Keywords
Evolution, function, gene expression, non-symbiotic, structure, symbiotic, truncated
This article is included in the Oxygen-binding and sensing proteins collection.
Evolution, function, gene expression, non-symbiotic, structure, symbiotic, truncated
Major differences between the revised and the published version of this review are: (1) a figure (Figure 3) for rice non-symbiotic Hb1-5 and rice and Arabidopsis tHbs sequence alignments was included, (2) a table (Table 2) for the rate and equilibrium constants for the reaction of oxygen from rice Hb1 and 2 and selected plant and non-plant Hbs was included, (3) structural and biophysical properties for rice tHb are discussed in a separate subsection within section “Structure and biophysical properties of rice hemoglobins”, (4) in all cases we indicated that NO binds to oxyHb, (5) care was taken when properties for predicted structures of rice Hb2-5 and tHb were postulated/hypothesized by preceding postulates/hypothesis with “possible”, “probable”, “suggest”, etc., (6) a statement on the biological relevance of the folding pathways for rice Hb1-5 was included, (7) similarity values for rice Hb1-5 and tHb between O. sativa var. japonica and O. sativa var. indica were included, and (8) the number of references was reduced.
See the authors' detailed response to the review by Juliette T. J. Lecomte and Eric Johnson
See the authors' detailed response to the review by Martino Bolognesi
2,4-D, 2,4-dichlorophenoxyacetic acid; ARR1, Arabidopsis response regulator 1; BCIP, 5-bromo-4-chloro-3´-indolyphosphate; Hb, hemoglobin; Lb, leghemoglobin; MIP1, macrophage inflammatory protein 1; mya, million of years ago; NBT, nitro-blue tetrazolium; nsHb, non-symbiotic hemoglobin; nsHb-1, non-symbiotic hemoglobin type 1; nsHb-2, non-symbiotic hemoglobin type 2; nsHb-I, clade I non-symbiotic hemoglobin; nsHb-II, clade II non-symbiotic hemoglobin; RT-PCR, reverse transcriptase-polymerase chain reaction; SNP, sodium nitroprusside; tHb, truncated (2/2) hemoglobin.
Two decades ago Taylor and co-workers reported the cloning and sequencing of a hemoglobin (Hb) cDNA from barley1. This was the first report about the existence of Hbs in monocotyledonous plants. Since then Hbs have been identified in a number of monocots, including rice2, maize3 and wheat4. Rice Hbs and genes coding for these proteins are rather well characterized, thus in some aspects rice Hbs are a model to understand monocot and other land plant Hbs. However, the accumulated information on rice Hbs over the last seventeen years is scattered. This review discusses major findings from the study of rice Hbs including a historical perspective, and proposes biochemical and physiological mechanisms for rice Hbs based on information available about rice Hbs and other monocot and land plant Hbs. For general aspects and the biochemistry, physiology and evolution of plant Hbs, we recommend to the reader reviews published elsewhere5–15.
Hb is known to the reader because this protein is responsible for the red color of vertebrates´ blood16. However, Hbs are widely distributed in living organisms, ranging from bacteria to mammals17,18. The tertiary structure of Hbs consists of a specific arrangement of 6 to 8 α-helices (designated with letters A to H) known as the globin-fold. This protein folding forms a hydrophobic pocket where a heme prosthetic group is located16,19. Two structural types of the globin-fold have been identified in Hbs: the 2/2- and 3/3-folding. In the 2/2-Hbs, helices B and E overlap to helices G and H and in the 3/3-Hbs helices A, E and F overlap to helices B, G and H. Likewise, three evolutionary families have been identified in Hbs: the M, S and T Hb families. The M Hbs, which exist in bacteria and eukaryotes, include flavoHbs and single domain globins, the S Hbs, which exist in bacteria and some fungi, include globin-coupled sensors, protoglobins and single domain globin sensors, and the T Hbs, which exist in bacteria, unicellular eukaryotes and plants, include truncated Hbs (tHbs). Canonical T Hbs from bacteria and unicellular eukaryotes are ~100 to 120 amino acids in length, however plant T Hbs are longer than canonical T Hbs because of the existence of extra amino acids at the N- and C-terminal. The M and S Hbs fold into the 3/3-folding whereas the T Hbs fold into the 2/2-folding18,20–25.
A variety of ligands bind to the heme iron of Hbs, including O2 and NO. Reversible binding of O2 is closely associated to the major function of Hbs in organisms, which is the transport of O216. Binding of NO by oxygenated Hbs is essential to NO-detoxification via a NO-dioxygenase activity26. Several additional functions have been reported for Hbs, including dehaloperoxidase activity and reaction with free radicals, binding and transport of sulfide and lipids, and O2-sensing27–32. This indicates that in vivo Hbs might be multifunctional proteins.
