Keywords
FDA approval, repurposed drugs, antivirals
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Ebola Virus collection.
FDA approval, repurposed drugs, antivirals
Edits to the manuscript focus on a simple approach to using the known actives to propose additional FDA approved compounds to test. Supplemental figures have been included which show similarity searches for the molecules in Fig 1 active against the Ebola virus. A reference to our recent commentary which suggests involving the physician's perspective is included as this addresses the reviewers comment about criteria for decisions in a selection process.
See the authors' detailed response to the review by Raymond Lin
See the authors' detailed response to the review by James Popp
As the Ebola outbreak continues and the costs spiral1 we should perhaps be considering what alternative treatments are close to hand in Africa to complement the public health measures that have been used to date2. Two independent studies funded by the US Defense Threat Reduction Agency in 2013 identified FDA approved drugs worthy of further evaluation. This work now seems prescient although it appears to have not been followed through to any public conclusion.
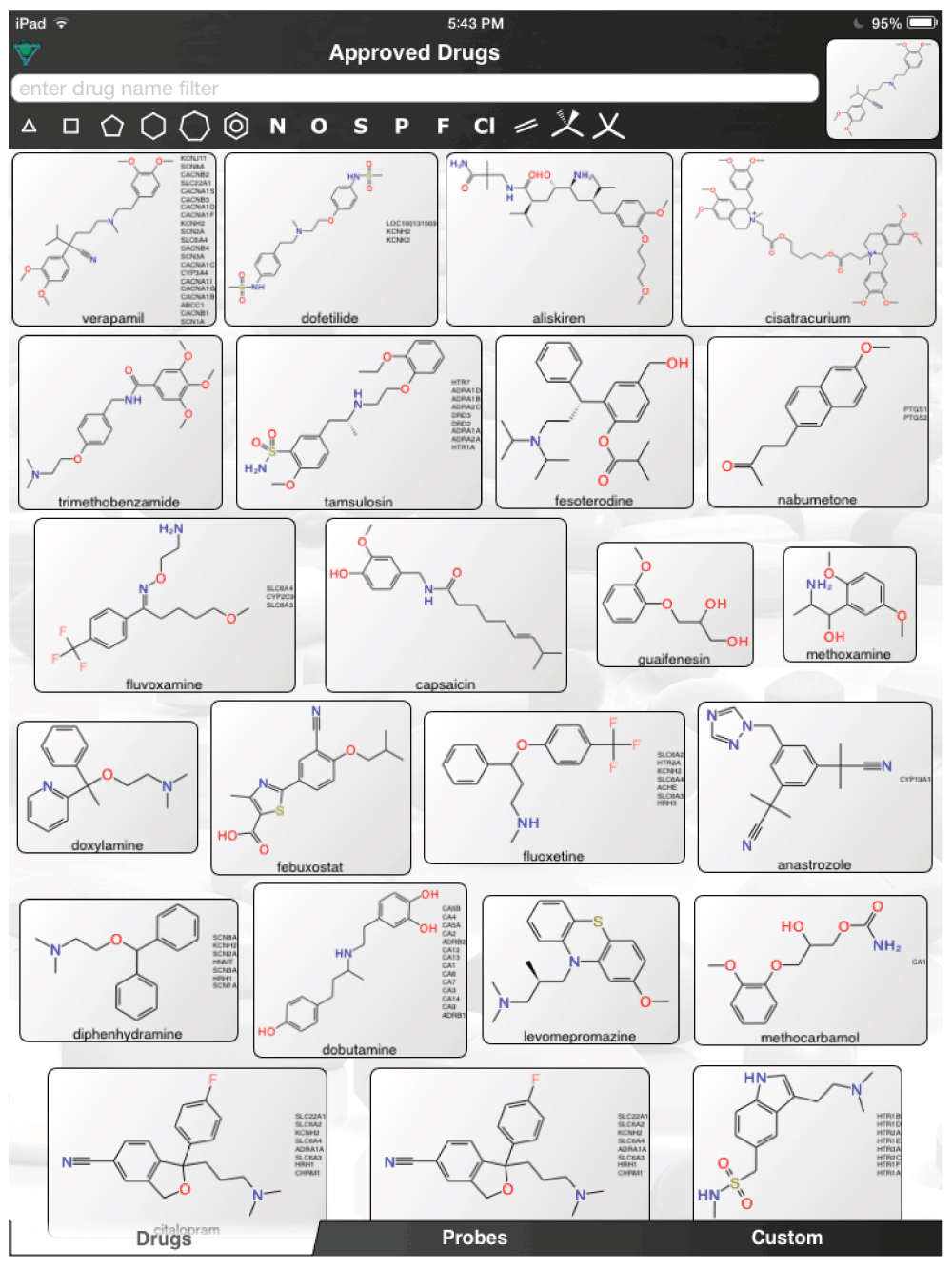
In one study, the antimalarials amodiaquine and chloroquine (Figure 1) were found to be active using in vitro cell culture assays and an in vivo mouse model3. Both drugs are cheap, generally safe, and likely readily accessible in Africa. These compounds have also shown relatively broad activity against other viruses in vitro and in vivo in