Keywords
antibody, network, thermodynamics, binding, entropy, energy landscape
antibody, network, thermodynamics, binding, entropy, energy landscape
Blood is a massive and critically important extracellular space of multicellular organisms. It is a fluid tissue with cellular, macro- and small molecular components that perfuses the whole multicellular organism, being in direct contact with vascular endothelial cells and blood cells. Its components are potentially derived from any cell of the organism via secretion and leakage (Anderson & Anderson, 2002). Such a hugely diverse molecular pool needs to be regulated with respect to the quality and quantity of its components. One of the mechanisms of regulation is the generation of antibodies by the humoral adaptive immune system (Prechl, 2017a; Prechl 2017b). Considering the diversity of antibodies and the diversity of molecular targets, the interaction landscape of the humoral immune system is presumably the most diverse in an organism. In this opinion article, I approach antibody homeostasis from the thermodynamic point of view, depicting antibody-antigen interactions in a novel energy landscape model. The currently used funnel energy landscape model is suitable for the description of folding and binding of one or a few molecules, but it would require landscapes of intractable sizes to depict a whole system, like adaptive immunity. I introduce the fountain energy landscape, a projection of the multidimensional binding landscape of antibodies to the dimensions of entropic penalty and energy of molecular interactions, to accommodate the vast range of interactions of antibodies.
Molecular interactions can be described by examining structural, kinetic and thermodynamic properties of the binding. Structural approaches aim to define the relative spatial positions of the constituting atoms of the interacting partners in the bound and unbound forms of a molecule. The advantage of the structural approach is the high resolution visual rendering of molecular structure that helps human perception. Systematic analysis of protein structures gives insight into the evolution of protein complexes and the dynamics of assembly and disassembly (Marsh & Teichmann, 2015). Structural information can reveal networks of protein interactions (Kiel et al., 2008). Kinetic studies follow temporal changes of association and dissociation of interacting partners. These observations are easily applicable to a simple system with a few components only, but it is difficult to describe complex systems and crowded molecular environments (Schreiber et al., 2009; Zheng & Wang, 2015). Thermodynamics examines the changes in free energy that accompany a binding event; providing statistical descriptions of enthalpic and entropic components of the interaction. Energy landscape theory resolves some shortcomings and integrates these approaches by assuming the presence of many different conformations that converge to thermodynamically stable forms -- the route taken to obtain this conformation dictating the kinetics of the events (Bryngelson et al., 1995). The intramolecular interactions of proteins lead to the emergence of the functional protein conformation, a process called folding. The energy landscape of folding is assumed to be funnel shaped, the stable form of the protein being at the bottom of the funnel with the lowest free energy state (Finkelstein et al., 2017; Wolynes, 2015).
The process of folding is obviously strongly dependent not only on general physical parameters, such as temperature and pressure, but on the quality and quantity of molecules present in the system. Water is the solvent of life and interactions with water molecules (Fogarty & Laage, 2014) are of key importance in all molecular interactions associated with life. The concentration of hydrogen ions (pH), cations and anions and small molecules modulate interactions. Macromolecules influence interactions not only by taking part in the interactions, but also by the excluded volume effect, restricting diffusional freedom (Zhou et al., 2008). A detailed definition of the binding environment is therefore indispensable for a realistic depiction of the binding energy landscape. Defining the antibody binding landscape in blood would therefore at least require a complete list of all constituents of blood, better involving abundance of each molecule.