Land plant Hbs were first identified by Kubo in soybean root nodules33. Few years after Kubo´s discovery these proteins were named as leghemoglobins (Lbs) by Virtanen and Lane34 because they were only found in the symbiotic (N2-fixing-) nodules of the leguminous plants. Lbs are the most abundant soluble proteins in nodules (e.g. in soybean nodules their concentration is as high as 3 mM)14,35. The x-ray analysis of lupin Lb revealed that the tertiary structure of Lbs was remarkably similar to that of the sperm whale myoglobin36. This evidence demonstrated that Lbs are plant Hbs and indicated that plant and animal Hbs evolved from a common ancestor more than 600 mya6. Subsequent work led to the identification of Lb-like (or symbiotic) Hbs in nodules of actinorhizal plants37–41, purification of an Hb from the root nodules of the dicotyledonous non-legume Parasponia andersonii42, cloning and sequencing of an hb gene from the non-nodulating dicot Trema tomentosa43,44 and detection of Hbs in non-symbiotic organs from several land plants, including primitive bryophytes and evolved angiosperms9,15,45–47. Until now three types of Hbs have been identified in land plants: the symbiotic Hbs, which include Lbs, that are specifically located within nodules of the N2-fixing land plants, and the non-symbiotic (nsHbs) and truncated (tHbs) Hbs, that are located within non-symbiotic and symbiotic organs of primitive and evolved land plants9,15. Based on sequence similarity the nsHbs are further classified into type 1 and type 2 nsHbs (nsHbs-1 and nsHbs-2, respectively)9,48,49.
Monocots are a large family of flowering plants50 that includes cereals. Cereals, such as rice, maize and wheat, are the main source of food for humans. Because of this, during that last decade the genomes of a number of cereals have been sequenced. This allowed the identification of novel cereal Hbs. The search of hb genes in databases by G. Rodríguez-Alonso and R. Arredondo-Peter51,52 revealed that nsHb and tHb sequences exist in the Brachypodium distachyon, Hordeum vulgare (barley), Oryza glaberrima (rice), O. rufipogon (rice), O. sativa (rice) var. indica, O. sativa (rice) var. japonica, Panicum virgatum (switchgrass), Setaria italica (foxtail millet), Sorghum bicolor (sorghum), Triticum aestivum (wheat) and Zea mays ssp. mays (maize) genomes. The highest number of nsHbs (5) exists in O. sativa var. indica and O. sativa var. japonica, whereas one to three nsHbs exist in barley, Brachypodium, foxtail millet, maize, O. glaberrima, O. rufipogon, sorghum, switchgrass and wheat. Also, with the exception of wheat, which contains two copies of the thb gene, a single copy of thb was identified in the genome of Brachypodium, barley, O. sativa var. indica, O. sativa var. japonica, switchgrass, foxtail millet, sorghum and maize. Little is known about Hbs from non-cultivated monocots. The only Hb reported from a non-cultivated monocot is that of teosinte (Z. mays ssp. parviglumis)3, which is postulated as the ancestor of maize53,54. Analysis by Southern blot using the teosinte hb gene as probe showed that apparently a single copy of hb exists in teosinte (J. Sáenz-Rivera and R. Arredondo-Peter, unpublished results). Sequence comparison revealed that maize and teosinte Hb polypeptides are identical3.
Monocots were a target for searching Hbs after these proteins were detected in non-symbiotic organs of dicotyledonous plants (see subsection above). At that time, monocot genomes had not been sequenced. Searching approaches consisted in detecting Hb polypeptides and hb genes by spectroscopy and molecular biology methods, respectively. Attempts to detect absorption maxima in the Soret (~410 nm) and Q (~500 to 550 nm) regions, which are characteristic of ferric (Fe3+), ferrous (Fe2+) and liganded Hbs55,56, were unsuccessful (R. V. Klucas and C. A. Appleby, unpublished results) mostly due to the very low Hb concentration (~50 to 100 nM) in plant non-symbiotic organs5,57. At the molecular level a consensus probe designed from legume and non-legume (T. tomentosa, P. andersonii and Casuarina glauca) Hb sequences58 hybridized with hb-like sequences from rice and other monocot total DNAs (Figure 1). This observation suggested that hb sequences exist in monocots, however hybridizing fragments were not subsequently cloned and sequenced in order to verify if they actually corresponded to hb genes.

Approximately 20 μg of undigested total DNA was used as template and a consensus oligonucleotide for legume and non-legume plant Hbs58 was used as probe. Sequence of the consensus probe was 5´-GTA GCC TAT GAT GAA TTG GCA GCT GCA ATT AAG-3´. The probe was labeled by nick translation with Biotin-dATP using a Bionick labeling system (Gibco BRL). The membrane was prehybridized with SSC 2× for 4h at 42°C, hybridized overnight at the same temperature, washed at high stringency (SSC 2×/SDS 0.1% for 3 min at room temperature, SSC 0.2×/SDS 0.1% for 15 min at room temperature and SSC 0.16×/SDS 0.1% for 15 min at 65°C) and incubated with the streptavidin-alkaline phosphatase conjugate and the BCIP/NBT mix to develop color. Animal (salmon sperm and calf thymus) and legume DNAs were included as negative and positive controls, respectively.
Rice Expressed Sequence Tags (ESTs) were first deposited in databases early in the 1990´s. The first rice Hb (Hb1 and Hb2) sequences were detected from ESTs deposited in the DNA Data Bank of Japan (DDBJ) database59. Rice Hb1 and Hb2 corresponded to clones C741 and C2576 with DDBJ accession number D15507 and D38931, respectively. Rice hb1 and hb2 genes were subsequently amplified by PCR, cloned and sequenced. Sequence analysis revealed that rice hb1 codes for non-symbiotic Hb1 and that rice hb2 codes for non-symbiotic Hb22. Afterwards, sequencing of the rice (O. sativa L. ssp. indica) genome more than a decade ago60 allowed the identification of a family of rice nshb genes and a single copy of the rice thb gene (see subsection below).
