Introduction
The skull of most vertebrates is characterized by the presence of articulated, asymmetric jaws which support the function of a muscularized oral cavity2,3. During embryonic development, the upper and lower jaws derive from the maxillary and mandibular processes of the first pharyngeal arch (PA1). Most cartilaginous and dermatocranial derivatives of PA1 are formed by Cranial Neural Crest Cells (CNCCs)4–9. During migration, signals emanating from the endoderm and possibly other PA1 components instruct the CNCCs to unfold the morphogenetic process of the jaws8,10,11. The nested expression of Dlx homeobox genes, vertebrate homologues of Drosophila Distal-less, has a fundamental role in the specification of the dorsoventral patterning of PA1 derivatives2,12. While Dlx1 and Dlx2 are expressed by CNCCs of the maxillary and mandibular components of PA1, Dlx5 and Dlx6 transcripts are present only in mandibular CNCCs. Targeted simultaneous inactivation of Dlx5 and Dlx613,14 results in the transformation of lower jaw into upper jaw-like structures, underlining the importance of these genes for lower jaw identity. This phenotype is more severe than those observed in both single mutants (for instance, see2,13,15,16). The activation of Dlx5 and Dlx6 by endothelin-1 signalling is necessary and sufficient to define lower jaw identity17–21. Interestingly it has been observed13,14 that, after inactivation of Dlx5 and Dlx6, maxillary components are also affected despite the fact that these genes are not expressed by maxillary CNCCs. This observation could be accounted for by the presence of shared Dlx5/6-dependent signalling centres in proximity to the extremities of both the mandibular and maxillary arches; this notion gave rise to the so-called “Hinge and Caps” model of jaw organization1,3,22. In its original formulation this model predicts the presence of two opposing morphogen gradients, one emanating from the region of the upper/lower jaw articulation (hinge) and one from the distal extremities of PA1 (caps); the origin and nature of these signals remain elusive. Here we revisit the effects of Dlx5 and Dlx6 double inactivation on jaw development and, using a transgenic lineage tracing approach, we reveal that the maxillary arch epithelium harbours a cellular contingent derived from frontonasal Dlx5-expressing progenitors. Our findings suggest that transient Dlx5/6 expression could program these epithelial cells to provide the cues needed for maxillary arch morphogenesis.
Material and methods
Mouse strains and breeding
All animal experimentation was performed in accordance to French national regulations and approved by the MNHN ethical committee (approval n° 68-028r1). For this study we used about 35 dams (including 10 WT, 5 Dlx5+/-; 3 Dlx5/6+/-; 12 B6.129S4-Gt(ROSA)26Sortm1Sor/J; 5 B6(Cg)-Dlx5tm1(cre/ERT2)Zjh/J) and analyzed about 120 embryos, the exact record of animals used, litters obtained, embryos genotyped and number of embryos per litter is on record in our animal house. WT animals were from Charles River France and were maintained in the MNHN mouse facility which is officially certified by the French National Animal well being committee.
Dlx5lacZ/+ knock-in mice were maintained on a mixed B6/D2 genetic background15. Double Dlx5 and Dlx6 (Dlx5/6) mutant mice were maintained and genotyped as reported23. The inducible Cre driver strain B6(Cg)-Dlx5tm1(cre/ERT2)Zjh/J (designed by Z. J. Huang24), and the Cre reporter strain B6.129S4-Gt(ROSA)26Sortm1Sor/J were purchased from Jackson Laboratory (#10705 and #003309 respectively; Maine, USA) through Charles River Laboratories (L’Arbresle, France) and maintained on a C57BL/6J genetic background through heterozygous mating. Double heterozygous embryos were obtained through bi-directional crosses. Induction of Cre recombinase activity was obtained upon single intraperitoneal injection of 5mg of tamoxifen (Sigma-Aldrich), in corn oil. Tamoxifen preparation and administration in pregnant dams followed the Jackson Laboratory’s Guidelines and CNRS/MNHN Animal Handling Guidelines. Dams were anesthetized in a chamber containing 2.5% isoflurane in oxygen, euthanized by cervical dislocation at indicated stages and embryos were collected in phosphate-buffered saline (PBS), then staged and fixed by immersion in ice-cold fixative (2% paraformaldehyde/0.2% glutaraldehyde) for 5 to 15 minutes (depending upon their developmental stage).
