 (see below). The summation was taken over the length of the promoter region as defined above (i.e., between -200 bp and +1200 bp from the TSS).
Here are the command line inputs used for generating the methylation values of CpG sites within the Bismark Software package19.
% bismark_genome_preparation \
–path_to_bowtie bowtie_dir \
–verbose ./hg19/ &
% R
>x <- scan("GSM375442_CpgMIP-IMR90.seq.txt",
sep="\n",what=character(0))
>write.table(file="sequence.fa",
paste(paste(">p",1:length(x),sep=""),
x,sep="\n"),sep="\n",row.names=F,
quote=F,col.names=F)
>q()
% bismark ,/hg19/ \
–path_to_bowtie bowtie_dir \
–bowtie2 -f sequence.fa
% methylation_extractor -s –comprehensive \
sequence.fa_bt2_bismark.sam
% genome_methylation_bismark2bedGraph_v3.pl \
CpG_content_sequence.fa_bt2_bismark.sam.txt \
> sequence.fa_bt2_bismark.sam.bed
where bowtie_dir is the directory where bowtie2 is installed. We also assumed that R22 was installed but the part executed by R can be perfomed by any other alternative script languages. GSM375442_CpgMIP-IMR90.seq.txt is a file downloaded from GEO and sequence.fa_bt2_bismark.sam.bed includes methylation percentages of each CpG sites, yj, which was explained above.
The inference of miRNA-targeting-specific promoter methylation/miRNA regulation of target genes was carried out using the MiRaGE method, which has been described previously21.
Although the MiRaGE method is typically used for datasets with two experimental conditions, each of which contains more than one replicate, I used this method for instances in which each condition consisted of only a single replicate. In each case, I based our analysis on the premise that for a given gene i, there were a pair of gene expression datasets or promoter methylation profile datasets, which were measured under a control condition xcontrol,i and a treatment condition xtreat,i. From this, I computed the logarithmic ratio
In such an example, when a difference between raw values is favorable, exponential values exp(xi) can be used instead of xi, in which case I get
When computing P-values that reject the null hypothesis by using the alternative hypothesis that Δxis of the target genes of the miRNA m are less (greater) than those that are off-target but form a target of any other miRNAs, I compute
where P[A < (>)B] is P-values computed by statistical tests when two set A and B are compared. The tests implemented within the MiRaGE Server/package are the t-test, Wilcoxon rank sum test, and Kolmogorov-Smironov test.
Thus, judging A < (>)B depends upon the selected statistical test used. Gm represents the set of genes targeted by miRNA m and G’m is the intersection of the set of off-target genes of miRNA m and the set of target genes of all other miRNAs. It should be noted that genes that were not the targets of any miRNAs were totally excluded from the analysis; however, all miRNAs were considered and no exclusions based on conservation were applied. When inferring promoter methylation, xcontrol,i = 1 and xtreat,i were used to represent the amount of promoter methylation. When inferring miRNA regulation of target genes during cell senescence in IMR90 and MRC5 cell lines, xcontrol,i was used to represent gene expression of young cell line and xtreat,i was used to represent gene expression in senescent cell lines. When inferring regulation of target genes during differentiation in BG02 and BG03 cell lines, xcontrol,i was used to represent gene expression in undifferentiated cell lines and xtreat,i for gene expression in differentiated cell lines.
I have used two types of P-values, P<(>)m:methy, which corresponded to the miRNA-targeting-specific promoter methylation, and P<(>)m:regul which corresponded to the miRNA regulation of target genes of miRNA m. When P<(>)m:methy[regul] is small enough, the target genes of miRNA m are significantly hypermethylated or hypomethylated and thus downregulated or upregulated. In order to see if these two types of P-values were correlated, I computed various correlation coefficients:
and accompanied P-values to reject null hypothesis that ρ = 0 by using the alternative hypothesis that ρ ≠ 0. ρ[a, b] is the Pearson’s correlation coefficients between a and b and rank(xm) is the rank order of xm among {xm}. Pm:z where z ∈ {methyl, regul} is either P<m, P>m, 1 – P<m or 1 – P>m. Thus, there are 4 × 4 = 16 possible combinations of Pm:methy and Pm:regul. ρs of Kolmogorov-Smirnov test and ρPearsonlogs for all tests can change when P<(>) is replaced with 1 – P>(<) because P<(>) ≠ 1 – P>(<) for Kolmogorov-Smirnov test and log(P<(>)) = log(1 – P>(<)) for all tests. Thus, optimal combinations with the maximum absolute correlation coefficients were employed.
