Keywords
network analysis, genetic variant, pipeline, next generation sequencing
This article is included in the Bioinformatics gateway.
This article is included in the Hackathons collection.
network analysis, genetic variant, pipeline, next generation sequencing
Traditionally, the goal of genome-wide association studies (GWAS) has been to associate single nucleotide polymorphisms (SNPs) and their respective haplotype blocks with disease status, allowing the eventual identification of particular genes responsible for disease phenotype. Unfortunately, only a small subset of diseases arise from variants within a single gene. For most complex diseases, it is likely that the disease arises due to the interactive effects of multiple genetic variants, and different collections of these variants may be present in different patients. Within a GWAS study, these variants individually will exhibit low predictive power making it difficult for researchers to obtain a sufficient sample size to identify them with high confidence. Therefore, tools that can help detect groups of interacting genetic variants are needed1–3.
One set of tools that has great potential for aiding in this problem is network analyses. Within these tools, the results from GWAS studies are overlaid on networks constructed from curated molecular interaction data, such as databases documenting protein-protein interactions (PPIs), protein-DNA interactions, metabolite interactions, and gene-gene co-expression1,2. Many of these tools are powerful, but somewhat inaccessible to users with weaker computational backgrounds. For example, installing, configuring, running, and comparing the output of multiple network analysis tools could require a working knowledge of command-line scripting, Python, R, and Perl. Therefore, the goal of our hackathon team was to create a single command-line pipeline within which a user could input the results of a GWAS study, execute existing network analysis tools, and then access results from multiple network analyses. This work was conducted as part of the NCBI January 2016 Hackathon.
The context of the hackathon event allowed only three development days to create the pipeline which impacted the scope and design of the tool. The focus was on allowing one input file to be directed towards multiple tools; consolidation of results from individual tools was out of scope. Similarly, each tool output was not post-processed for unified output. We envision that future improvement to the pipeline may offer advanced visualisation options; however, this was not part of this pilot implementation. A working instance of the pipeline is also published as an Amazon Machine Image ami-4510312f.
As much as possible, the MetaNetVar pipeline uses existing tools for network analysis. We only considered tools that are freely available, with no license restrictions. We describe briefly each tool that is integrated into the MetaNetVar pipeline. Tools vary in scope, and some include additional functions that include network analysis.
FunSeq2 is an existing tool for prioritizing variants using several different approaches, including network-based analysis4,5. FunSeq2 identifies hub genes and provides the measure of centrality for those hub genes. Inference of the network analysis results requires further processing of the program’s output. We chose to include FunSeq2 in our pipeline because of its capability to identify functionally important, non-coding variants in the context of biological networks.
NetworkX is a network analysis framework available in a Python language software package. It allows for “the creation, manipulation, and study of the structure, dynamics, and functions of complex networks”6. It contains many standard graph algorithms and accepts and outputs 13 file formats, where nodes can be anything and edges can hold any type of data. NetworkX was used to calculate the degrees and betweenness centrality of nodes (genes) and to create XML format and static PNG figures of subnetworks containing the input genes. The degrees and the betweenness centrality gives you a measurement of how important the gene is in the network. A network analysis framework similar to NetworkX is CytoscapeJS7. We chose to include NetworkX in our pipeline because of our experience with Python.
HotNet2 is an algorithm for detecting “significantly altered subnetworks in a large gene interaction network”8–10. The algorithm uses heat diffusion kernel to capture the local topology of the interaction network. The subnetworks in genome scale interactions that have non-random mutations are identified using this approach. The limitation of HotNet2 are the challenges in getting the scripts running straight out of the box, along with the long computational time involved in the preliminary influence matrix creation process. We chose to include HotNet2 in our pipeline because of our experience with Python.