Antibodies are globular glycoproteins secreted into the blood and other biological fluids by plasma cells (Nutt et al., 2015). Antibodies are actually a family of oligomeric proteins, with distinct constant regions that qualify them into classes and subclasses, and with distinct variable domains that determine their binding specificity (Schroeder & Cavacini, 2010). While most of us think of antibodies as molecules with a well-defined specificity, in fact the majority of the circulating antibodies (especially of the IgM class) is not monospecific (specific to one target), but rather poly-specific and cross-reactive (Kaveri et al., 2012; Seigneurin et al., 1988). Any comprehensive systems approach to describe antibody function therefore must account for the presence of both highly specific and poly-specific antibodies. Our quantitative model of antibody homeostasis accordingly attempts to provide a unified framework for the complete humoral adaptive immune system (Prechl, 2017b). Antibodies are secreted by plasmablasts and plasmacells, descendants of B cells that had been stimulated by antigen. B cells are thus raised in an antigenic environment, the function of the immune system being the selection and propagation of B cells, which can respond to the antigenic environment. The essence of humoral immunity is therefore the definition and control of this antigenic environment by regulating molecular interactions. Thermodynamically this translates to the generation of a binding energy landscape suitable for maintaining molecular integrity of the host organism.
The funnel energy landscape is a theoretical approach used for the depiction of conformational entropy and free energy levels of one particular molecule (Bryngelson et al., 1995). Besides the description of intramolecular binding (folding) it can also be applied for the interpretation of homo- or hetero-specific binding, such as aggregation or ligand binding (Zheng & Wang, 2015). If we tried to describe antibody binding by the binding funnel energy landscape, we would face two interconnected problems, one deriving from antibody heterogeneity and the other from target heterogeneity. Antibody variable domains constitute the most diverse repertoire of all the proteins present in the organism, estimates being in the range of 109–1011 different primary structures at any particular time of sampling, the hard upper limit being the number of B cells in a human body, around 1012 cells (Bianconi et al., 2013). Even if the tertiary structures show orders of magnitude of lower diversity, we still face an immense variability. On the other side, poly-specific antibodies bind to a multitude of targets, with limits to the number of known targets being posited only by experimentation. A combination of these two factors implies that the binding funnel approach would not allow a clearly comprehensible yet thorough description of antibody-antigen binding. To resolve this issue, here I develop the concept of a binding fountain energy landscape model.
Free energy decrease associated with binding can be resolved into components that act against or act favorably for binding. The loss of conformational entropy, a.k.a. entropic penalty, acts against binding while binding energies (enthalpic component), hydrophobic effect (conformational entropy of water molecules), contribute positively. The net difference between these events determines binding energy and protein stability (Figure 1). Conformational entropy loss of the antibody molecule thereby sets a minimum energy level that needs to be exceeded for any binding event to be stable. First, let us virtually collect all antibody binding events taking place in our system under examination, blood, and sort these events according to the entropic penalty of binding. For the sake of simplicity let us only consider entropic penalty of the variable domains of the antibodies. Second, let us plot free energy changes against conformational entropy. Since entropic penalty sets a minimum, all stable binding events should appear below the theoretical line, representing a gradually increasing entropic penalty (Figure 1). We can also set arbitrary limits for the free energy decrease, as the range of equilibrium constants for reversible antibody binding are known (Figure 1B) and we can obtain ΔG from Keq by the equation:
ΔG = -RT ln Keq
where R is the universal gas constant and T is the thermodynamic temperature. The resulting plot will show the distribution of binding energies against conformational entropy loss. This latter entity is itself associated with the number of atoms at the binding interface and the buried surface area (Marillet et al., 2017). Experimental evidence suggests that reversible binding is characterized by a range of energies, limits observed both for maximal and minimal values, which are dependent on the magnitude of the interacting surface, whether characterized by the number of atoms or by buried surface area (Brooijmans et al., 2002; Smith et al., 2012).