β-galactosidase detection
For lacZ expression, embryos were fixed for 15–30 min in 4% paraformaldehyde; X-gal staining was performed as described previously15,25. Vehicle (corn oil) injection in double heterozygous mice did not yield leaking β-galactosidase activity.
Histology and 3D reconstruction
Heads from 18.5dpc (days post coitum) Dlx5/6-/- and wild type mouse embryos were fixed in Bouin’s solution (Sigma, France), embedded in paraffin and complete sets of frontal or parasagittal serial sections (12µm) were prepared. All sections were stained by Mallory’s trichrome as in21 and photographed (Nikon Digital Site DS-FI1). Pictures were aligned, piled and registered using the Fiji plug-in of NIH ImageJ “Register Virtual Stack Slices” (http://fiji.sc/wiki/index.php/Register_Virtual_Stack_Slices). 3D segmentation was performed with Mimics (Materialise, Belgium: http://biomedical.materialise.com/mimics) and visualized using Adobe Acrobat 9 pro.
Results
Dlx5/6 inactivation results in upper and lower jaw transformation with gain of symmetry
Previous reports suggest that double inactivation of Dlx5 and Dlx6 results in lower-to-upper jaw transformation; these reports also indicated that the upper jaw of these mice is not normal13,14. To better visualize the jaw phenotype of Dlx5/6 mutants, we performed 3D reconstructions of craniofacial elements of 18.5dpc (days post coitum) embryos. Frontal view of the mutant jaws (Figure 1, upper panel) shows an obvious gain of symmetry compared to a WT animal. Examining the defects of the lower and upper jaws separately (Figure 1, middle and lower panels), it is evident that both are transformed. In the absence of Dlx5 and Dlx6 the dentary and the upper jaw bones do not form correctly and are replaced by remarkably similar skeletal structures. In the mutant embryos, both the upper and lower jaw skeletal elements are reduced in size, are not fused in the midline, and display a lateral process positionally homologous to the wild type zygomatic arch. Thus the upper and lower jaw mutant bones resemble each other more closely than usually found in their normal counterparts.