In contrast to the cell senescence study15 in which miRNA expression was investigated by NGS, only microarray measurements were available for differentiation in BG02 and BG03 cell lines. Due to issues related to the accuracy and quality of microarray data and the relatively small amount of miRNA expression, very few miRNAs were found to be differentially expressed between undifferentiated and differentiated cell lines. Thus, miRNAs differentially expressed between undifferentiated and differentiated cell lines were selected based on two criteria before being subjected to further analyses;
Absolute differential expression |xdiff,i – xundiff,i|> Δxc, where xdiff,i and xundiff,i are the normalized expression of gene i of differentiated and undifferentiated cell lines, respectively. Δxc is the threshold value that can be used to select genes associated with significant differential expression during differentiation.
For this method, it was required that adjusted P-values based on the BH criterion23 were less than 0.05 for significant upregulation or downregulation. Here, P-values were computed using a t test between two sets of probe values attributed to gene i.
After Δxc was suitably selected, the correlation coefficient between differential expression xdiff,i – xundiff,i and log (P<m:regul) were computed. Positive values were taken to indicate a reciprocal relationship between miRNA expression and miRNA regulation of target genes; smaller P<m:regul were assumed to signify that target genes were upregulated (and vice versa); thus, a reciprocal relationship required that miRNA should be downregulated, i.e., xdiff,i – xundiff,i < (>)0, which should result in a positive correlation between xdiff,i – xundiff,i and log (P<m:regul). Since miRNA names in GSE14473 were old (miRBase, release 9.1), miRNA names were converted to miRNA names used in the present version of the MiRaGE software package (miRBase release 18) and by the miRConverter implemented in miRSystem24.
miRNAs were ranked based on P>m in each of 283 samples in GSE30653, after excluding 12 control samples. Each miRNA was ranked based within each sample, and then, the cumulative rank was determined for 271 samples.
miRNAs were ranked based on P-values for either downregulation during cell senescence (IMR90 and MRC5) or upregulation during differentiation (BG02 and BG03) for each statistical test. Then, each miRNA was ranked based on order summed up over three statistical tests.
Based on the inference from the data produced by the MiRaGE method21 (see Methods), the promoters of genes that are targets of 70–90% of human miRNAs were significantly hypomethylated, dependent on the statistical tests used, and the definition of promoter methylation levels: the β-value or the amount of methylation (see Table 1 and Additional files). This finding was consistent with conclusions made by Su et al.11, who stated that miRNAs had a tendency to target genes with hypomethylated promoters. Given that promoter methylation patterns do not change drastically between certain cell types, the amount of miRNA-targeting-specific promoter methylation is also highly cell-type-independent. Mean correlation coefficients of P>m range between 0.8 and 0.9, again depending on the statistical test used and the definition of promoter methylation levels (Table 2).
The summation of the number of miRNAs that target genes with significantly hypomethylated promoters in each cell line. The total numbers of miRNAs and cell lines were 1921 and 271, respectively (the number of cell lines was out of 283 in GSE30653, excluding 12 control samples). Thus, the total number of combinations of an miRNA and a cell line is 1921 × 271 = 520591. Promoter methylation levels were defined as either the amount of methylation or the β-values. Observed P-values based on the BH criterion23, P>m of < 0.05 were used to signify the promoters of the genes targeted by miRNAs that significantly hypomethylated. P-values were adjusted using the p.adjust function in R22. A complete list of raw (not adjusted) P-values is available in additional file 1. Cell line names can be also identified as column names of the additional file 1.