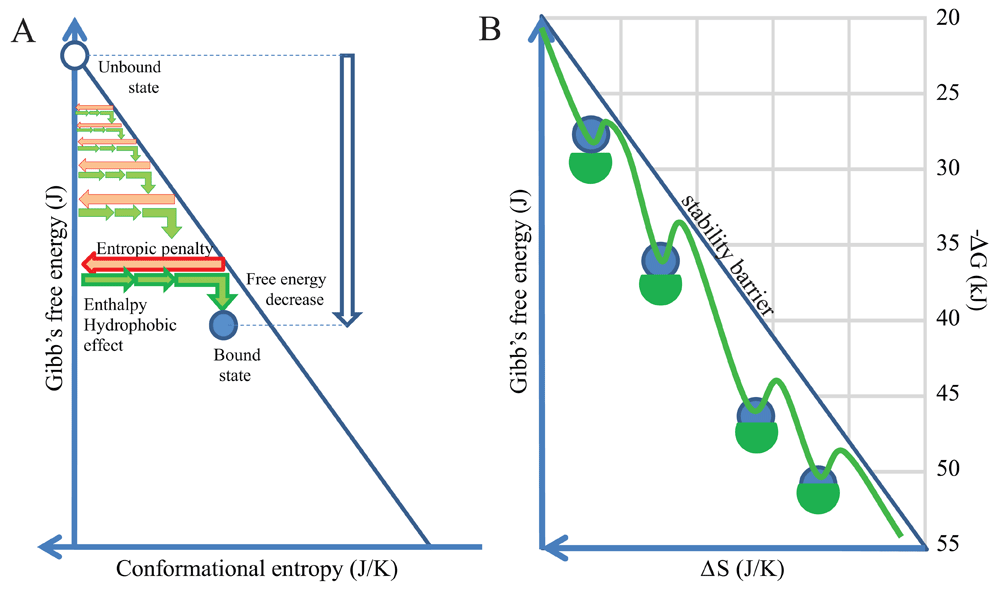
(A) By anchoring the axis of free energy change at zero entropic penalty we normalize binding events. Any antibody entering the binding landscape appears at the top can search for binding partners with favorable thermodynamic characteristics. (B) These binding events take place below the stability barrier, the line representing equality of entropic penalty and energies favoring binding. Theoretically we can collect and position all antibody interactions in this energy landscape.
A binding funnel energy landscape focuses on the one (or few) native conformation(s) that can be reached via various conformational routes, as represented by a hypersurface of conformation, entropy and energy. The contribution of binding energies and conformational changes to free energy is described by the equation:
ΔG =ΔH -TΔS
where ΔH is enthalpy change and ΔS is entropy change.
If instead of focusing on the one or few native conformations we would like to focus on the multitude of different conformational routes taken by several different antibodies while binding to different targets, we need a different kind of representation. To this end, we assume that all native unbound antibodies enter our landscape at the top of the energy landscape plot, where their starting conformational entropic penalty represents that associated with folding. In order to get a better resolution of the binding landscape let us spin our two-dimensional plot around the energy axis at the maximal entropy to obtain a conical hypersurface in three dimensions (Figure 2). Native unbound antibody molecules entering our landscape will move down along a path, while interacting with their targets with an increasing binding energy. This gradual increase in ΔG is accompanied by an increasing involvement of the binding site, called antibody paratope. All stable binding events take place under the theoretical conical surface generated from the stability barrier line. A binding path ends when the antibody finds its lowest state of energy, corresponding to binding to a target with the highest affinity. Where this point is located depends both on the antibody and the nature of its target (e.g. size, chemical characteristics). The hypersurface of conformations in the space of conformational entropy and free energy generated by this approach we shall call a binding fountain energy landscape.
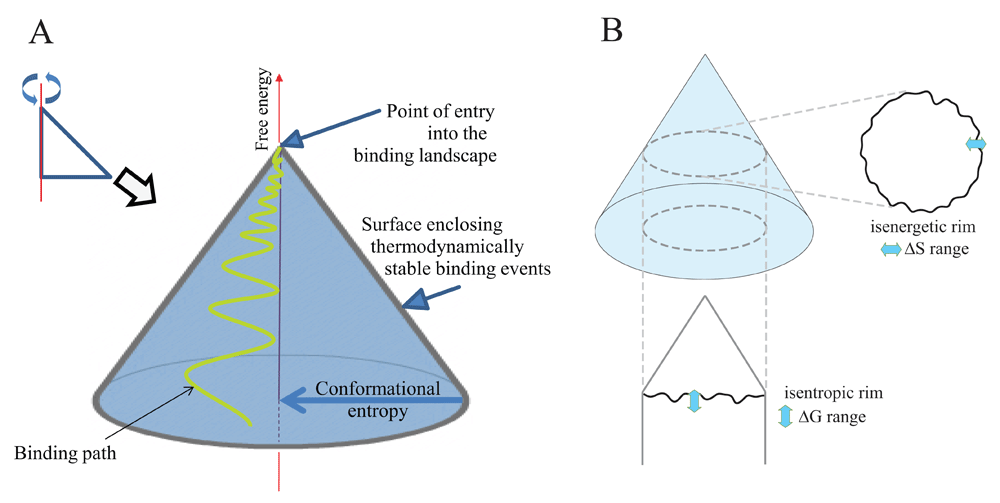
(A) By spinning the former representation in Figure 1 around the anchored axis we obtain a quasi-conical surface. This surface encloses all stable binding events of an antibody molecule and is suitable for displaying a binding path. (B) Thermodynamically defined subsets of binding events in the binding fountain can be obtained by looking at events in the isenergetic or isentropic rim.