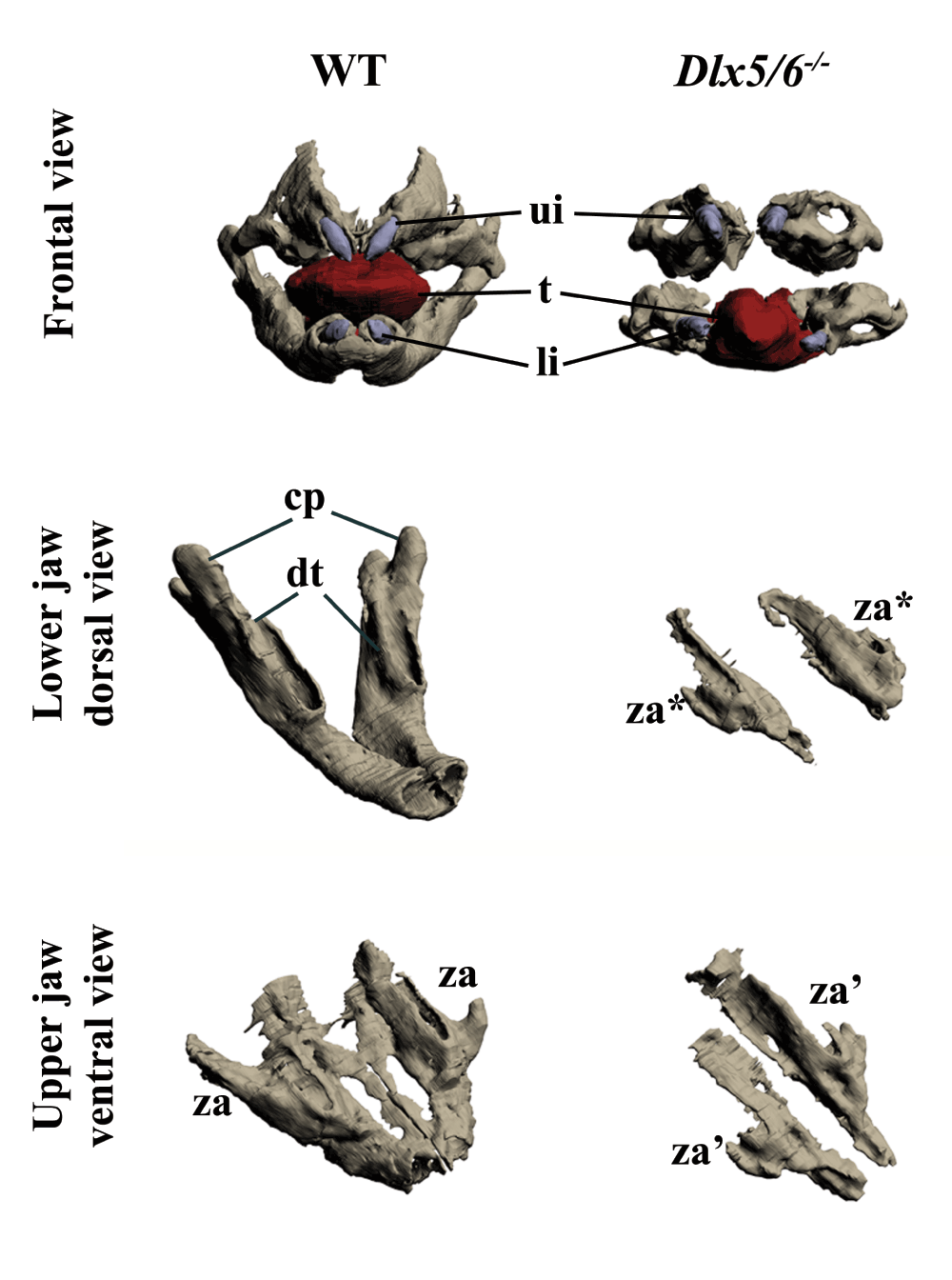
Figure 1. Three-dimensional reconstruction of the dentary and maxillary bones of 18.5dpc wild type and Dlx5/6-/- mouse embryos.
Upper row: Frontal view of WT and Dlx5/6-/- oral apparatus. Skeletal elements are grey, the tongue is red and incisors are violet. Middle row: Dorsal view of the dentary bone of WT and Dlx5/6-/- 18.5dpc mice. Lower row: Ventral view of the maxillary components of WT and Dlx5/6-/- 18.5dpc mice. Note that the inactivation of Dlx5/6 results in the transformation of both lower and upper jaw skeletal elements into new structures which appear more similar to each other than to their WT counterpart. cp, coronoid processes; dt, dentary bone; li, lower incisor; t, tongue; ui, upper incisor; za, zygomatic arch; za*, zygomatic arch-like structure deriving from lower jaw transformation; za’, zygomatic arch-like structure deriving from upper jaw transformation.
Transient Dlx5 expression in maxillary arch progenitors
In Dlx5-lacZ heterozygous Theiler stage (ts) 19 (12 dpc) embryos the reporter is active in the olfactory pit and mandibular arch, but not in the maxillary arch; this pattern of expression does not change upon tamoxifen treatment of the pregnant dam (Figure 2A, A’). To understand the origin of the Dlx5/6-dependent defect of the upper jaw we used a genetic approach to follow the lineage of Dlx5-precursors in the head. To this end we brought the R26R-lacZ reporter into the Dlx5-creERT2 driver background and we activated cre-recombinase activity by tamoxifen treatment of the pregnant dam at ts9 (7 dpc). We monitored β-Gal reporter activity from ts15 (10 dpc) to ts20 (12.5 dpc) (n=10 embryos per stage). At ts15 we observed a stream of β-Gal-positive cells extending from the lambdoidal junction, which joins the olfactory pit with the distal maxillary arch1,26, towards the body of the maxillary arch (Figure 2B, B’). At ts19 and ts20 (Figure 2C, C’; D, D’) reporter-expressing cells are found in the upper epithelial lining of the maxillary arch (arrowheads in Figure 2C’, 2D’) and in two distinct proximal and distal territories of the arch body (red asterisk in Figure 2C’).