Mean Pearson’s correlation coefficients of P>m between pairs of cell lines.
In order to see if miRNA-targeting-specific promoter methylation was genuinely related to miRNA regulation of target genes, I compared P-values of miRNA-targeting-specific promoter methylation to P-values of miRNA regulation of target genes during the senescence of IMR90 and MRC5 cell lines15,16 and during the differentiation of BG02 and BG03 cell lines20. It was clear that promoter methylation and miRNA regulation of target genes were significantly correlated during both cell senescence and differentiation (Table 3), despite the fact that correlation coefficients exhibited opposite directionalities in cell senescence and differentiation. This means that genes with miRNA-targeting-specific promoter hypomethylation are downregulated during cell senescence, but upregulated during differentiation.
Various correlation coefficients between P-values of promoter methylation and regulation of target genes in IMR90 and MRC5 during cell senescence and in BG02 and BG03 during differentiation; P-values associated with the correlation coefficients are indicated. P>ms were employed for cell senescence while P>ms of promoter methylation and 1 – P>m of regulation of target genes were employed for differentiation as shown, as these combinations exhibited the most significant correlations. Genes with miRNA-targeting-specific promoter hypomethylation were recognized to be downregulated during cell senescence based on positive correlations and upregulated during differentiation based on negative correlations (for details, see Methods).
To confirm that the significant correlation noted above between miRNA-targeting-specific promoter methylation and miRNA regulation of target genes was not a false-positive finding, I also tested for this association in IMR90 cell lines by using a different microarray and, NGS approach, as described by another group of researchers18 (Table 4). Again, I observed a significant correlation between promoter methylation status and miRNA regulation, suggesting that the relationship found between these two mechanisms was independent of experimental conditions.
Correlation coefficients between miRNA-targeting-specific promoter methylation and miRNA regulation of target genes during cell senescence shown in Table 3 were evaluated by a comparison with the results produced by different array design (GSM868008), distinct research group, alternative measures of promoter methylation (GSM739940), and NGS (GSM375442). Independent of these factors, miRNA-targeting-specific promoter methylation was found to be always positively correlated to miRNA regulation of target genes (i.e., genes with miRNA-targeting-specific promoter hypomethylation were downregulated during cell senescence).
In addition to these analyses, I also confirmed a significant reciprocal relationship between miRNA expression and target gene expression. Because it is usually believed that miRNAs downregulate target gene expression, downregulated genes should be targeted by upregulated miRNAs and vice versa. In fact, the reciprocal relationship during the senescence process of IMR90 cells has been reported15,16. In this study, I observed a similar reciprocal relationship during the differentiation of BG02 and BG03 cells20 (Table 5).
| Cell lines | Δxc |
Correlation coefficients | P-value |
|---|---|---|---|
| BG02 | 0.00 | 0.18 | 1.9 × 10-4 |
| BG03 | 0.04 | 0.51 | 0.04 |
The reciprocal relationship between target gene regulation by miRNA and miRNA expression is evaluated in BG02 and BG03 cell lines during differentiation. Given that only a limited number of miRNAs are differentially expressed during differentiation, miRNAs were screened as described in the Methods section before computing correlation. Then the correlation coefficients between differential expression of miRNA and logarithmic value of P-values of miRNA regulation of target genes were computed A positive correlation implied the reciprocal relationship, that is, if the target genes are expressive, then, the miRNA itself should be suppressive. (for details, see Methods).
Thus, I conclude that miRNA-targeting-specific promoter methylation is not an artifact, but a genuine biological process. One may doubt this conclusion given the finding that miRNA-targeting-specific promoter methylation is cell-line-independent and miRNAs are believed to regulate target gene expression in a cell-line-dependent manner. Although this implies an apparent discrepancy, the correlation coefficients obtained, which were at most 0.7 to 0.8, indicate that miRNA-targeting-specific promoter methylation governs at most 50% to 60% of miRNA regulation of target genes. Thus, cell line specific miRNA regulation of target genes can act within the remaining 50% to 40%. In actuality, as can be seen in Table 3, the correlations between miRNA-targeting-specific promoter methylation and miRNA regulation of target genes have opposite signs dependent on the biological processes being considered. Therefore, the fact that cell-line-independent and miRNA-targeting-specific promoter methylation partially governs cell line dependent miRNA regulation of target genes is not a discrepancy.