While the conical surface enclosing stable binding events is a theoretical surface, we can obtain descriptors of real binding events by looking at subsets of events of the interaction space. By cutting the binding fountain horizontally at a given ΔG value we obtain the isenergetic rim (Figure 2B). The isenergetic rim is the collection of binding events with identical ΔG and a range of corresponding ΔS. Thus, its ΔS distribution shows the range of entropic penalties that give rise to binding at the given ΔG in our system of study. By cutting the skirt of the cone at a given ΔS value, we obtain the isentropic rim (Figure 2B). The isentropic rim is the collection of binding events with identical ΔS and a range of corresponding ΔG values. Thus, its ΔG distribution shows the range of free energy changes and corresponding affinity values that give rise to binding at the given ΔS in our system of study. It shows how enthalpy and hydrophobic effects exceed entropic penalty. Please note that as these lines are derived from a hypersurface the lines are theoretical hyperlines themselves, comprising high-dimensional data that cannot be properly visualized in a simple 2D plot.
We have so far worked out an energy landscape interpretation tool, which helps map all the binding events that occur in a molecularly complex environment, such as blood. We assumed that antibodies secreted into the blood gain their native unbound conformations then engage in binding events of various energies until they reach their specific target. The path leading to thermodynamic equilibrium can be rugged, caused by less specific contacts, or smooth, with few intermediate binding states (Figure 3A). It is important to note, however, that blood is the most heterogenous biological fluid, comprising potentially all molecules found in the organism (Anderson & Anderson, 2002). Besides a huge number of secreted molecules, any leakage from tissues, debris of cell death and foreign molecules may be present in blood. This vast molecular diversity generates a binding site diversity that we may assume to approach a randomized structural space, representing all potential variants of an antibody binding site covering up to 3000 Å2 (Marillet et al., 2017). Such a diverse binding space should approach a power law distribution of binding partners, with decay of partners as we increase binding energy or affinity (Figure 3B) (Zheng & Wang, 2015). A rugged start is therefore expected for all antibodies, with the path smoothing out depending on the paratope properties and the content of the binding landscape. As we approach higher energy and higher entropy, loss regions the epitope “sharpens”, as Irun Cohen termed (Cohen & Young, 1991) the gradually increasing affinity of antibodies (Figure 3C). This sharpening involves both a gradually increasing buried surface area and better fitting surfaces and various combinations of these components. It is also apparent that sectors of conformational entropy contain structurally related binding sites, since sharpening reveals more details of epitopes that appear identical at lower resolution (Figure 3D), later maturing into distinct conformational entities. This relationship also reflects the clonal relationship of antibodies going through affinity maturation, gaining sharper but constrained vision of targets by improving their fit (Kang et al., 2015).