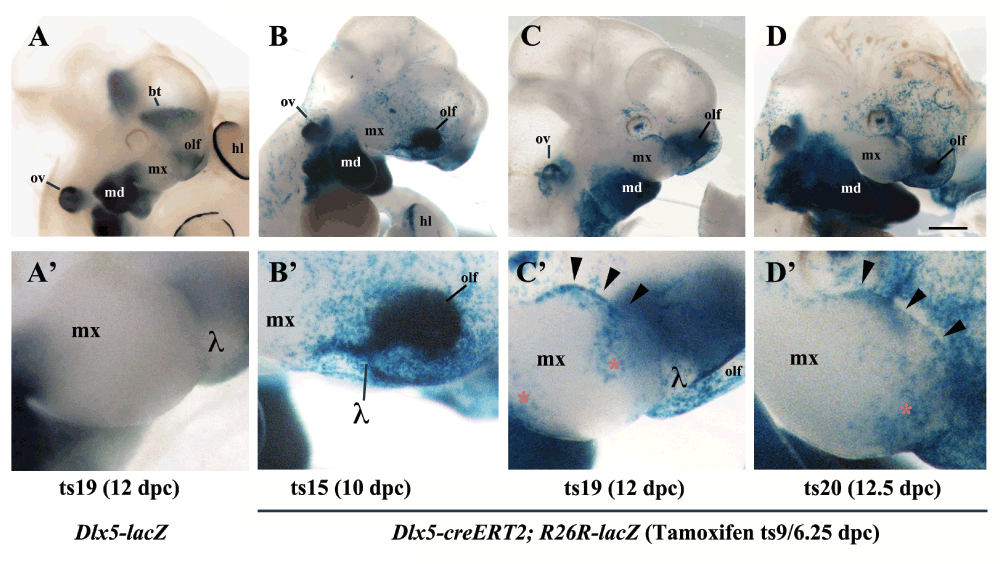
Figure 2. Lineage of Dlx5-expressing cells in the maxillary arch.
β-Galactosidase activity in the cephalic region of Dlx5-lacZ (A, A’) and Dlx5-creERT2; R26R-lacZ mouse embryos (B–D’). In all cases pregnant dams were treated with tamoxifen at 7dpc/Theiler stage 9 (ts9) and embryos were collected at the indicated Theiler stage. A, A’) As expected, even after tamoxifen treatment, Dlx5 is expressed in the mandibular arch (md), in the olfactory pit (olf), in the otic vesicle (ov), in the striatum (st) and in the hind limb (hl), but not in the maxillary arch. B, B’) Permanent activation of lacZ reporter expression in derivatives of Dlx5-expressing early progenitors (ts9) reveals the presence of a positive cellular contingent in the ts15 lambdoidal junction (λ) between the olfactory pit and the maxillary process. C, C’; D, D’) At later developmental stages (ts19, ts20) a contingent of lacZ positive cells populates the distal domain of the maxillary arch. hl, hind limb; md, mandibular arch; mx, maxillary arch; olf, olfactory pit; ov, otic vesicle; bt, basal telencephalon; λ, lambdoidal junction; red asterisk/black arrowheads, territories of the maxillary arch colonized by derivatives of Dlx5-expressing progenitors. Bar: A–D 1mm; A’–D’ 250µm.
To determine more precisely the tissue distribution of craniofacial derivatives of Dlx5-positive cells, we set apart two illustrative among the ten embryos per stage, and performed serial paraffin sections of Dlx5-creERT2; R26R-lacZ β-Gal-stained mouse embryos (12.5 dpc) after tamoxifen treatment of pregnant dams at 7dpc/Theiler stage 9 (ts9) (Figure 3). While in the mandibular arch β-Gal staining is limited, as expected, to CNCCs derivatives, the analysis of the complete set of serial sections shows that only epithelial cells lining the maxillary arch are positive. As no Dlx5-positive epithelial cells are present in the maxillary arch of normal embryos, we conclude that a population of epithelial cells derived from the Dlx5-positive frontonasal process participates to the formation of the maxillary arch.