The O. sativa var. indica and O. sativa var. japonica genomes are fully sequenced, and the O. glaberrima and O. rufipogon genomes are partially sequenced. Rice genome sequences are mainly available from the GenBank (www.ncbi.nlm.nih.gov) and Phytozome (http://www.phytozome.org/) databases. Search of Hb sequences in the above databases showed that a family of the nshb genes, consisting of hb1, hb2, hb3, hb4 and hb5, and a single copy of the thb gene exist in the O. sativa var. indica and O. sativa var. japonica genomes. A single copy of the nshb gene was detected in the O. glaberrima and O. rufipogon genomes, however thb genes have not yet been detected in these plants52. Given that the sequencing of the O. glaberrima and O. rufipogon genomes is in progress the identification of hb genes in these genomes is incomplete. Thus, the following discussion will focus on the O. sativa var. indica and O. sativa var. japonica hbs. However, we must clarify to the reader that the sequence of Hb1, Hb2, Hb3, Hb4 and tHb and Hb5 polypeptides are 100% and 97% identical between O. sativa var. indica and O. sativa var. japonica, respectively. Therefore, the subsequent discussion on the O. sativa Hbs will indistinctively correspond to either O. sativa var. indica or O. sativa var. japonica.
The structure of known rice hb genes corresponds to four exons and three introns, with introns located at similar position as all of the known plant hb genes61. Canonical TATA boxes and a variety of potential promoters exist upstream of the rice hb genes which suggests that rice hbs are functional and that the regulation of the hb genes in this plant is complex62–64. Figure 2 shows the localization of hbs in the O. sativa chromosomes and mapping of hbs in the O. sativa genome. Rice hb1, hb3 and hb4 cluster forming the hb1-hb4 cluster63 which is localized in chromosome 3. Rice hb2 is also localized in chromosome 3 but 467 kb upstream of the hb1-hb4 cluster. In contrast, rice hb5 and thb genes are localized in chromosomes 5 and 6, respectively (Figure 2A). Rice hbs are flanked by a variety of genes with known and unidentified functions (Figure 2B). However, with the exception of genes coding for a ternary complex factor macrophage inflammatory protein MIP1 and an ubiquitin fusion protein which are located 239 and 411 nucleotides up- and downstream of the hb1-hb4 cluster, respectively, distance of flanking genes to hbs is >1 kb. This suggests that co-expression of hb and flanking genes is unlikely.

The hb genes were localized in the rice chromosomes by BlastN analysis using the rice (O. sativa) genome resource from the GenBank database as template and the sequence for the rice hb1, hb3 and hb4 (GenBank accession number AF335504), hb2 and hb5 (GenBank accession numbers AF335503 and EF061459, respectively) and thb (GenBank accession number NM_001064507) genes as probes. The hb (black boxes) and flanking (gray boxes) genes were mapped into 50 kb fragments of the O. sativa genome by BlastN2.2.26+ analysis using the Phytozome V9.1 server (www.phytozome.org) and the above hb sequences as probes. Arrows indicate the transcription orientation. Information for each gene corresponds to predicted protein (following the Phytozome nomenclature), locus name in the O. sativa genome and position at the O. sativa chromosome. Gene sizes and distance between genes are not shown at scale. Pltd, chloroplast chromosome; MT, mitochondrial chromosome.
The expression of hb genes and localization of Hb polypeptides have been analyzed in rice growing under normal and stressed conditions. Under normal conditions the expression level of rice nshbs was low2,62. However, analysis by RT-PCR revealed that hb1, hb2 and hb5 genes were expressed in embryonic and vegetative organs obtained from rice plants grown under a normal environment2,62,65. Specifically, transcripts for rice Hb1 were detected in embryos, seminal roots, leaves and roots, transcripts for rice Hb2 were detected in embryos, coleoptiles, seminal roots and leaves, and transcripts for rice Hb5 were detected in embryos, coleoptiles, seminal roots, leaves and roots. Likewise, evaluation of the β-glucuronidase (GUS) activity from a construct containing the rice nshb2 gene promoter that is responsive to the cytokinin-regulated ARR1 trans-acting factor showed that this promoter is activated in roots, the vasculature of young leaves, flowers and the pedicel/stem junction of transgenic Arabidopsis64. In addition, a variety of potential promoters was identified upstream of the rice nshb genes, such as those involved in the ethylene synthesis, photoregulation, heat shock response and plant defense signaling57,62–64. However the activities of these promoters have not been determined.
Transcriptomic analyses revealed that nsHb and tHb transcripts coexist in rice embryonic and vegetative organs (Table 1). This evidence suggests that nsHb (i.e. Hb1, Hb2, Hb3, Hb4 and Hb5) and tHb polypeptides coexist and probably function in rice organs. Immunoanalysis by Western blot and confocal microscopy using a polyclonal anti-rice Hb1 antibody revealed that Hb polypeptides exist in rice seeds and in rice leaves and roots from 2 to 14 weeks after seed germination. These analyses also revealed that Hb polypeptides exist in the cytoplasm of differentiating cells of the root cap, schlerenchyma, aleurone, and in the vasculature, principally in the differentiating xylem14,57,66. However, the anti-rice Hb1 antibodies cross-react with different rice Hbs (G. Sarath and E. J. H. Ross, unpublished results) and thus it is not known which Hb polypeptides were detected in the above analyses by the anti-rice Hb1 antibodies.