dmGWAS_3.0 is an existing tool for overlaying gene-level summaries of case-control association p-values onto an existing network (in this case, we use the network extracted from GeneMania detailed below) and then identifying subnetworks that are particularly enriched for strong associations using a greedy algorithm11,12. Unlike the previous version of dmGWAS (2.0), dmGWAS_3.0 also has the ability to incorporate differential gene coexpression data (in other words, the difference in gene co-expression between cases and controls) as weights for the edges in the network, but for the sake of simplicity we did not make use of this new functionality in our pipeline. Due to this choice, we discovered that the dense-module search output (ResList.RData) took the format of the previous version dmGWAS_2.013 and could not be manipulated using the tools referenced in the current documentation. Therefore, we created our own short script to extract out the basic statistics and subnetwork nodes associated with each input gene present in the network (see below: ModuleStrengthSummaryByGene.txt and Top1000ModuleScores.txt). We later discovered that some of the old tools capable of manipulating dmGWAS_2.0 output (ResList.RData) were preserved in the current code package and could be used for further data exploration by a motivated user by loading the output file (ResList.RData) into R and installing the requisite packages (dmGWAS, igraph), although some of the tools did not appear to be fully functional anymore (such as the subnetwork plotting capability in simpleChoose()).
Overall, the primary limitation that we observed for dmGWAS was computing time, so we adapted the existing code to make use of parallel computing using the BiocParallel package in R14.
To utilize dmGWAS_3.0, it is first necessary to convert the input file containing the case-control association p-values for each SNP to a gene-level summary. Within our pipeline, we complete this conversion using VEGAS, an existing command line (Linux/Unix) based tool recommended within the dmGWAS documentation15,16. It should be noted that by default, VEGAS uses the HapMap2 CEU (Central Europeans, Utah) population to estimate patterns of linkage disequilibrium for each gene.
VEGAS is written in Perl but also makes use of two R packages (corpcor and mvtnorm) and depends on functions provided by PLINK, a commonly-used whole genome analysis toolset17,18. The output of VEGAS requires further processing before input into dmGWAS. We found that several of the VEGAS gene-level p-values were rounded to either 0 or 1, which was incompatible with dmGWAS, so we substituted the minimum p-value present in our test file (1e-06) for 0 and 0.999 for 1.
Table 1 provides a summary of the tools used in this pipeline, including notes about their advantages and disadvantages.
Network construction based on a user-provided list of variants required accessing molecular interaction network data from external databases. We describe the network databases utilized by each tool. Some networks (such as Multinet) are used by multiple tools (FunSeq2 and HotNet2).
FunSeq2 utilizes multinet19, which is an integrated network consisting of regulatory interactions from the ENCODE regulatory network20,21, phosphorylation interactions from the SignaLink database22–24, protein-protein interactions from BioGRID (release 3.1.83)25, and metabolic interactions from KEGG26. While there are options for users to bring in their own network for use with FunSeq2 analysis, this pipeline prototype uses the pre-packaged multinet.
The NetworkX and dmGWAS are libraries and do not include particular network data.
We paired GeneMania with NetworkX and dmGWAS. The GeneMania network is a protein-protein interaction network27,28. Two genes are connected if they are found to interact in a protein-protein interaction study. The network was created from various protein-protein interaction databases, including BioGRID and Pathway Commons29,30. We used version 2014-10-15 of Homo_sapiens.COMBINED network.
HotNet2 uses mutation data to prioritize subnetworks by identifying significantly mutated subnetworks in genome scale interaction networks. In our pipeline, we have used the 2012 version of HINT (High-quality INTeractomes) a database of high-quality protein-protein interactions31.
As a sample input for our pilot, we searched NCBI dbGaP for a sample study that provided a real world list of variants. We used data from a clinical study of age-related macular degeneration32 with dbGAP identifier phs000182.v3.p1.
As an additional input example, we used data from ClinVar33. ClinVar is a database of interpretations of clinical significance of variants for reported conditions, hosted by the National Center for Biotechnology Information (NCBI). It includes germline and somatic variants of any size, type, or genomic location with interpretations from several sources (such as clinical testing laboratories, research laboratories, or locus-specific databases). It includes a link of variants to phenotypes. For this example, we identified variations submitted by LabCorp and extracted disease-variant pairs, for diseases with 30+ variants. The example dataset is provided on the MetaNetVar GitHub page.
We implemented four network analysis programs or platforms into our pipeline (FunSeq2, NetworkX, HotNet2, dmGWAS), utilizing molecular interaction data from several external knowledge databases (listed above). Figure 1 provides an overview of the pipeline.