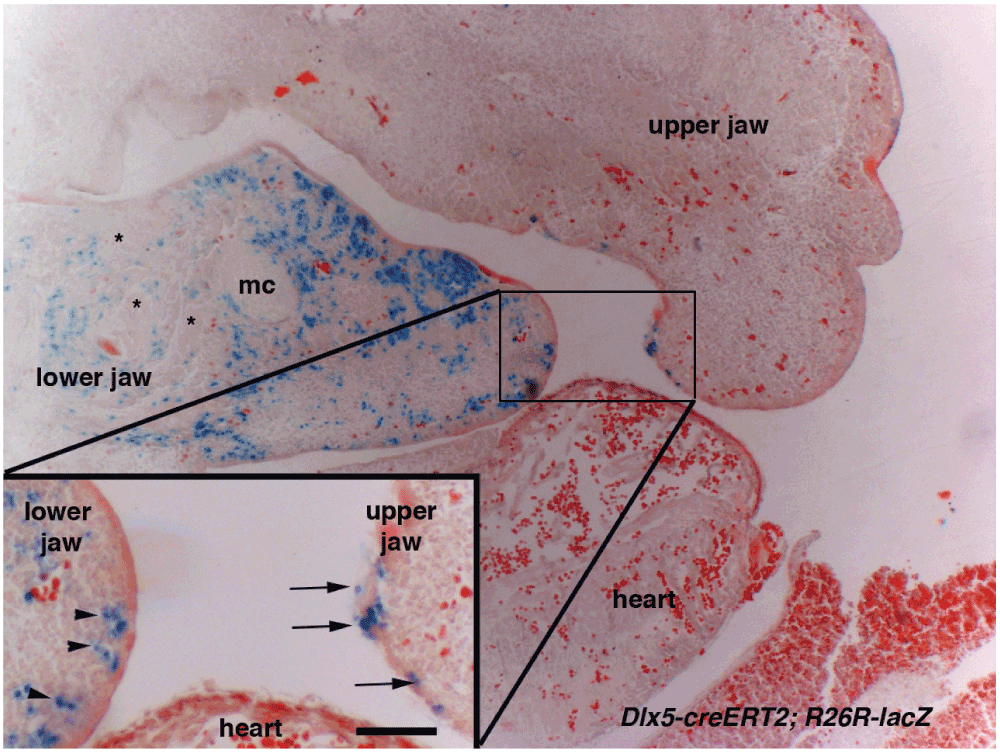
Figure 3. Derivatives of Dlx5-expressing progenitors in the upper and lower jaw primordia.
Sagittal section of Dlx5-creERT2; R26R-lacZ through the cephalic region of a 12.5dpc/Theiler stage 22 (ts22) mouse embryo. The pregnant dam was treated with tamoxifen at 7dpc/ts9. β-Galactosidase activity is found in CNCCs derivatives of the lower jaw (arrowheads). In contrast, in the upper jaw positive cells are only present in the overlying epithelium (arrows). mc, Meckel’s cartilage; asterisks, lower jaw muscles. Scale bar 50µm in insert, 150µm in the main figure.
Discussion
In this study we have re-examined the skeletal jaw phenotype of Dlx5/6 mutant mice. We confirm that both the mandibular and maxillary arches are transformed. The profound change in the shape of the maxillary arch is difficult to explain, as this region does not derive from a Dlx5/6-expressing territory. Indeed, in normal embryos maxillary CNCCs and the overlying epithelium do not express Dlx5 and Dlx6. Lineage analysis to identify derivatives of Dlx5-positive progenitors reveals a new population of cells extending from the olfactory pit through the lambdoidal junction towards the maxillary arch1,22,26. These derivatives of Dlx5-positive cells have lost Dlx5 expression as seen by Dlx5 in situ hybridization (see for example14,15,27,28) and by lacZ-Dlx5 knock-in (15, and Figure 2A’). We have previously shown that early Dlx5 and Dlx6 expression in the anterior neural fold is essential for nasal capsule patterning29; our present findings suggest that the same population of cells could also contribute to maxillary patterning. The epithelial cell contingent might well exert a patterning role upon the maxillary arch providing spatial cues to the underlying mesenchyme. A further argument supporting the notion that Dlx5/6 patterning of the upper jaw does not require their expression in CNCCs derives from our recent observation that selective ablation of these genes in CNCCs does not affect upper jaw morphology (Gitton et al., in preparation). This observation fits with the prediction of the ‘Hinge and Caps’ model1,3,22, and suggests that ‘cap’ signals could originate from derivatives of Dlx5-expressing frontonasal progenitors. Even if, after migration in the maxillary arch, these cells lose Dlx5 expression, it appears that the early expression of Dlx5 confers them the capacity to pattern maxillary arch CNCCs, which do not themselves express Dlx5 and Dlx6. As the compound Dlx5/6 mutant displays an upper jaw malformation which is not observed in either single mutant16, it appears that Dlx5 and Dlx6 exert redundant functions not only on lower, but also on upper jaw morphogenesis. Whether there is a specific and transiently-expressing Dlx6 cell population during upper jaw morphogenesis remains, ideally, to be formally determined using an inducible Cre-targeted Dlx6 strain. Our lineage strategy is based upon a strain with an inducible Cre recombinase inserted into the Dlx5 open reading frame. Based on previous work it is likely that the resultant reporter expression reflects the activity of jaw-specific regulatory elements known to control both Dlx5 and Dlx6 transcriptions, as observed for instance through similar transgenic analysis30. It appears, therefore, that Dlx5 and Dlx6 pattern the upper and lower jaw through very different mechanisms, which must be coordinated to generate asymmetric, articulated, muscularized jaws.