In order to better understand the biological significance of our findings regarding miRNA-targeting-specific promoter methylation, I sought to identify the specific miRNAs that target genes with hypomethylated promoters. I found that most gene promoters targeted by miR-548 miRNAs were highly hypomethylated (Table 6; here, I define miRNAs called "miR-548" as miR-548 miRNAs). At present, little is known about miR-548 miRNAs, which likely results from the fact that miR-548 miRNAs are primate-specific miRNAs (ps-miRNAs). Because of this, ps-miRNAs were not expected to play critical roles in fundamental biological processes like cell differentiation and development, and it has so far been widely assumed that such basic biological processes are conserved among mammals. This assumption has resulted in the exclusion of ps-miRNAs from consideration as candidates for basic and important biological processes. Thus, it is thought that the roles of these ps-miRNAs may be limited to primate-specific biological processes. Another reason that miR-548 miRNAs have not been extensively investigated is that they are generally expressed at low levels; miRNAs that play critical roles are thought to be highly expressed. For example, although miRBase stores the amount of short reads detected by next generation sequencing (NGS) for various miRNAs, miR-548 miRNAs have at most 100 short reads; this is in comparison to more abundant miRNAs like those from the let-7 family, which are typically represented by several millions reads. This makes it difficult to detect miR-548 miRNAs using microarray technology or sequencing. Actually, although there have been a few reports noting that miR-548 family members, including miR-548 miRNAs, play critical roles, these data come exclusively from quantitative PCR (qPCR) experiments25,26.
| Statistical Test | Number of miR-548 miRNAs | Mean rank | |
|---|---|---|---|
| Within top 100 ranked | Total number | ||
| t test | 43 | 68 | 216.2 |
| Wilcoxon rank sum test | 433 | 68 | 214.0 |
| Kolmogorov-Smirnov test | 433 | 68 | 215.0 |
68 miR-548 miRNAs were ranked as described in the Methods (promoter methylation was defined as the amount of methylation). Independent of the statistical methods used for computation of P>m, 43 out of 68 miR-548 miRNAs were ranked within the top-ranked 100 miRNAs. The mean rank of all miR-548 miRNAs is ~200 independent of the statistical method used. Given that the total number of miRNAs considered was 1921, these values are highly significant, P = 2 × 10-35.
In this study, here I found that promoters of genes targeted by miR-548 miRNAs were hypomethylated. Because it is generally assumed that genes with hypomethylated promoters are expressed, the fact that promoters of target genes of miR-548 miRNAs are hypomethylated may mean that, compared to genes that are targets of other miRNAs, target genes of miR-548 miRNAs are more sensitive to changes in miRNA expression. This could explain why promoters of target genes of non-expressed miR-548 mIRNAs are specifically hypomethylated.
miR-548 miRNAs represent a large set of miRNAs, with 68 family members; thus, although these miRNAs exhibit low levels of expression, the fact that there are many family members may imply that they have important biological roles. Indeed, it has been reported that potential target genes of miR-548 miRNAs play critical roles in various biological processes27,28. Since it is also suggested that they originate from transposable elements (TEs), miR-548 miRNAs exhibit high sequence similarities while maintaining significant sequence diversity27.
During senescence processes of IMR90 and MRC5 cell lines, the target genes of miR-548 miRNAs were significantly downregulated (Table 7), whereas during differentiation of the BG02 cell line, the target genes of miR-548 miRNAs were significantly upregulated (Table 7). The fact that I observed significant changes in expression of target genes without significant changes in expression of miRNAs that target these genes implicates a role miRNA-targeting-specific promoter hypomethylation.
miRNAs were ranked as described in the Methods. Target genes of miR-548 miRNAs were significantly downregulated during cell senescence (IMR90 and MRC5 cell lines) and upregulrated during differentiation (BG02). Insignificant upregulation of target genes of miR-548 miRNAs in the BG03 cell line was weakly correlated to miRNA expression and miRNA regulation of target genes (Table 5). Given that the total number of miRNAs considered was 1921, the values for IMR90, MRC5, and BG02 cell lines are highly significant.