To lower the adoption threshold for potential users, we offer the snapshot of our working instance as an Amazon Machine Image. The collection of tools and the pipeline script can be executed using an instance of our publicly available Amazon Machine Image: ami-4510312f. The accompanying supplementary file describes the step-by-step procedure for running our pipeline using the published Amazon Machine Image.
We discuss below the results from individual tools integrated into our pipeline. Results of all of these tools, using the example dataset, is also provided on the MetaNetVar GitHub page.
The parsed input file required for the FunSeq2 analysis, the PHP script that generates this parsed input file from the original dbGaP association data, and the output files (using default parameters) are provided in the GitHub project page. An example file generated from a filtered list of SNPs from the ClinVar database, for the Cardiomyopathy phenotype is also provided for testing purposes and can be found at http://github.com/NCBI-Hackathons/Network_SNPs/blob/master/test/sample_output/funseq2/cardiomyopathyfunseqoutput.
We took the NetworkX library and created a script that we refer to as SNPsNet. This script generates one output file containing the degrees and betweenness centrality measure of genes that are input into the pipeline, as well as creating two directories (see Figure 2). The two directories contain figures of subnetworks with the input genes and the XML format of the subnetworks. With these results, the user can prioritize the input variants or genes by sorting how important each gene is based on degreeness or centrality, as well as visualizing the subnetwork. Since NetworkX is not primarily a visualization tool, the XML file can be input into several other tools to better visualize the graph.
The influence matrix for HINT was pre-computed and then used in the current version of our pipeline. Influence matrix creation is a one-time process for a given network and, if required, advanced users may use custom influence matrices with MetaNetVar by modifying the path to the input influence matrix file and corresponding gene index file. For evaluation of MetaNetVar, we generated heat scores from a test mutation file. The .json file containing heat scores on each gene, which was used in subsequent steps, may be accessed at https://github.com/NCBI-Hackathons/Network_SNPs/blob/master/heat_score.json.
The final step of weighted graph generation uses the influence matrix for HINT, the HINT index file, and the heat score .json file, to remove edges with weight less than the delta value, and extract the resulting connected components. Two output files were generated: components.txt (available at https://github.com/NCBI-Hackathons/Network_SNPs/blob/master/components.txt) and results.json (available at https://github.com/NCBI-Hackathons/Network_SNPs/blob/master/results.json)
The sample association file from the age-related macular degeneration dataset (phs000182) was parsed down to a two-column text file containing only SNP identifiers (“rs numbers”) and case-control association p-values. This file was fed into VEGAS and a gene-level summary file was created, which was further parsed into a simple two-column text file containing gene identifiers (gene symbol) and “weight” (an integrated p-value for the gene). Within dmGWAS, this input was overlaid onto a network provided by GeneMania to produce a network of weighted nodes from which particularly “dense” subnetworks are identified (full output: ResList.RData). Finally, our program summarizes the data into two easily navigable tab-delimited .txt files which can be viewed within accessible programs such as Microsoft Excel (ModuleStrengthSummaryByGene.txt, Top1000ModuleScores.txt). Figure 3 and Figure 4 demonstrate example output files.