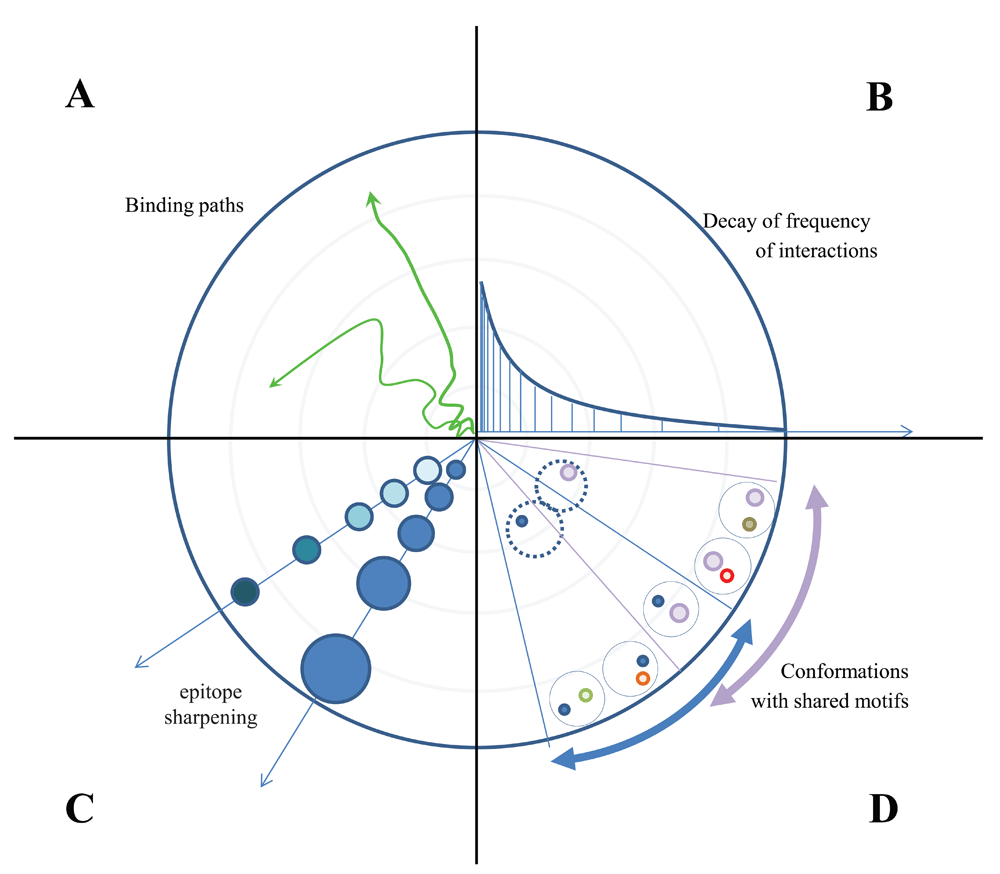
(A) Sequential binding of a given antibody appears as a path with more or less rugged track. (B) The frequency of interactions decreases by power law decay as we approach high energy binding with high entropic penalty. (C) The levels of contact accounting for the entropic penalty increase by improved fit with stronger binding forces and by increased buried interface area. (D) Conformations of binding surfaces share common origin with identical structural motifs closer to the “source” of the fountain, the region of low energy interactions.
The binding landscape is the set of all potential interactions in a given fluid with given constituents, each interaction being positioned according to the entropic penalty, conformation and free energy decrease. In the binding fountain representation we can trace the fate of a particular antibody in time as a binding path (Figure 4A) or display several different antibodies at an imaginary thermodynamic equilibrium (Figure 4B). Owing to the fact that blood is a highly heterogenous fluid with a vast diversity of potential binding sites, the frequency of low energy interactions is very high. At the tip of the fountain, antibodies are “surfing” along the ripples of low affinity interactions. Moving down the surface they encounter interaction partners with gradually improved fit, spending more and more time in an interaction, until the target with best fit, that is highest free energy decrease and largest entropic penalty, is found (Figure 4A).