Figure 1 schematically summarises the principal findings of this study. To the best of my knowledge, this is the first time that miRNA-targeting-specific promoter methylation in many types of cell lines have been observed (Table 1 and Table 2). miRNA-targeting-specific hypomethylation was correlated (Table 3 and Table 4) with regulation of miRNA target genes, which had a reciprocal relationship with miRNA expression (Table 5); genes with miRNA-targeting-specific hypomethylated promoters were downregulated during cell senescence and upregulated during differentiation. I also found that genes with miRNA-targeting-specific hypomethylated promoters were specific targets of miR-548 miRNAs (Table 6 and Table 7). Regulation of target genes by miR-548 miRNAs, which were typically expressed at low levels are likely the result of miRNA-targeting-specific promoter hypomethylation.
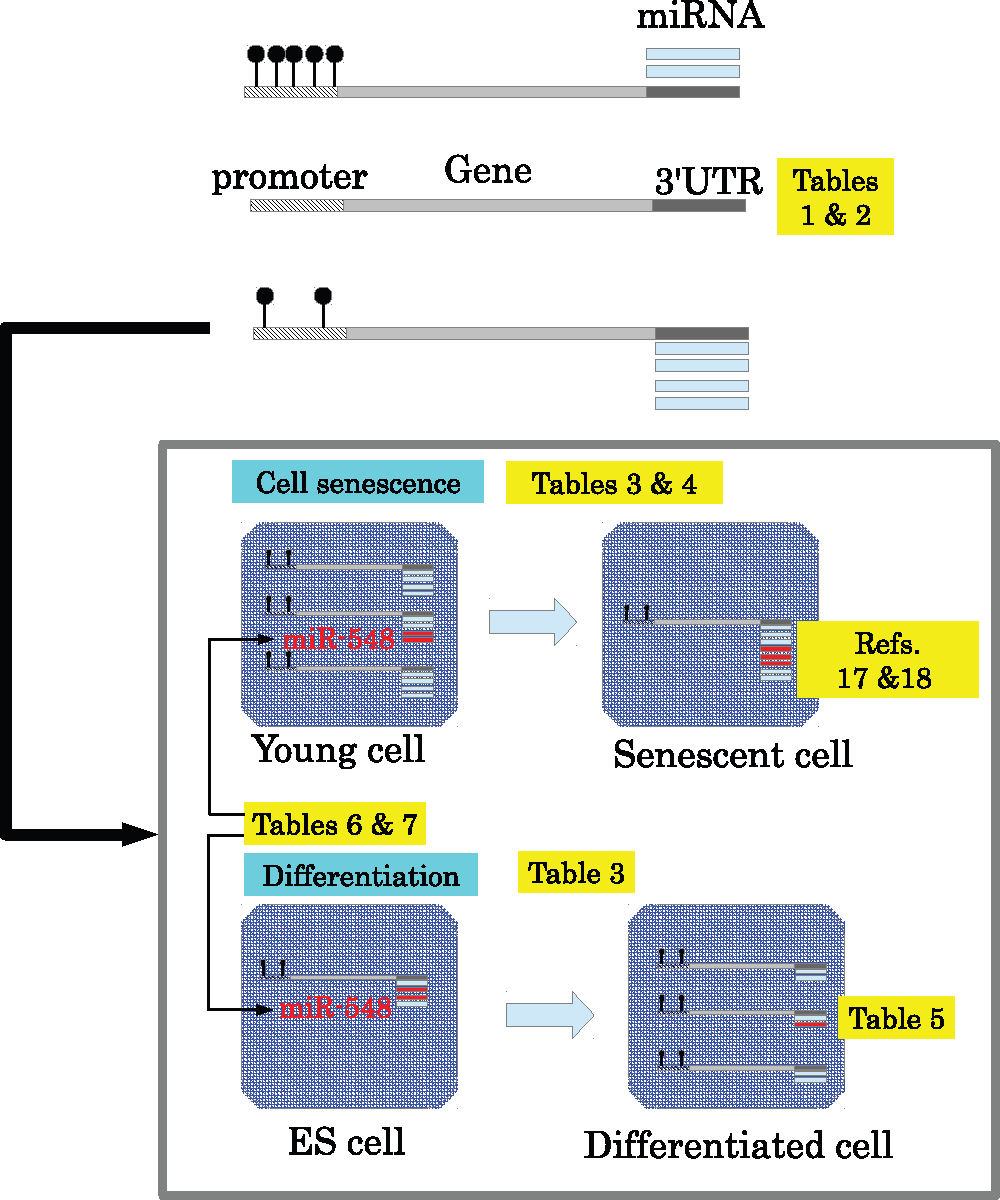
Promoters are methylated in a miRNA-targeting-specific manner (Table 1 and Table 2). Genes with miRNA-targeting-specific hypomethylated promoters are downregulated during cell senescence and upregulated during cellular differentiation (Table 3 and Table 4), whereas miRNAs that target genes with hypomethylated promoters were upregulated during cell senescence17,18 and downregulated during differentiation (Table 5). miR-548 miRNAs, which are expressed at low levels, were suggested to regulate target genes with the assistance of miRNA-targeting-specific promoter hypomethylation (Table 6 and Table 7).
TSS: transcription start sites;
GEO: gene expression omnibus;
bp: basepair;
miRNA: microRNA;
ps-miRNA: primate-specific miRNAs;
TE: transposable elements;
NGS: next generation sequencing.
Conceived and designed the experiments: YHT. Analyzed the data: YHT. Wrote the paper: YHT.
The author thanks the anonymous reviewer16 who suggested the investigation of the promoter methylation of miRNA target genes. This study was supported by KAKENHI (23300357).