Rice Hb transcripts were detected in plant organs using The Rice Genome Annotation Project database (http://rice.plantbiology.msu.edu/) and hemoglobin as keyword (S. Castro-Bustos and R. Arredondo-Peter, unpublished).
It is well documented that land plant hb genes are either up- or down-regulated by stress conditions1,45,66–69. Table 1 shows that Hb transcripts coexist in rice growing under cold, drought and salt stress conditions. Also, Ohwaki and co-workers70 reported that nshb1 and nshb2 are induced by nitrate, nitrite and NO in cultured rice cells. These observations indicate that rice hb genes response to a variety of stress conditions. However, the detection of Hb polypeptides by Western blot using the anti-rice Hb1 antibodies showed that level of Hbs increased in rice etiolated leaves and flooded roots, but not in rice plants subjected to oxidative (H2O2), nitrosative (SNP) and hormonal (2,4-D) stresses. These observations suggest that rice Hbs do not appear to be part of a generalized stress response, but may be functional in plant organs subjected to specific stress conditions66.
Rice hb genes are functional and code for Hb polypeptides with a predicted molecular mass of ~16 to 19 kDa. Also, sequences among rice nsHb polypeptides are highly similar: Hb1 and Hb2 are 93% similar to each other, Hb3 and Hb4 are 87.1% similar to each other, and 85.5% and 84.7%, and 79.2% and 82.2% similar to Hb1 and Hb2, respectively, and rice Hb1 and Hb5 are 67% similar to each other2,62,63 (Figure 3A).
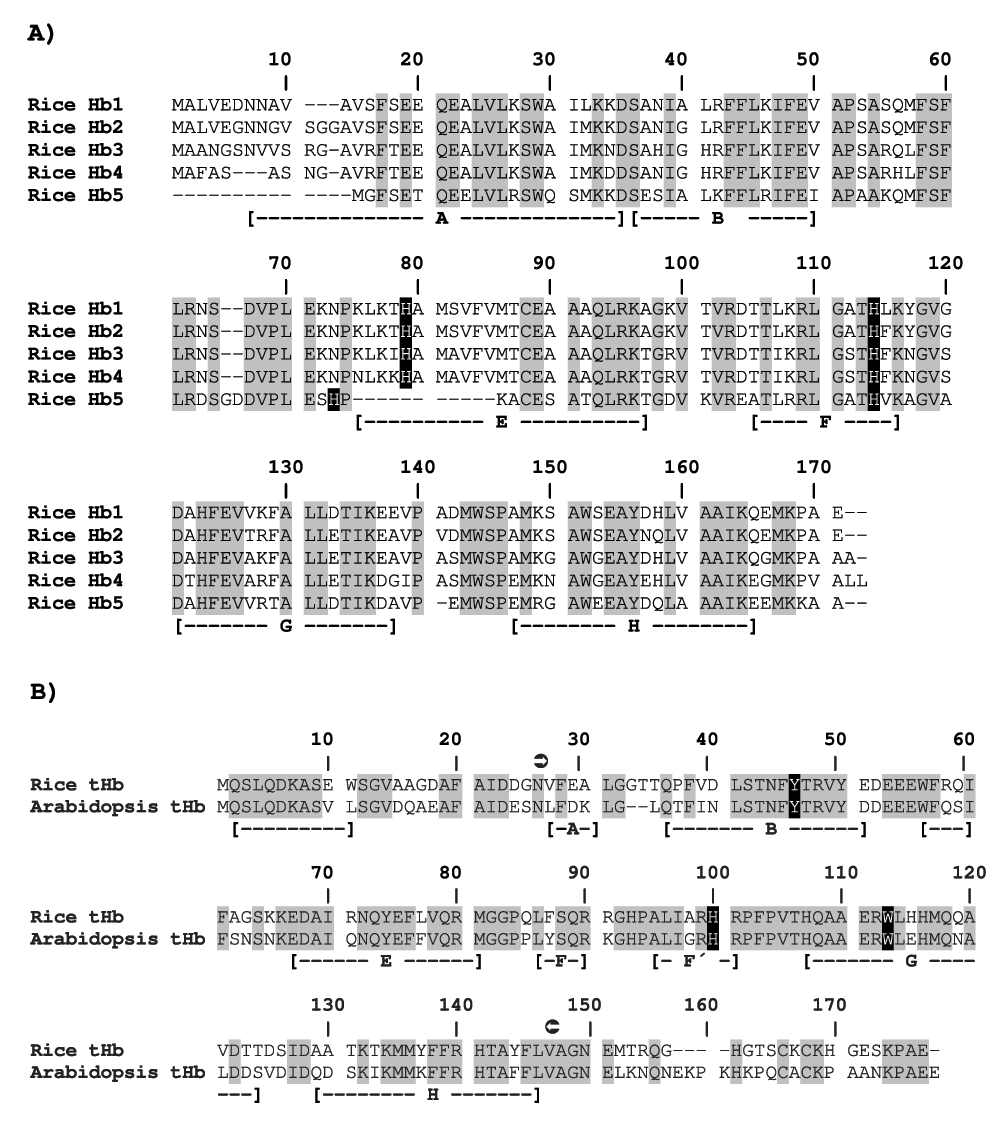
(A) Sequence alignment of rice nsHbs. Note the 11 amino acids deletion in rice Hb5 at position 75–85. Modified from Garrocho-Villegas et al.62. (B) Sequence alignment of rice and Arabidopsis (GenBank accession number AAK55409) tHbs. Distal and proximal His in rice nsHbs (H79 and H114, respectively) and proximal His (H100) and proposed distal Tyr/Tre (Y46/W113) in Arabidopsis86 and rice tHbs and conserved amino acids are shown with black and gray background, respectively. Helices are indicated with letters A to H based on the crystal structure of rice Hb171 in rice nsHbs and on the crystal structure of Arabidopsis tHb86 in rice tHb. Left- and right-oriented arrows within a black circle in the rice and Arabidopsis tHbs sequence alignment delimit the globin domain.