Author contributions
GL and YG conceived the study and designed the experiments. YG and NN-N carried out the research. GL and YG prepared the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
Competing interests
No competing interests were disclosed.
Grant information
This research was partially supported by the EU Consortium IDEAL (HEALTH-F2-2011-259679) to GL.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
This work was made possible thanks to the excellent technical assistance of Mss. Anastasia Fontaine, Aurélie Hagneau, Ocilia Fernandes and Gladys Alfama.
Faculty Opinions recommendedReferences
- 1.
Depew MJ, Compagnucci C:
Tweaking the hinge and caps: testing a model of the organization of jaws.
J Exp Zool B Mol Dev Evol.
2008; 310(4): 315–35. PubMed Abstract
| Publisher Full Text
- 2.
Depew MJ, Simpson CA, Morasso M, et al.:
Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development.
J Anat.
2005; 207(5): 501–61. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 3.
Compagnucci C, Debiais-Thibaud M, Coolen M, et al.:
Pattern and polarity in the development and evolution of the gnathostome jaw: both conservation and heterotopy in the branchial arches of the shark, Scyliorhinus canicula.
Dev Biol.
2013; 377(2): 428–48. PubMed Abstract
| Publisher Full Text
- 4.
Tan SS, Morriss-Kay GM:
Analysis of cranial neural crest cell migration and early fates in postimplantation rat chimaeras.
J Embryol Exp Morphol.
1986; 98: 21–58. PubMed Abstract
- 5.
Couly GF, Coltey PM, Le Douarin NM:
The triple origin of skull in higher vertebrates: a study in quail-chick chimeras.
Development.
1993; 117(2): 409–29. PubMed Abstract
- 6.
Creuzet S, Couly G, Vincent C, et al.:
Negative effect of Hox gene expression on the development of the neural crest-derived facial skeleton.
Development.
2002; 129(18): 4301–13. PubMed Abstract
- 7.
Kontges G, Lumsden A:
Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny.
Development.
1996; 122(10): 3229–42. PubMed Abstract
- 8.
Noden DM:
Vertebrate craniofacial development: novel approaches and new dilemmas.
Curr Opin Genet Dev.
1992; 2(4): 576–81. PubMed Abstract
| Publisher Full Text
- 9.
Trainor PA, Tam PP:
Cranial paraxial mesoderm and neural crest cells of the mouse embryo: co-distribution in the craniofacial mesenchyme but distinct segregation in branchial arches.
Development.
1995; 121(8): 2569–82. PubMed Abstract
- 10.
Ruhin B, Creuzet S, Vincent C, et al.:
Patterning of the hyoid cartilage depends upon signals arising from the ventral foregut endoderm.
Dev Dyn.
2003; 228(2): 239–46. PubMed Abstract
| Publisher Full Text
- 11.
Couly G, Creuzet S, Bennaceur S, et al.:
Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head.
Development.
2002; 129(4): 1061–73. PubMed Abstract
- 12.
Merlo GR, Zerega B, Paleari L, et al.:
Multiple functions of Dlx genes.