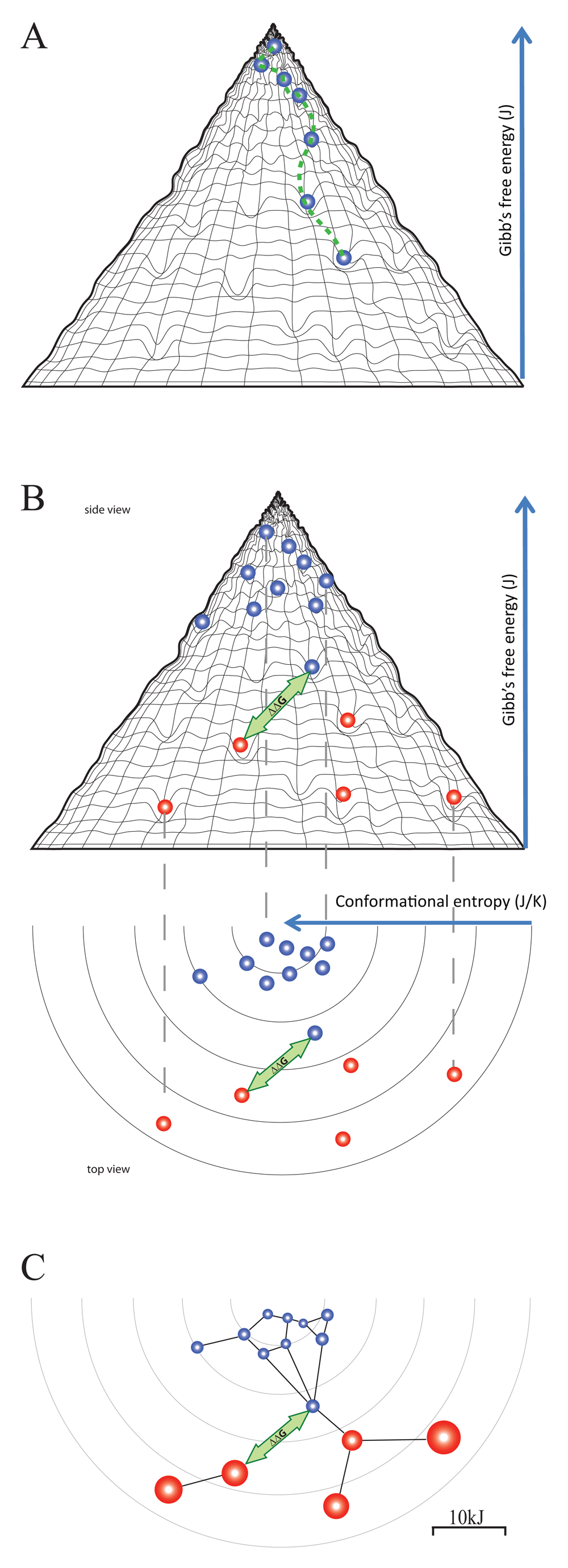
(A) An antibody entering the binding landscape engages in serial interactions with increasing energy, taking the molecule down a binding path. (B) At an imaginary equilibrium natural antibodies (blue beads) and affinity matured thymus dependent antibodies (red beads) fill the holes of binding, arranged according to their conformation, entropic penalty and free energy level. The distance between any two binding events can be expressed as ΔΔG, which represents the cross-reactivity of the two antibodies concerned. (C) We can further project these events into an interaction space where a network is formed based on distance and binding capacity.
Interactions in the blood cannot reach thermodynamic equilibrium; molecules are continuously entering and leaving this compartment. On the other hand, due to the constant turbulent mixing, the distribution of molecules is constantly approaching homogeneity. Thus, we may display antibodies at an imaginary equilibrium where their position reflects their potential energy minimum in the system. This is where actually target antigen-bound antibody molecules are accumulating (Figure 4B). Registering the position of all the copies of a given antibody species should show a distribution of bound forms determined not only by ΔG, but also by the availability of the target molecules, which is antigen concentration [Ag]. The disappearance of the target ([Ag]≈ 0 M) will lead to the disappearance of the low energy position in the landscape. As a consequence, the antibody will accumulate in the interaction with the next available energy level, albeit the ratio of bound to free form will be lower as dictated by the higher KD value. Alternatively, the antibody can search the neighboring conformational space along the isenergetic rim for a binding site with similar ΔG. High concentrations of the target ([Ag]>>KD) will deplete antibody resulting in the potential overflow of related antibodies from the neighboring conformational space. The distance ΔΔG between any two interactions has three components: a free energy component, a conformational component and an entropic penalty component. These components are perceptible from the side view, top view and both views of the binding fountain, respectively (Figure 4B).