Rice Hb1 was the first monocot nsHb whose crystal structure was elucidated71. This protein crystalizes as a dimer when its concentration is ≥1 mM72. After the elucidation of the rice Hb1 structure the tertiary structure of rice Hb273, Hb3, Hb4 and Hb562 (CASPUR PMDB ID PM0075009, PM0075873, PM0076005 and PM0075011, respectively) was predicted using computational methods and rice Hb1 (PDB ID 1D8U) as the structural homolog. The crystal structure of rice Hb1 and that of predicted rice Hb2, Hb3 and Hb4 is highly similar. The tertiary structure of these proteins consists of six helices that fold into the 3/3-folding (see subsection on Generalities on hemoglobins). However, the structure of rice Hb1 to 4 is characterized by the existence of a short pre-helix A located at the N-terminal and an extended and poorly ordered CD-loop. The heme pocket in these proteins differs from that in “traditional” Hbs because the proximal and distal His side chains coordinate the heme iron forming a hemichrome (Figure 4), resulting in that heme iron from rice Hb1 to 4 is hexacoordinate. Also, the amino acid residues (V50, S53, E125, V126, F129 and A130 from Figure 3A) located at the monomer-monomer interface of dimeric rice Hb171 are highly conserved in rice Hb2 to 463. This suggests that rice Hb1 to 4 can potentially form homo- or hetero-dimers if the hb1 to 4 genes coexpress in rice organs. The tertiary structure of rice Hb5 also consists of six helices that fold into the 3/3-folding. However, rice Hb5 differs from rice Hb1 to 4 in missing 11 amino acids in helix E (Figure 3A) which results in that the length of the CD-loop and helix E in the predicted Hb5 structure are unusually long and short, respectively. An apparent consequence from this characteristic is that distal His is located far away (13.92 Å, compared to 2.11 Å in rice Hb1) from the heme iron within the predicted Hb5 structure, resulting in that heme iron from rice Hb5 could be pentacoordinate62. The amino acid residues located at the monomer-monomer interface of dimeric rice Hb171 are poorly conserved in rice Hb562 (Figure 3A) which suggests that rice Hb5 exists in vivo as a monomer.

The tertiary structure of rice tHb was modeled using the automated mode of the I-Tasser server (http://zhanglab.ccmb.med.umich.edu/I-TASSER/)131–133 and the crystal structure of the Thermobifida fusca tHb (PDB ID 2BMM) as the structural homologue. Model for the rice tHb is deposited in the Caspur Protein Model Database (http://bioinformatics.cineca.it/PMDB/main.php) under the ID number PM0079484. Helices are indicated with letters A to H. Note the overlapping of helices A, E and F to helices B, G and H in (3/3-folding) rice Hb1, and overlapping of helices B and E to helices G and H in (2/2-folding) rice tHb. Heme prosthetic group is shown in dark green color and proximal and distal His are shown in light brown color.
The folding pathway and kinetics of rice nsHbs were predicted using the Average Distance Map (ADM) method74–76. This analysis indicated that rice Hb1 and Hb2 could fold in the C → N direction at a moderate rate, that rice Hb3 could fold in the N → C direction at a fast rate, and that rice Hb4 and Hb5 could fold in the N → C direction at a moderate rate. Thus, it appears that the predicted folding pathway and kinetics among rice nsHbs are diverse. Also, the ADM analysis showed that pre-helix A and CD-loop apparently do not play a role during the folding of rice nsHbs77. The physiological relevance of the folding pathways for rice nsHbs, including the polypeptide association with the heme, is still not known.
Visible spectroscopy (see subsection Early search and identification of rice hemoglobins) is a tool to analyze the redox state of and ligand-binding to the heme iron of Hbs56,78,79. Rice Hb1 is the only rice nsHb that has been spectroscopically characterized2. This protein exhibits spectral characteristics that are similar to other Hbs. However, rice Hb1 exhibits distinctive absorption maxima in the deoxyferrous form: the unligated ferrous state exhibits maxima at 526 and 556 nm2 which are characteristic of hexacoordinate heme iron80. This is in contrast to pentacoordinate Hbs which display a broad peak centered at 556 nm in their deoxyferrous form7,81,82. The distal ligand that coordinates the heme iron in rice Hb1 was identified as His74 by site directed mutagenesis. Absorbance spectra of the ferric and deoxyferrous forms of an H74L mutant of rice Hb1 showed no evidence of His coordination. Also, the addition of exogenous imidazole to ferric and deoxyferrous H74L mutant resulted in a spectrum identical to that of the wild-type rice Hb12. This evidence indicated that in rice Hb1 the distal ligand to heme iron is His74. A similar case can be predicted for rice Hb2 to 4. In contrast, distal His appears to be located far away from the heme iron in the predicted structure of deoxyferrous rice Hb5, resulting in that heme iron in rice Hb5 could be pentacoordinate62.