animal models (Dengue, Coronavirus OC43, SARS etc.)4–7. A second study suggested selective estrogen receptor modulators (SERM) clomiphene and toremifene (Figure 1) as inhibitors of Ebola virus8. The latter compounds are likely more accessible in the west and indicates that other FDA or EMEA approved drugs may be worth testing including those with hormonal effects that are SERMs. More recent work from 2014 in Europe identified a further 3 FDA drugs, amiodarone, dronedarone and verapamil (Figure 1) that inhibit filovirus entry at plasma levels attainable in humans9. The mechanism of action for most of these drugs is unknown although, using computational methods we have recently shown that the antimalarials and SERMs may share some pharmacophore features which may be important to infer a potential common target or targets10. To our knowledge likely well over 100 small drug-like molecules have now been identified with activity against the Ebola virus including over 50 FDA drugs derived from a reporter assay at NCATS11–15.
As we await the development of a vaccine or biologic could we consider assessing the efficacy of the antimalarials or the other ‘FDA approved drugs’, as either treatments or prophylactics to prevent the Ebola virus from spreading further? While there can be no guarantee they will work (perhaps requiring adjusted dosage) they may be a last resort. It is possible there are other “non-antivirals” that are widely used in Africa that may also be effective against Ebola. Another example of where ‘non-antiviral’ FDA approved drugs have been found to have ‘anti-viral