As suggested above, besides the presence of targets with a given ΔG, the actual concentrations of both Ab and Ag determine the frequency of their interactions and the development of the imaginary equilibrium. To appreciate these factors we can project the interactions of a binding fountain into a space where the distance of the interactions is defined by ΔΔG and the availability of antibody is expressed as the ratio of free antibody to the dissociation constant ([Ab]/KD). This latter value can be visualized as the radius of the circle representing the interaction (Figure 4C). Please note that this value corresponds to [AbAg]/[Ag], the ratio of bound and free antigen concentrations. This visualization corresponds to the network representation of antibody-antigen interactions as I recently described (Prechl, 2017b).
The adaptive immune system responds to an antigenic stimulus by the production of antibodies reacting with the eliciting antigen. In our binding landscape an antigenic stimulus appears as an impression on the hypersurface representing antibody interactions, the position of the impression being determined by both the conformation of antigen and the conformation of fitting antibodies. The fact that an antigen can stimulate the humoral immune system implies that secreted antibodies that could efficiently bind to the antigen are not present. The antigen therefore binds to the membrane antibodies (B-cell receptors, BCR) of specific B cells (Figure 5). If BCR engagement reaches a threshold the affected B cells proliferate, differentiate and secrete antibodies (Prechl, 2017a). Depending on the nature of the antigen, the route of entry into the host, the presence of costimulatory signals, the ensuing response can proceed basically in two distinct ways. A thymus independent (TI) response will result in the generation of antibodies with binding properties identical to the parental B cell, since there is no affinity maturation. The structure of the binding site does not change, conformation, entropic penalty and ΔG of binding will be identical to the original interaction (Figure 5A). These interactions take place in regions with moderate conformational entropy loss and high interaction frequency, meaning that of the huge repertoire of BCRs several will respond. The response appears as a standing wave, the appearance of antigen showing as the development of the impression, the response of antibody secretion as the disappearance of the impression as free antigen is replaced by bound antigen and immune complexes are removed. This kind of response seems suited for keeping concentrations of target molecules stable. We can think of the response as a closely knit elastic net that regains its original shape after applying pressure to a point (Figure 5A). Thymus dependent (TD) responses will involve the affinity maturation of the antibody binding site, the sequential generation of antibodies with increasing affinity. As the binding site matures, the entropic penalty and ΔG increase. The interactions will take place at different positions of the binding landscape (Figure 5B). The response appears as a propagating wave sweeping down the slope of the binding fountain energy landscape. This wave is taking along the antigen, resulting in the efficient elimination of antigenic molecules.