Analysis of ligand-association and -dissociation rate constants of penta- and hexacoordinate Hbs using stopped-flow methods indicated that these proteins exhibit low to moderate and high affinity for O2, respectively. Rice Hb1 is hexacoordinate and apparently rice Hb2 to 4 are hexacoordinate and rice Hb5 is pentacoordinate. The O2-association rate constants for rice Hb1 and 2, and possibly for rice Hb3 and 4, are rather similar to those of other O2-transport and -storage proteins, such as the sperm whale myoglobin and soybean Lba (Table 2). However, in rice Hb12 and 214, and possibly in rice Hb3 and 4, the bound O2 is stabilized by distal His after binding to the heme iron, which results in very low O2-dissociation rate constants. The O2-association and -dissociation rate constants of hexacoordinate rice Hb1 and 2, and possibly of rice Hb3 and 4, result in that the affinity of these proteins for O2 is very high (Table 2). In the absence of biochemical data it becomes difficult to evaluate the O2-binding characteristics of rice Hb5, however, its predicted pentacoordinate structure would suggest a low to moderate affinity for O2.
Constants for arbitrarily selected plant and non-plant Hbs are included for comparison.
The bis-histidyl hexacoordinated form of rice Hb1 displays a hydrophobic distal cavity which appears to be connected with the external solvent through the position of Phe44 (also known as FB10 because it occupies the tenth position in helix B). It was suggested that this amino acid regulates the migration of small ligands in rice Hb1, for example in ligand binding to the heme iron, ligand migration through internal docking sites and ligand release into the external solvent83,84. Kinetic analysis after laser flash photolysis of rice Hb1 encapsulated in silica gel combined with computational analysis revealed the existence of two channels in the rice Hb1 CO-bound species. The first channel is located in the distal region of the heme pocket and is connected with a secondary channel that is directly connected with the external solvent. Apparently, the position of FB10 in hexacoordinated rice Hb1 leaves the distal heme pocket accessible to the external solvent, however after the ligand entrance the phenyl ring rotates closing the cavity and thus hindering the exit of the bound ligand85. Thus, together with distal His (see subsection Spectroscopic characteristics of rice non-symbiotic hemoglobins) and aromatic amino acids that are located in the distal region of the heme pocket, FB10 appears to regulate hexacoordination and functioning of rice Hb1.
Rice (O. sativa) tHb (GenBank accession number NP_001057972) is 172 amino acids in length, which corresponds to a globin domain (position 26 to 147) flanked by N- and C-terminal extensions (Figure 3B). No monocot tHb has been analyzed by x-ray crystallography, however the tertiary structure of a rice tHb was predicted using computational methods (Figure 4). The predicted structure of rice tHb is highly similar to the crystal structure of an Arabidopsis thaliana tHb86. The globin domain from rice and A. thaliana tHbs folds into the 2/2-folding (see subsection on Generalities on hemoglobins). Similarly to the A. thaliana tHb structure, flanking regions to the globin domain of predicted rice tHb correspond to an N-terminal helical extension and a C-terminal unfolded extension (Figure 4). The high similarity between the crystal structure of A. thaliana tHb and the predicted structure of rice tHb suggests that the biochemical properties and function of dicot and monocot tHbs are similar.
Rice tHb has not been subjected to spectral analysis, however the predicted structure of this protein (Figure 4) is highly similar to the crystal structure of an A. thaliana tHb86 (see above). The absorption spectra of an A. thaliana tHb showed that heme iron from this protein is pentacoordinate69,86. Thus, it is likely that heme iron in rice tHb is pentacoordinate and that the rate and equilibrium constants for the reaction of O2 of rice tHb are similar to those of the Arabidopsis tHb (Table 2), i.e. the O2-association and -dissociation rate constants are low to moderate.
While data on the localization, kinetics, regulation and structure of rice Hbs have accumulated, little work has been performed to fully understand the function of these proteins in rice organs. However, previous work from other plant and non-plant Hbs provides data that enable us to propose potential functions for rice Hbs. Rice Hbs could potentially function within cells through O2-transport and -signaling, binding to small molecules (most notably NO) and other as yet undetermined mechanisms. Here we evaluate the evidence for and against these modes of action.
Oxygen transport is a major function of many Hbs. This process requires that the kinetics of O2-binding do not limit the O2-diffusion process87–90. Based on the concentration of Hb polypeptides in rice organs (~50 to 100 nM)57, the O2-association rate constant of rice Hb1 and 2 (Table 2) and possibly that of rice Hb3 to 5 and tHb (see subsections Rate and equilibrium constants for the reaction of oxygen from rice non-symbiotic hemoglobins and Rice truncated hemoglobin, predicted structure and properties), and the free O2 concentration in aerated rice roots (<1.4 μM)91, it is likely that Hbs would be substantially oxygenated in rice organs. However, the O2-dissociation rate constants of rice Hb1 and 2 (Table 2), and possibly that of rice Hb3 and 4, are extremely low. These data do not support the O2-transport function for rice Hb1 to 4 because these proteins would not release O2 after oxygenation.