activity’ is for Hepatitis Virus B and D where the sodium taurocholate co-transporting polypeptide (NTCP) was identified as a receptor16 and screening produced drugs such as azelastine, pioglitazone, glyburide, irbesartan and ezetimibe that inhibited the transporter and may provide potential treatments17,18. Of these compounds, azelastine has been shown to possess in vitro activity against Hepatitis Virus B to date18.
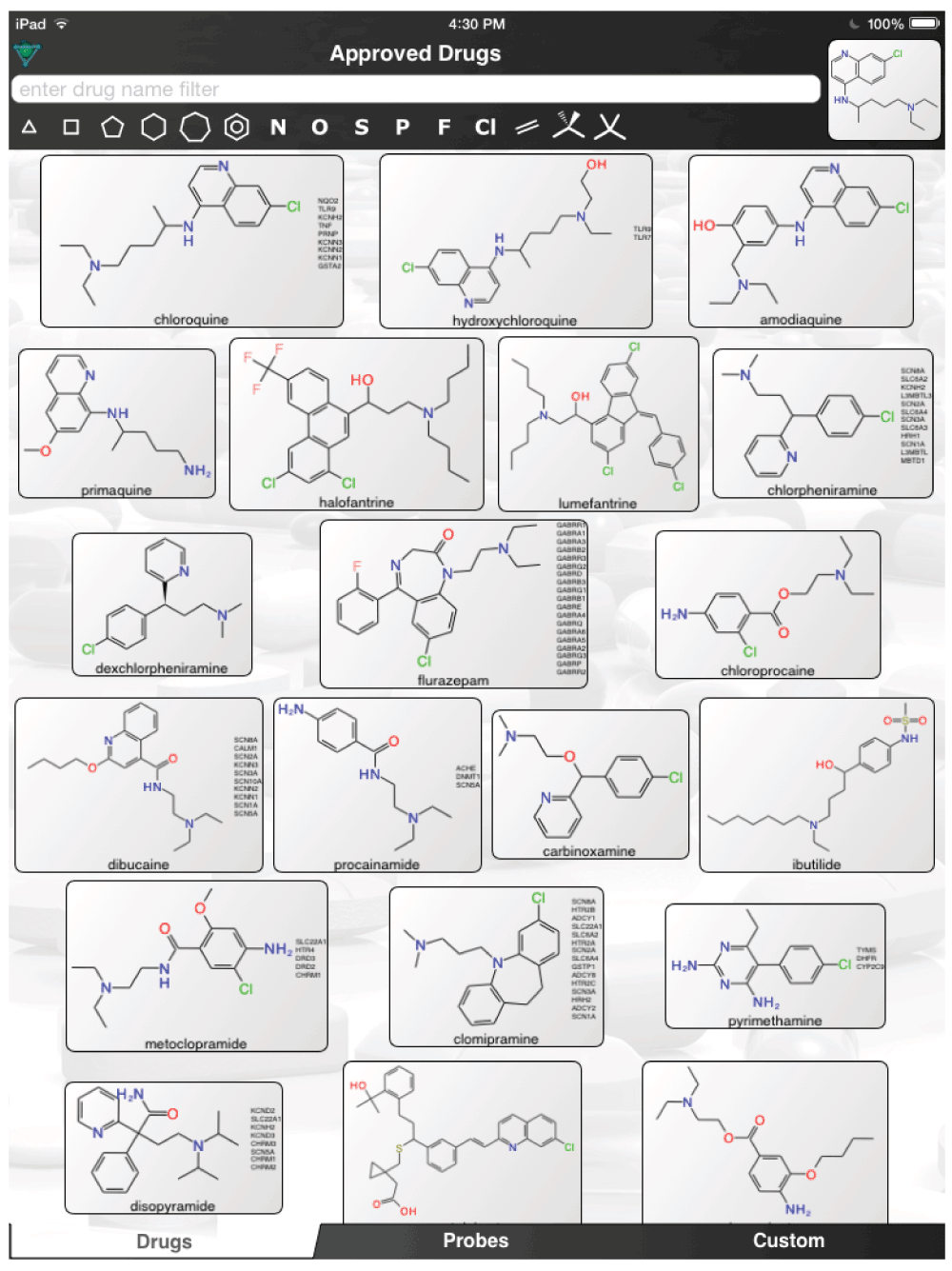
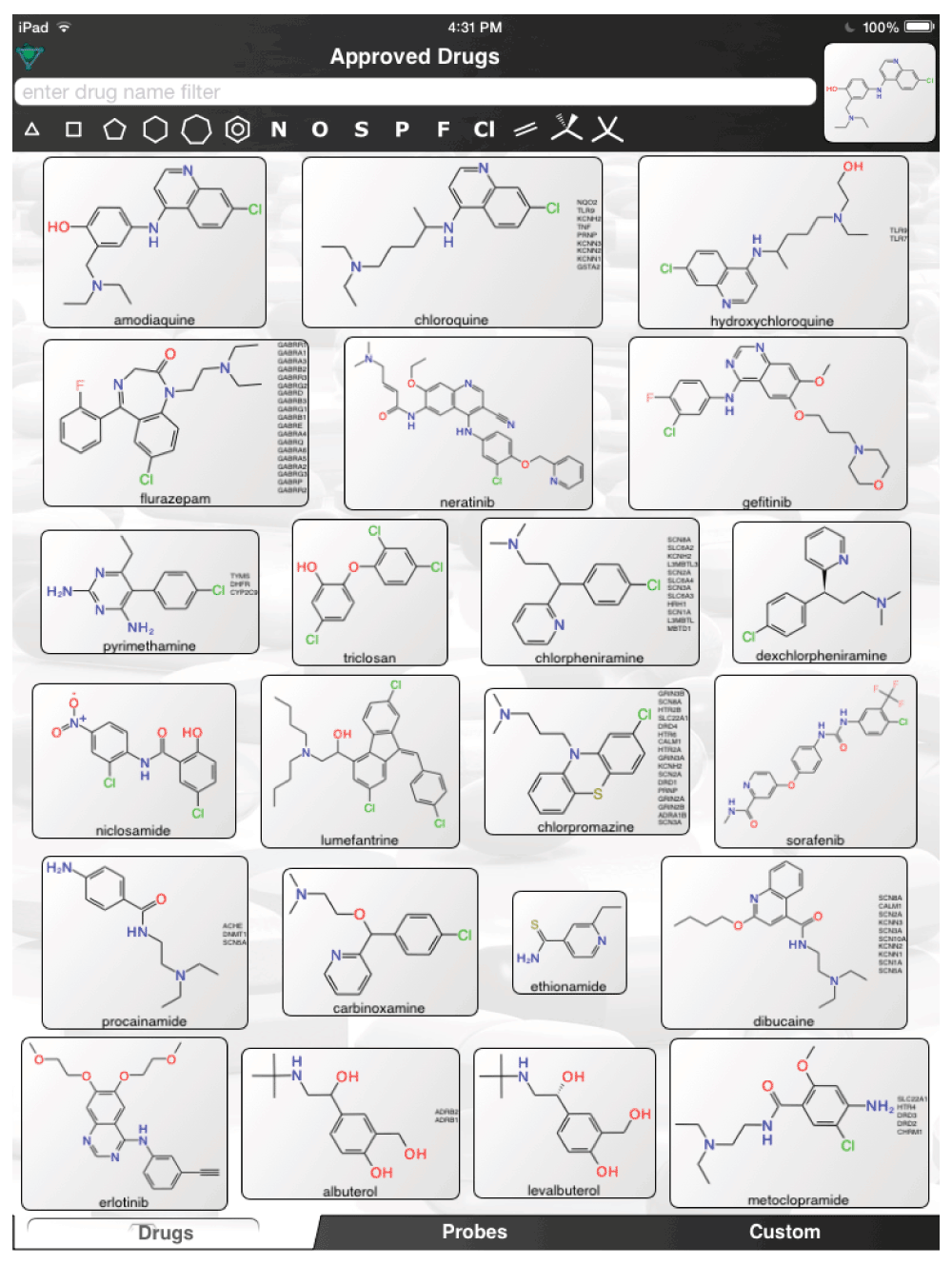
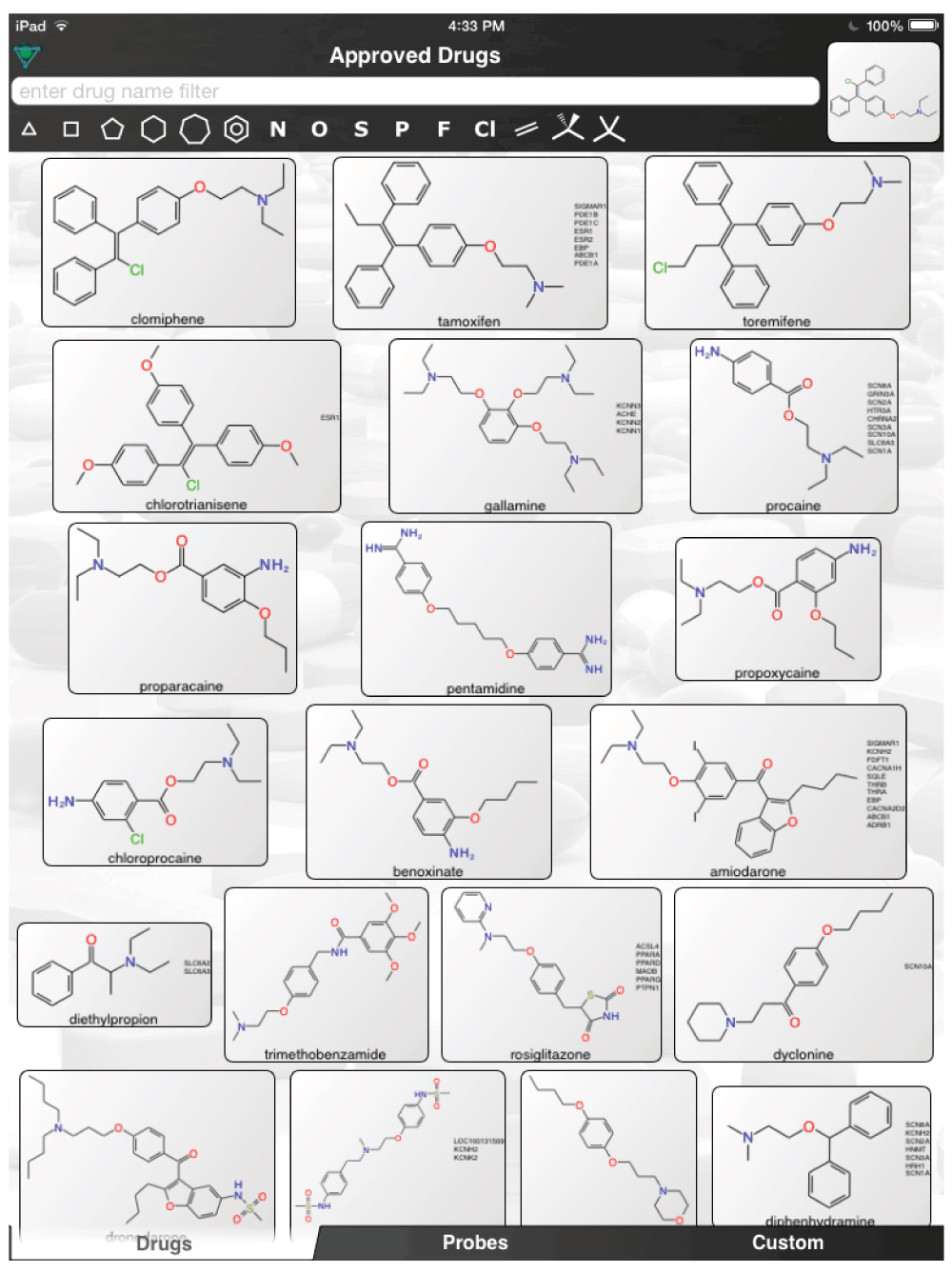
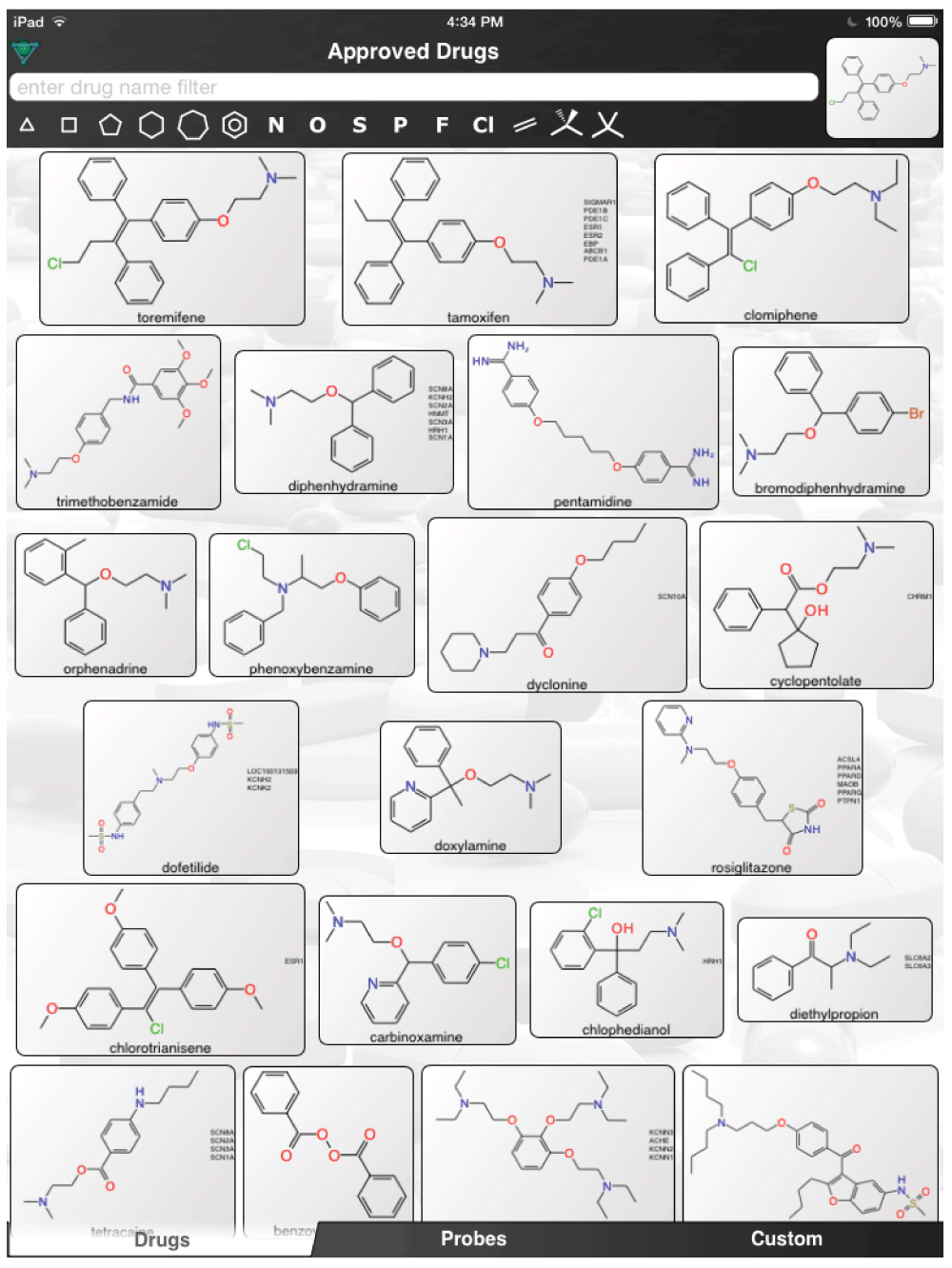
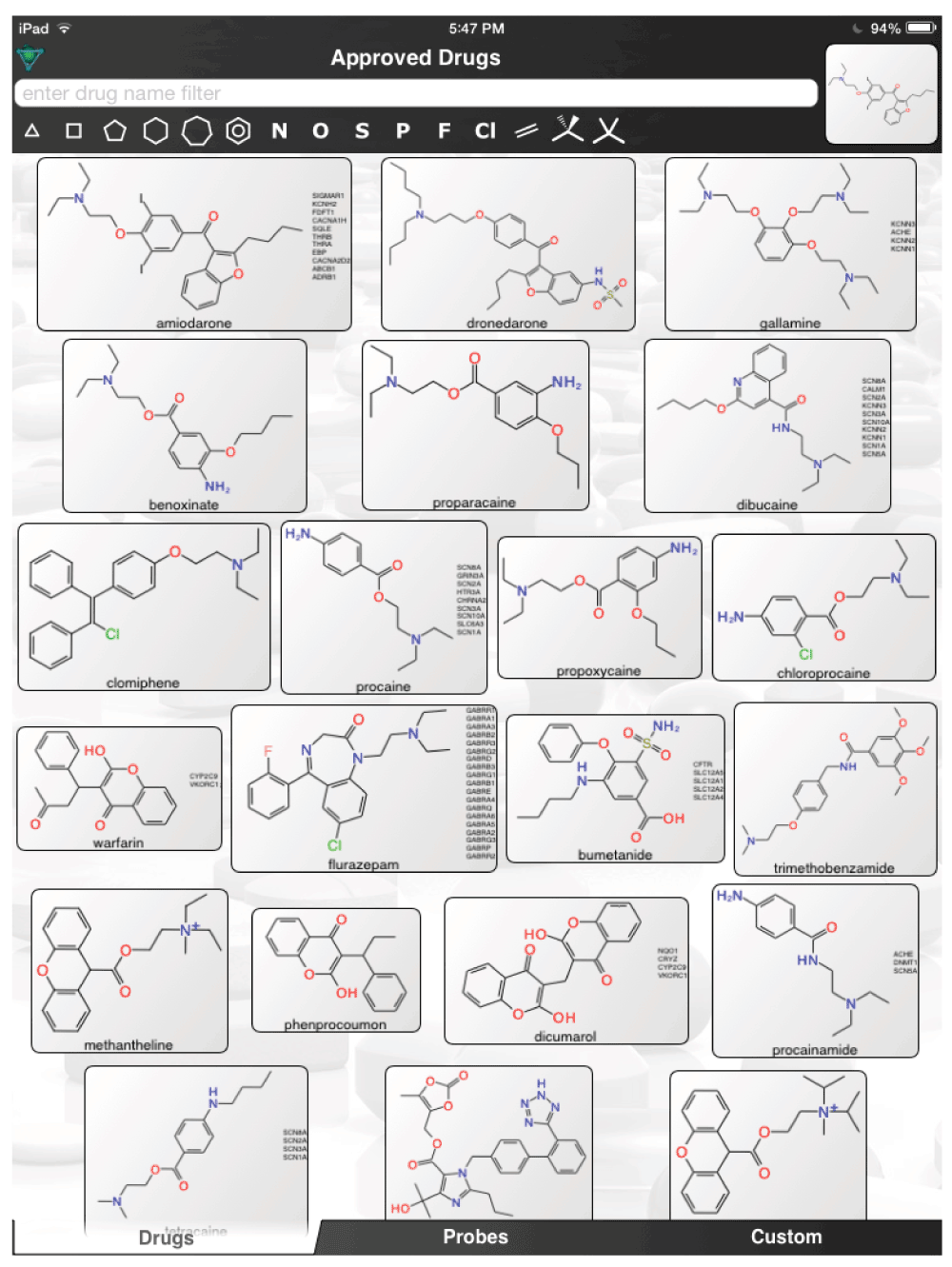
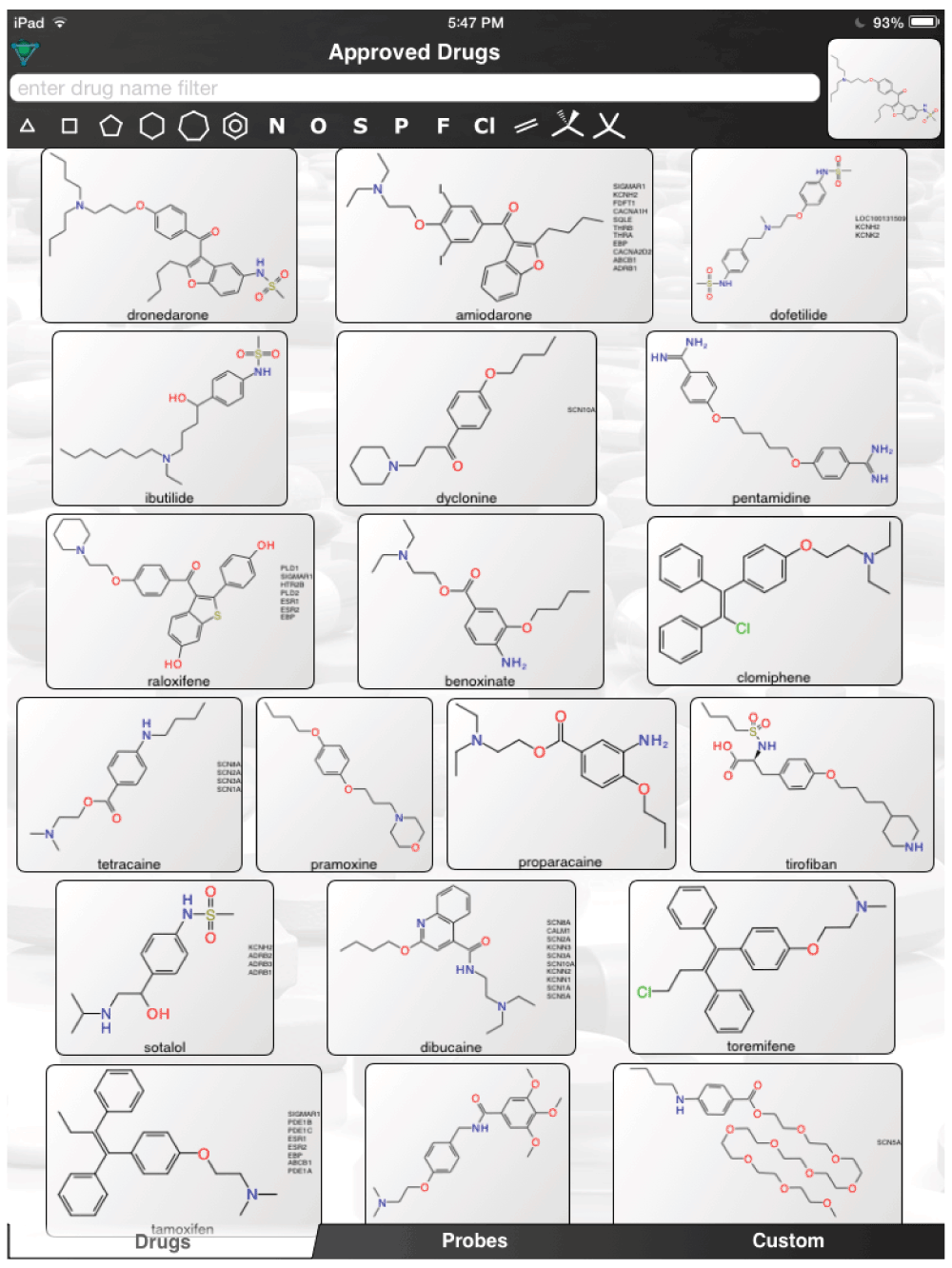
The aforementioned screens of ‘FDA approved drugs’3,8,9 for Ebola virus activity, were far from comprehensive, covering only some of the