Int J Dev Biol.
2000; 44(6): 619–26. PubMed Abstract
- 13.
Beverdam A, Merlo GR, Paleari L, et al.:
Jaw transformation with gain of symmetry after Dlx5/Dlx6 inactivation: mirror of the past?
Genesis.
2002; 34(4): 221–7. PubMed Abstract
| Publisher Full Text
- 14.
Depew MJ, Lufkin T, Rubenstein JL:
Specification of jaw subdivisions by Dlx genes.
Science.
2002; 298(5592): 381–5. PubMed Abstract
| Publisher Full Text
- 15.
Acampora D, Merlo GR, Paleari L, et al.:
Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5.
Development.
1999; 126(17): 3795–809. PubMed Abstract
- 16.
Jeong J, Li X, McEvilly RJ, et al.:
Dlx genes pattern mammalian jaw primordium by regulating both lower jaw-specific and upper jaw-specific genetic programs.
Development.
2008; 135(17): 2905–16. PubMed Abstract
| Publisher Full Text
- 17.
Clouthier DE, Hosoda K, Richardson JA, et al.:
Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice.
Development.
1998; 125(5): 813–24. PubMed Abstract
- 18.
Ozeki H, Kurihara Y, Tonami K, et al.:
Endothelin-1 regulates the dorsoventral branchial arch patterning in mice.
Mech Dev.
2004; 121(4): 387–95. PubMed Abstract
| Publisher Full Text
- 19.
Ruest LB, Xiang X, Lim KC, et al.:
Endothelin-A receptor-dependent and -independent signaling pathways in establishing mandibular identity.
Development.
2004; 131(18): 4413–23. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 20.
Fukuhara S, Kurihara Y, Arima Y, et al.:
Temporal requirement of signaling cascade involving endothelin-1/endothelin receptor type A in branchial arch development.
Mech Dev.
2004; 121(10): 1223–33. PubMed Abstract
| Publisher Full Text
- 21.
Sato T, Kurihara Y, Asai R, et al.:
An endothelin-1 switch specifies maxillomandibular identity.
Proc Natl Acad Sci U S A.
2008. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 22.
Fish JL, Villmoare B, Köbernick K, et al.:
Satb2, modularity, and the evolvability of the vertebrate jaw.
Evol Dev.
2011; 13(6): 549–64. PubMed Abstract
| Publisher Full Text
- 23.
Merlo GR, Paleari L, Mantero S, et al.:
Mouse model of split hand/foot malformation type I.
Genesis.
2002; 33(2): 97–101. PubMed Abstract
| Publisher Full Text
- 24.
Taniguchi H, He M, Wu P, et al.:
A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex.
Neuron.
2011; 71(6): 995–1013. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 25.
Gitton Y, Cohen-Tannoudji M, Wassef M:
Specification of somatosensory area identity in cortical explants.
J Neurosci.
1999; 19(12): 4889–98. PubMed Abstract
- 26.
Tamarin A, Boyde A:
Facial and visceral arch development in the mouse embryo: a study by scanning electron microscopy.
J Anat.
1977; 124(Pt 3): 563–80. PubMed Abstract
| Free Full Text
- 27.
Depew MJ, Liu JK, Long JE, et al.:
Dlx5 regulates regional development of the branchial arches and sensory capsules.
Development.
1999; 126(17): 3831–46. PubMed Abstract
- 28.
Debiais-Thibaud M, Metcalfe CJ, Pollack J, et al.:
Heterogeneous conservation of Dlx paralog co-expression in jawed vertebrates.
PLoS One.
2013; 8(6): e68182. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 29.
Gitton Y, Benouaiche L, Vincent C, et al.:
Dlx5 and Dlx6 expression in the anterior neural fold is essential for patterning the dorsal nasal capsule.
Development.
2011; 138(5): 897–903. PubMed Abstract
| Publisher Full Text
- 30.
Ruest LB, Hammer RE, Yanagisawa M, et al.:
Dlx5/6-enhancer directed expression of Cre recombinase in the pharyngeal arches and brain.
Genesis.
2003; 37(4): 188–94. PubMed Abstract
| Publisher Full Text
| Free Full Text



Comments on this article Comments (0)