Additional file 1 – Full list of P-values from the analysis of miRNA-targeting-specific promoter hypomethylation: t test
t test.xlsx: List of P-values from analysis of promoter hypomethylation of miRNA target genes calculated using the t test based on the amount of methylation.
Additional file 2 – Full list of P-values from the analysis of miRNA-targeting-specific promoter hypomethylation: Wilcoxon rank sum test
Wilcoxon rank sum test.xlsx: List of P-values from the analysis of promoter hypomethylation of miRNA target genes calculated using the Wilcoxon rank sum test based on the amount of methylation.
Additional file 3 – Full list of P-values from the analysis of miRNA-targeting-specific promoter hypomethylation: Kolmogorov-Smirnov test
Kolmogorov Smirnov test.xlsx: List of P-values from the analysis of promoter hypomethylation of miRNA target genes calculated using the Kolmogorov-Smirnov test based on the amount of methylation.
Additional file 4 – Full list of P-values from the analysis of miRNA-targeting-specific promoter hypomethylation: t test
beta t test.xlsx: List of P-values from the analysis of promoter hypomethylation of miRNA target genes calculated using the t test based on β-values.
Additional file 5 – Full list of P-values from the analysis of miRNA-targeting-specific promoter hypomethylation: Wilcoxon rank sum test
beta Wilcoxon rank sum test.xlsx: List of P-values from the analysis of promoter hypomethylation of miRNA target genes calculated using the Wilcoxon rank sum test based on β-values.
Additional file 6 – Full list of P-values from the analysis of miRNA-targeting-specific promoter hypomethylation: Kolmogorov-Smirnov test
beta Kolmgorov Smirnov test.xlsx: List of P-values from the analysis of promoter hypomethylation of miRNA target genes calculated using the Kolmogorov-Smirnov test based on β-values.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (update) 11 Sep 13 |
|||
|
Version 2 (update) 27 Mar 13 |
read | read | |
|
Version 1 23 Jan 13 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)