It was reported that hexacoordinate Hbs interact with either organic molecules or protein partners27,92 and thus a possibility is that such interactions could impact the kinetic constants, particularly the O2-dissociation rate constants, of hexacoordinate nsHbs93. There have been no direct biochemical evaluations of this hypothesis in rice or in other plants, precluding definitive answers. However, their unique structural features could result in as yet undiscovered interactions.
Rice Hbs may function in O2-signaling if they easily bind and release O2. Appleby and co-workers5 proposed that under normal conditions Hbs would be oxygenated and under O2-limiting conditions the concentration of deoxyHb would increase triggering an anaerobic response. It was reported that levels of Hbs increase in rice roots from flooded plants indicating that the synthesis of rice Hbs increases under O2-limiting conditions66. Rice is a flooding resistant crop, thus under flooding (i.e. hypoxia) conditions rice Hbs could sense low O2-concentrations and trigger an anaerobic metabolism for rice growth. To act as a signaling molecule, rice Hbs will need to bind directly to the DNA, to additional proteins, such as transcription factors, or catalyze some unique reactions that can influence key downstream events. To date there are no reports of immunoprecipitation experiments specially targeting rice Hbs coupled to further proteomic analysis. It is thus uncertain if rice Hbs bind to other partners. There is also no structural evidence that indicates that rice Hbs can bind directly to DNA. In planta, they appear to be soluble and essentially contained within the cytoplasm57. There are reports of nuclear-localized Hbs94, but no direct evidence for a function arising from translocation of Hbs from the cytoplasm to the nucleus currently exist.
The NO dioxygenase activity exhibited by oxygenated Hbs is well documented95–97. NO is a hormone-like radical that modulates several aspects of the plant physiology, including plant immunity, seed germination, de-etiolation, apoptosis, stomata guard cells opening/closure and the rhizobia-legume symbiosis98–100. Scavenging of NO is considered a function of plant Hbs10,101–104. During this process, oxygenated plant Hbs react with NO producing nitrate and ferric Hb. Ferric plant Hbs are subsequently reduced to ferrous Hb by enzymatic105,106 and non-enzymatic107–111 mechanisms. This process regenerates (oxy) ferrous Hb which is able to bind NO in a cyclic pathway referred to as the Hb/NO cycle104,112. The operation of this cycle appears to be involved in maintaining an active metabolism in the plant cells10. Rice Hb1 exhibits NO dioxygenase activity (kobs, NOD = 90 s-1)113 thus a possible function of Hbs into the rice physiology is modulating levels of NO by scavenging NO. However, the inability of rice Hb1 to substitute the NO scavenger activity in a flavoHb knockout Escherichia coli113 and the observation that levels of Hbs did not change in rice seeds germinated under nitrosative stress66 suggest that the NO dioxygenase activity of rice Hbs is limited in vivo.
A consequence of the operation of the Hb/NO cycle could be the maintenance of cell respiration and energy status. Based on the studies on over- and under-expressing barley nsHb in maize cells, it was proposed that under hypoxic conditions barley nsHb is involved in the ATP metabolism, particularly in maintaining the energy status under O2-limiting conditions114. Immunolocalization data showed that rice Hbs are localized in differentiating cells (see subsection on Gene expression and localization of hemoglobins in rice organs)57. The metabolism of these cells is redirected in response to differentiation signals, such as a change in the cell redox state. Rice Hbs could be involved in redox signaling if the redox state of the heme is functional71. Thus, under these conditions rice Hbs may function by sensing or maintaining redox environments that promote specific cell metabolisms14.
It was proposed that one of the functions of plant Hbs could be related to the peroxidase activity8,93. This is of interest because peroxidase activity modulates the levels of reactive oxygen species and a variety of cellular processes115–121. In plants, evaluation of the peroxidase activities of Arabidopsis Hbs (AtGLB1, AtGLB2 and AtGLB3) revealed that these proteins oxidize Amplex Red, DHR123 and guaiacol substrates122 and overexpression of AtGLB1 increased tolerance of Arabidopsis to H2O2 stress123. These observations suggested that Arabidopsis Hbs function as antoxidants. However, levels of Hb polypeptides did not change in rice seeds germinated under H2O2 stress66. Also, the analysis of the peroxidase activity of rice Hb1 compared to that from horseradish peroxidase (HRP) showed that the catalytic efficiency of rice Hb1 for the oxidation of guaiacol using H2O2 as electron donor is several orders of magnitude lower than that of HRP (kcat/Km = 15.8 and 44,833 mM-1min-1, respectively). Additionally, it was observed that recombinant rice Hb1 poorly protects E. coli from H2O2 stress124. This evidence indicates that it is unlikely that rice Hbs function in vivo as peroxidases.
Based on gene expression (Table 1), protein localization and structural and kinetic properties of rice Hbs and data from the analysis of other plant and non-plant Hbs it is likely that Hbs play a variety of roles in rice plants growing under normal and stressed conditions. These functions may include O2-transport, O2-sensing, NO-scavenging and redox-signaling. Future work on rice Hbs should focus on testing the above potential functions as well as newly proposed functions that emerge from novel observations.