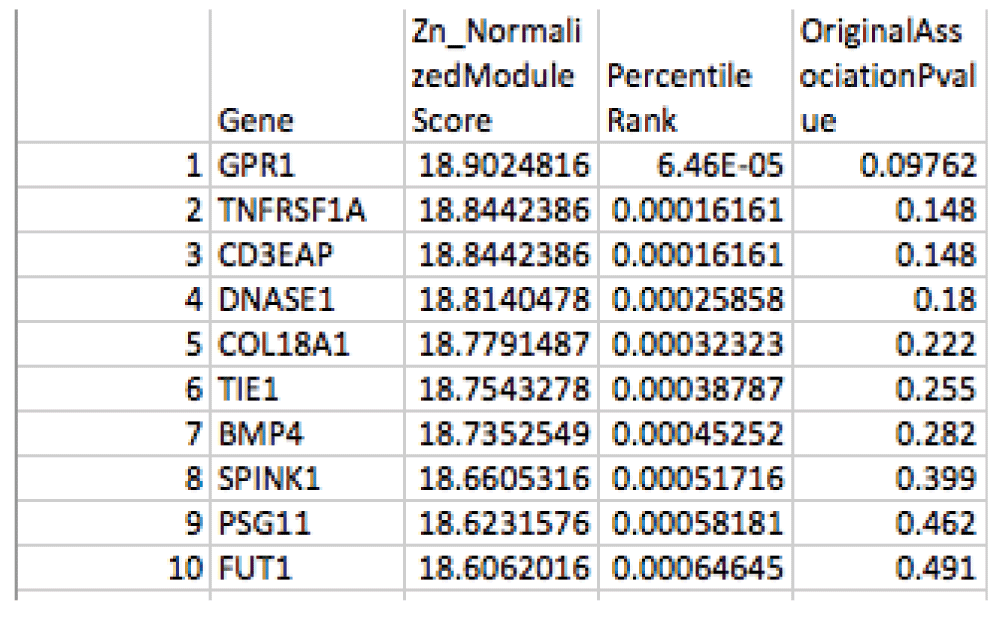
This file provides the Normalized Module Score for each gene included in the network (“Zn”, where a larger value indicates the gene is more enriched for significant case-control associations), and the gene-level summary case-control association p-value provided by VEGAS. It is ordered by percentile rank to allow comparison across different network analysis programs.
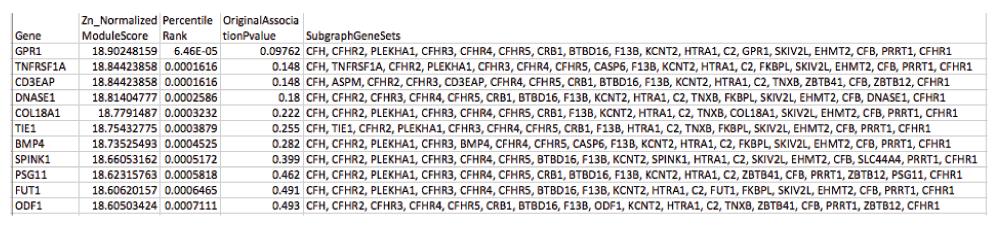
This second output provides similar information as the first output file, but expands it to include the list of genes (nodes) present in each gene of interest subnetwork. Only subnetwork output for the top 1000 seed genes is provided (as determined by percentile rank).
The current version of the pipeline is set to use data from dbGaP and ClinVar out-of-the-box. However, advanced users could tweak the provided scripts to make it run using other input formats. Some of the components of the pipeline use processes that are parallel and compute-intensive in nature. Using the provided working implementation of the pipeline through Amazon Web Services requires some computing skills.
Our tool, MetaNetVar, allows researchers with limited computational experience to access a host of powerful network analysis tools for application to genomic datasets. This platform is intended for use in a variety of future hackathons, including work on cancer and evolutionary biology, but will most likely also be used by participants from the current hackathon, as well as other interested individuals. Since this work was a pilot project, we expect further modification of the pipeline as new users provide feedback. Ideally, the future pipeline would include a unified output summary, better network visualization tools, and the ability to integrate known disease-related variants into the analysis, such as from ClinVar33, from PheGeni34, or from the output of epistasis analyses35.
Latest source code: https://github.com/NCBI-Hackathons/Network_SNPs
Archived source code as at time of publication: http://dx.doi.org/10.5281/zenodo.4820236
Amazon instance ID: ami-4510312f
Amazon instance name: NCBI-Hackathon-20160122-Network-SNPs
License: CC0 1.0 Universal
All of the authors participated in designing the study, carrying out the research, and preparing the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
The work on this project by Vojtech Huser, Eric Moyer and Ben Busby was supported by the Intramural Research Program of the National Institutes of Health (NIH)/National Library of Medicine (NLM)/Lister Hill Center (VH) and NCBI (EM and BB). Megan Hagenauer’s work on this project was supported by the Pritzker Neuropsychiatric Disorders Research Consortium.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors thank Lisa Federer, NIH Library Writing Center, for manuscript editing assistance.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 13 Apr 16 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)