Introduction
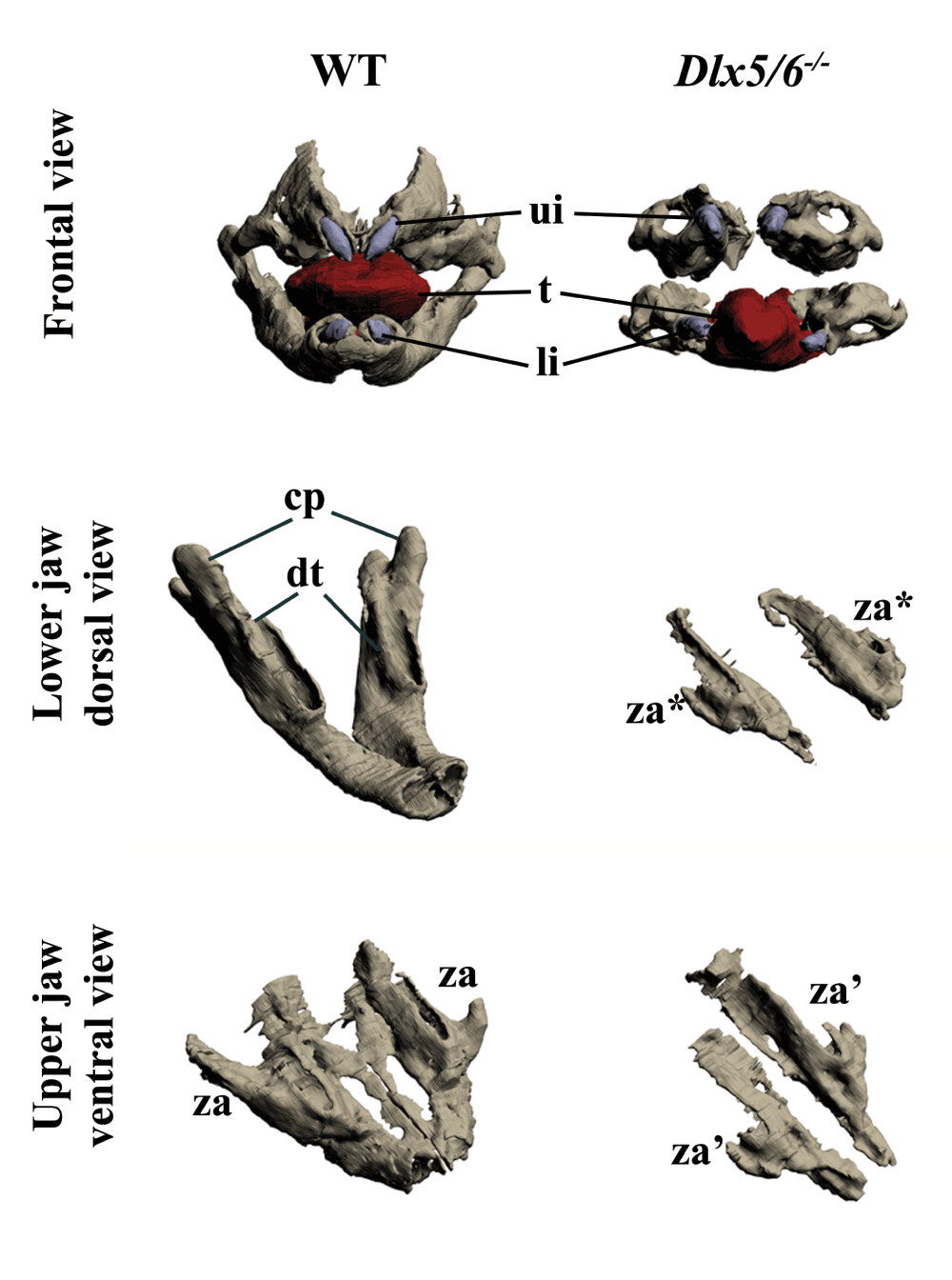
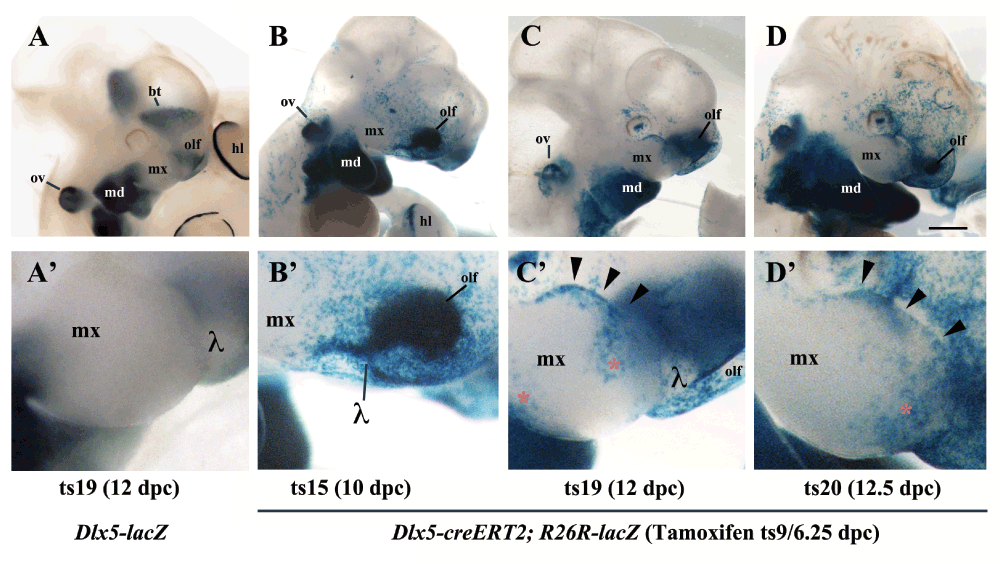
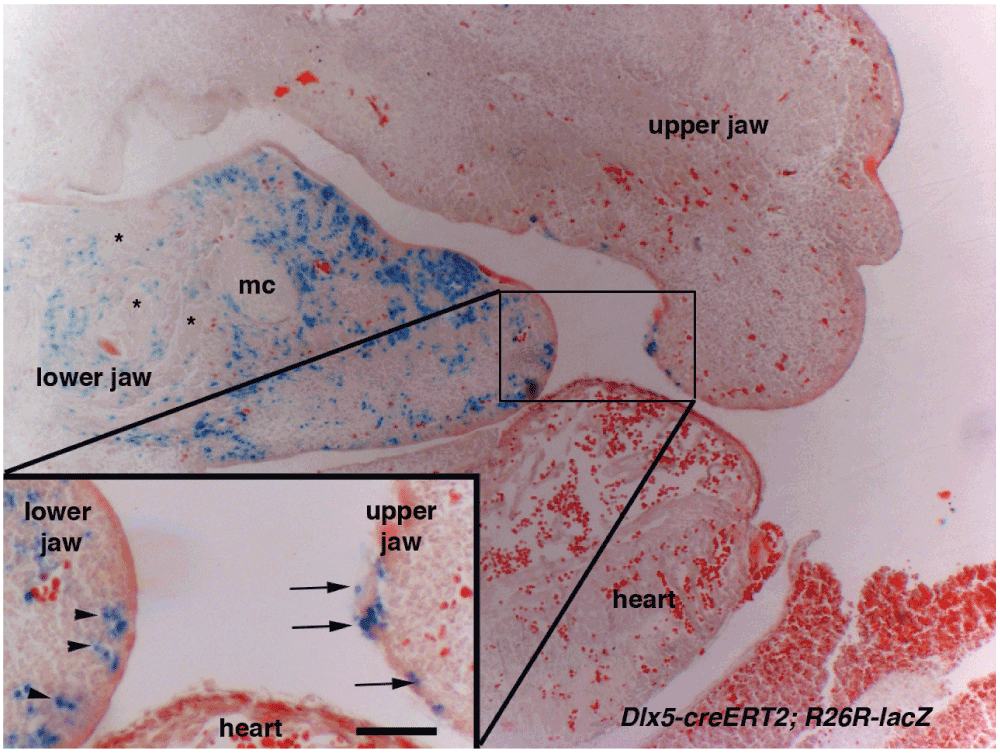
The skull of most vertebrates is characterized by the presence of articulated, asymmetric jaws which support the function of a muscularized oral cavity2,3. During embryonic development, the upper and lower jaws derive from the maxillary and mandibular processes of the first pharyngeal arch (PA1). Most cartilaginous and dermatocranial derivatives of PA1 are formed by Cranial Neural Crest Cells (CNCCs)4–9. During migration, signals emanating from the endoderm and possibly other PA1 components instruct the CNCCs to unfold the morphogenetic process of the jaws8,10,11. The nested expression of Dlx homeobox genes, vertebrate homologues of Drosophila Distal-less, has a fundamental role in the specification of the dorsoventral patterning of PA1 derivatives2,12. While Dlx1 and Dlx2 are expressed by CNCCs of the maxillary and mandibular components of PA1, Dlx5 and Dlx6 transcripts are present only in mandibular CNCCs. Targeted simultaneous inactivation of Dlx5 and Dlx613,14 results in the transformation of lower jaw into upper jaw-like structures, underlining the importance of these genes for lower jaw identity. The activation of Dlx5 and Dlx6 by endothelin-1 signalling is necessary and sufficient to define lower jaw identity15–19. Interestingly it has been observed13,14 that, after inactivation of Dlx5 and Dlx6, maxillary components are also affected despite the fact that these genes are not expressed by maxillary CNCCs. This observation could be accounted for by the presence of shared Dlx5/6-dependent signalling centres in proximity to the extremities of both the mandibular and maxillary arches; this notion gave rise to the so-called “Hinge and Caps” model of jaw organization1,3,20. In its original formulation this model predicts the presence of two opposing morphogen gradients, one emanating from the region of the upper/lower jaw articulation (hinge) and one from the distal extremities of PA1 (caps); the origin and nature of these signals remain elusive. Here we revisit the effects of Dlx5 and Dlx6 double inactivation on jaw development and, using a transgenic lineage tracing approach, we reveal that the maxillary arch epithelium harbours a cellular contingent derived from frontonasal Dlx5-expressing progenitors. Our findings suggest that transient Dlx5/6 expression could program these epithelial cells to provide the cues needed for maxillary arch morphogenesis.
Comments on this article Comments (0)