Hbs are widely distributed in land plants, ranging from primitive bryophytes to evolved angiosperms9. The outline of plant Hb evolution subsequent to land colonization was clarified15. Briefly, a phylogenetic analysis showed that plant and animal hb genes diverged 900–1400 mya, that land plant nshb and thb genes vertically evolved through different lineages from algal ancestors, that nsHbs-1 and nsHbs-2 are monophyletic and evolved via a gene duplication event prior to the divergence of monocots and dicots at ca. 140 mya, and that symbiotic hbs originated from nshb genes at ca. 94 mya. Likewise, the structural analysis of primitive nsHbs and Lbs revealed that changes during the evolution of nsHbs to Lbs were a hexacoordinate to pentacoordinate transition at the heme prosthetic group, a length decrease at the CD-loop and N- and C-terminal regions, and a compaction of the protein into a globular structure47,125.
In contrast, the evolution of rice Hbs is partially understood owing to the limited availability of Hb sequences from a wide variety of wild and cultivated rice. However, the outline of monocot Hb evolution is rather well understood. Thus, in this section we will discuss the evolution of rice Hbs within the context of major events that occurred during the evolution of monocot Hbs. A major event during the evolution of land plant nsHbs was the duplication of an ancestral nshb into nshb-1 and nshb-2 prior to the monocot-dicot divergence15,126. Sequence analysis revealed that nshb-1 and nshb-2 genes exist in dicots and that apparently only nshb-1 genes exist in monocots9,80,127. Earlier Garrocho-Villegas and co-workers62 reported the existence of a nsHb (Hb5) divergent from rice (Hb1 to 4) nsHbs-1 and suggested that nsHbs divergent from nsHbs-1 evolved within monocots. Subsequent phylogenetic analysis of monocot nsHb sequences revealed that apparently only nshb-1 evolved within monocots, that nshb-1 duplicated early in the evolution of monocots originating clade I and clade II nshbs (nshbs-I and nshbs-II, respectively), that nsHbs-I correspond to dicot nsHbs-1, and that nsHbs-II diversified into regular nsHbs-II, post-helix H-containing nsHbs-II and 11 amino acids deletion-containing nsHbs-II51. This analysis also showed that O. sativa var. indica and O. sativa var. japonica Hb1 to 4 and Hb5 cluster within clade I and clade II, respectively, and that O. glaberrima and O. rufipogon (whose all nshb copies remain unidentified because their genome sequencing is in progress) nsHbs cluster within clade I. Thus, apparently clade I and clade II lineages remain conserved during the evolution of rice nsHbs51.
Evaluation of the rate of divergence of selected land plant Hbs revealed that evolutionary rates slowed down previous to the origin of magnoliophyta and that the rate of divergence was slower in rice Hb1 than in rice tHb128. This observation suggested that rice Hb1 (and conceivably other rice nsHbs) evolved under the effect of the stabilizing selection. However, the estimation of the variability of the O. sativa var. indica, O. sativa var. japonica, O. glaberrima and O. rufipogon nshb and thb genes revealed that in these plants variability is higher in nshbs than in thbs and that these genes evolved under the effect of neutral selection52. Currently the effect of rates of divergence and gene variability on the Hbs function during the rice evolution is not known.
In the preceding sections of this review we summarized major findings from the study of rice Hbs. This review also reveals some major lacunae in our ability to completely understand rice Hbs, more specifically the lack of information about the precise functions of Hbs in rice organs. The proposed functions for rice Hbs are mostly based on the analysis of other plant and non-plant Hbs. Thus, future work should evaluate the Hb activities (e.g. the NO-binding and -detoxifying activities) in either rice organs or rice cell cultures under a variety of growing conditions. Elucidating the functions of rice Hbs also requires the identification of organic molecules and protein partners that interact with rice Hbs. Other lacunae are the absence of biochemical, biophysical and cellular data on the properties of rice Hb2 to 5 and tHb. Generating recombinant rice Hb2 to 5 and tHb should provide Hb polypeptides for a variety of analyses that reveal the biochemical and biophysical properties of these proteins.
With the exception of rice hb2, a lacuna is the absence of experimental information about the cis-elements and trans-acting factors that regulate the expression of rice hbs. This information may help to integrate the hb gene expression into the rice metabolisms, including those that are modulated by plant hormones.
A final lacuna is the incomplete understanding of the evolution of rice Hbs. Sequencing of the O. glaberrima and O. rufipogon genomes will be completed soon and most likely a number of rice genomes (including that of O. barthii, which is postulated as the ancestor of O. glaberrima129,130) will be sequenced within the near future. This will provide new Hb sequences for phylogenetic analysis and the understanding of the evolution of rice Hbs, including the identification of ancestral rice Hbs and the evaluation of the effect of rice domestication and breeding during the evolution of rice Hbs.
RAP conceived this review and prepared the first draft of the manuscript. RAP, JFM and GS were involved in the revision of the draft manuscript and prepared the revised version from the Reviewers´ evaluation.
Authors wish to express their gratitude to the Referees (Drs. Juliette T. J. Lecomte, Eric Johnson and Martino Bolognesi) of this review for helpful and constructive comments and suggestions.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 11 Dec 14 |
read | |
|
Version 1 27 Oct 14 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)