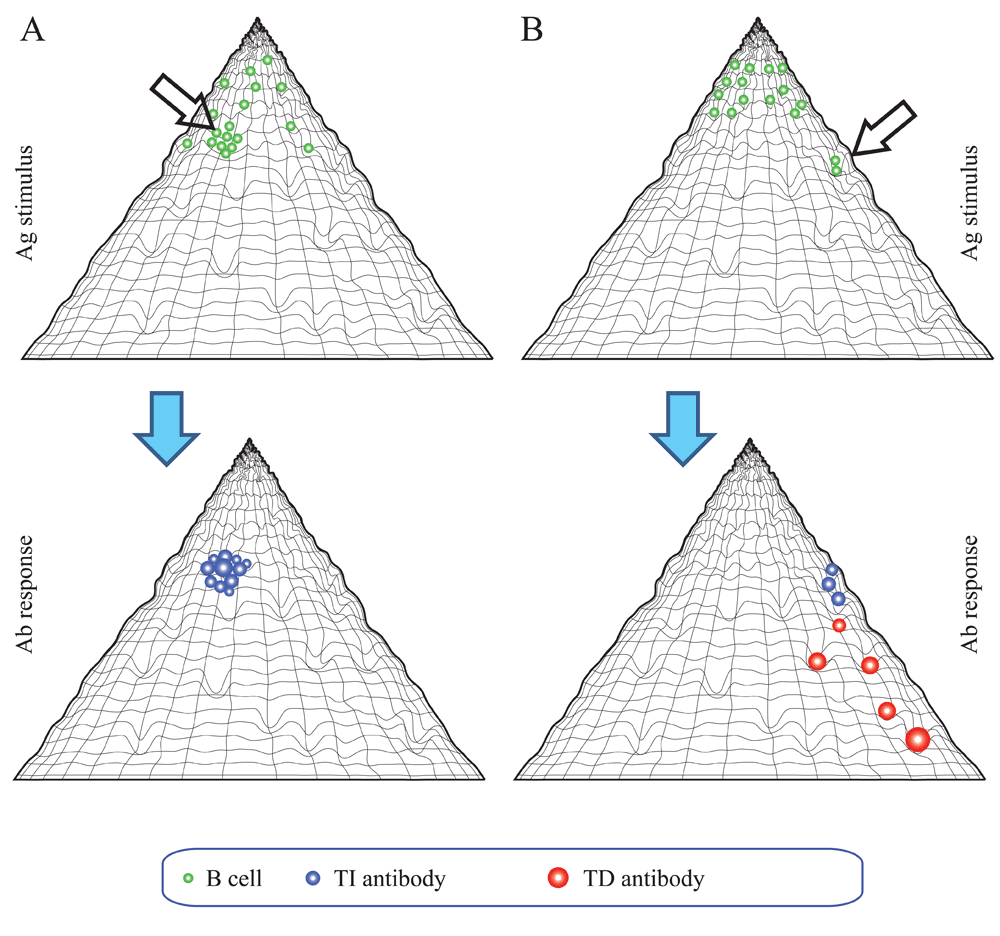
(A) Thymus-independent responses are characterized by antibodies of lower affinity. A closely knit network of antibody forming cells respond as an elastic net. (B) Thymus-dependent responses are characterized by the development of antibodies with increasing affinity. This corresponds to a wave of interactions sweeping down the slope of the fountain.
It is important to note the relative identity of binding partners in this landscape: an antibody can bind to antigens but can also be the target of another antibody. The unique binding site of an antibody, the paratope that determines idiotype (identity as a binder), is itself part of the binding landscape. This can be especially important for antibodies with high intrinsic specificity rate (Zheng & Wang, 2015) that are eager to bind and reach their conformation with lowest energy level. I suggest that in the absence of antigen these high affinity binders could be refrained from non-specific binding by engaging their binding sites in lower affinity interactions.
Blood carries potentially all the molecules expressed in the host, along with those originating from the environment. To ensure that all these molecules find their intended binding partners a regulated binding landscape evolved: the clonal immune system. The clonal humoral immune system generates a regulated binding landscape by constantly sampling the molecular environment via a huge repertoire of B-cell receptors and by the generation of antibodies with a wide range of specificities and affinities. To allow the thermodynamic representation of this multitude of interactions, I show here that this landscape can be visualized as a binding fountain, in an analogy with the folding funnel energy landscape. The binding fountain landscape is an anchored conformation landscape with the conformational entropic penalty of binding anchoring the axis of free energy. Binding sites appear as impressions of a hypersurface, which represents thermodynamically favorable binding events with negative ΔG values. This landscape can be further projected into a multidimensional space of the antibody-antigen interaction network. This systemic perception and interpretation of antibody function is expected to help reveal how the immune system actually functions as a whole, a thermodynamic network of interactions, taking us closer to the systems level understanding of adaptive humoral immunity.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the opinion article discussed accurately in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
Partly
Are arguments sufficiently supported by evidence from the published literature?
Partly
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Thermodynamics, immunology, kinetics
Is the topic of the opinion article discussed accurately in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Are arguments sufficiently supported by evidence from the published literature?
Yes
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 15 Feb 18 |
read | |
|
Version 1 11 Sep 17 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)