known approved drugs currently in use. In an age where drug repurposing is in vogue19–23 and it can be facilitated by computational methods24–26, it would seem a valuable resource for finding compounds active against the Ebola virus. For example, the recent pharmacophores developed for Ebola10 and virtual screens11 could be used to computationally search larger datasets of FDA approved drugs and prioritize additional compounds for testing in vitro. Even using the known actives (Figure 1) to perform simple similarity searches in a set of over 1300 Approved Drugs in a mobile app (http://molmatinf.com/approveddrugs.html) could prioritize further compounds for testing (Figure S1–Figure S7). For example molecules with structural similarity to chloroquine (Figure S1) not only includes known actives like amodiaquine and hydroxychloroquine3 but also suggests the antimalarials primaquine, halofantrine and the antihistamine chlorpheniramine. Molecules with similarity to amodiaquine include the kinase inhibitors neratinib and gefitinib while other kinase inhibitors have been suggested as having activity against Ebola virus15, these may not be readily accessible in Africa. Other compounds retrieved by similarity include the antimicrobial pentamidine (Figure S3, Figure S4, Figure S7), the antiemetic trimethobenzamide (Figure S3–Figure S7) and the antihistamine doxylamine (Figure S5). Certainly more sophisticated and exhaustive searches than this could be tried. Deciding which molecules to use or test should also involve the physician’s perspective27. Alternative treatments may also be found by studying those close to patients who may not have contracted the disease and are taking a drug for another chronic disease. Whether we can find a treatment for Ebola by serendipity is questionable but some of the published studies with known drugs might point us in the right direction of where to look. The opportunity to put already available drugs like those already identified3,8,9,11–14 back on the table may be a useful tool for frontline doctors to have and is worthy of more urgent discussion and research.
Dr. Christopher D. Southan, Dr. Joel S. Freundlich, Dr. Peter Madrid and Dr. Nadia Litterman are acknowledged for discussions on Ebola.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 10 Mar 15 |
read | |
|
Version 1 19 Feb 15 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)