Keywords
placental malaria, birth weight, leptin, Insulin-like growth factor 1, IGF-1,
This article is included in the Emerging Diseases and Outbreaks gateway.
placental malaria, birth weight, leptin, Insulin-like growth factor 1, IGF-1,
Malaria during pregnancy is a major public health concern, especially in sub-Saharan Africa where there are approximately 125 million pregnant African women living in malaria-endemic regions. Almost one fifth of these pregnant women are at risk of malaria (Dellicour et al., 2010; Desai et al., 2007). Malaria during pregnancy is the main cause of maternal, perinatal and neonatal adverse effects, especially anemia and low birth weight (LBW) (Ahmed et al., 2014; Menendez et al., 2000; Rogerson et al., 2003).
The pathogenesis of placental malaria and LBW is not fully understood. Leptin is a hormone secreted mainly by adipocytes (Zhang et al., 1994) that can potentiate inflammation by enhancing macrophage phagocytosis (Loffreda et al., 1998; Pacifico et al., 2006). Previous reports have shown that leptin levels were decreased during malarial attack in pregnant women (Conroy et al., 2011), and that these decreased leptin levels were associated with placental malaria infection, as well as low birth weight (Kabyemela et al., 2008a; Kabyemela et al., 2008b).
Insulin-like growth factor-I (IGF-1), also called somatomedin C, is a polypeptide with a sequence similar to that of insulin (Rinderknecht & Humbel, 1978). Recently, maternal and umbilical cord blood levels of IGF-1were investigated in malaria during pregnancy as possible determinants of birth weight (Ayoola et al., 2012; Umbers et al., 2011).
Research on malaria during pregnancy and its associated adverse effects e.g. LBW is highly valuable for researchers and clinicians as it can yield basic data needed for the future vaccine.
Pregnant Sudanese women are susceptible to malaria regardless of their age and parity, and malaria is associated with increased maternal mortality, anemia, LBW, and stillbirths (Adam et al., 2005a; Ali et al., 2011; Bader et al., 2010; Mohammed et al., 2013).
Central Sudan is characterized by unstable malaria transmission, and P. falciparum is the main malaria parasite species reported in the area (Malik et al., 2004). To add to the research on placental malaria during pregnancy that has been carried out already (Alim et al., 2015; Mostafa et al., 2015; Salih et al., 2011), the current study was conducted in central Sudan to investigate the maternal and umbilical cord levels of leptin and IGF-1in placental malaria infection.
A cross-sectional study was conducted from August to October 2014 in the labor ward of the Medani Maternity Hospital. After signing an informed consent form, women with singleton pregnancies were approached to participate in the study. Women with twins, hypertension, diabetes mellitus and antepartum hemorrhage were excluded from the study. Socio-demographic data (age, parity, residence and gestational age) and data on obstetric history, medical history, and bed net use were gathered using a structured questionnaire that was completed by a trained medical officer in the local language (Arabic). Maternal weight and height were measured and body mass index (BMI) was calculated and expressed as weight(kg)/height(m)2. Maternal hemoglobin concentrations were estimated (HemoCue AB, Angelhom, Sweden). Newborns were weighed immediately following birth using the Salter scale and the sex of each newborn was recorded. The total sample size was calculated assuming that at least 23% of parturient women would have placental malaria infection. To have over 80% power to detect a difference of 5% at α = 0.05, we recruited 175 women. We assumed that 10% of women might not respond or have incomplete data.
Maternal, placental, and umbilical cord blood films were prepared for testing. Slides were stained by 10% Giemsa. In the slides positive for malaria the number of asexual parasites was counted per 200 leukocytes, assuming a leukocyte count of 8000 leukocytes per μl (for thick films) or per 1000 red blood cells (for thin films). Blood films were considered negative if no parasites were detected in 100 oil immersion fields of a thick blood film.
The maternal and umbilical cord blood was then allowed to clot, centrifuged for 10 minutes at 3000 rpm and the serum separated and stored at - 20°C until further analysis.
The details on placental histology have been mentioned previously (Alim et al., 2015; Mostafa et al., 2015; Salih et al., 2011). In summary, a 3cm3 full thickness sample was obtained from the maternal surface approximately half the distance between the umbilical cord and the edge of the placenta. The placental biopsy samples were immediately placed in 10% neutral buffered formalin. Buffer was used to prevent formation of formalin pigment, which might be difficult to differentiate from malaria pigment (Bulmer et al., 1993a). The placental biopsy samples were then embedded in paraffin wax/sections. In every case, the thick paraffin sections were stained with hematoxylin-eosin and Giemsa stains. Slides were read by a pathologist who remained blind to the clinical characteristics of each of these samples. Placental malaria infection was characterized using parameters previously described by Bulmer et al.: uninfected (no parasites or pigment), acute (parasites in intervillous spaces), chronic (parasites in maternal erythrocytes and pigment in fibrin, or cells within fibrin and/or chorionic villous syncytiotrophoblast or stroma), and previous (no parasites, and pigment confined to fibrin or cells within fibrin) (Bulmer et al., 1993b).
Maternal and umbilical cord serum levels of leptin and IGF-1 were measured using ELISA Kits and the manufacturers' instructions were strictly followed (DRG Diagnostics, Marburg, Germany).
The data analyses were performed using SPSS statistical software for windows (version 18.0). Statistical significance was set at P value < 0.05. To compare means and proportions between groups, student’s t-test and Chi-square test were used, respectively. For non-parametric data significant differences in means between two groups were calculated using the Mann-Whitney test. Univariate and multivariate analyses were performed with a logistic regression model where placental malaria infection was the dependent variable and expected risk factors (mother’s age, parity, mother’s weight, mother’s haemoglobin, educational level, residence, use of bed net, antenatal care attendance, use of folic acids supplements and mother’s serum leptin and IGF-1) were the independent variables. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. Linear regression models were set to investigate the factors associated with the level of mother's haemoglobin and birth weight. Predictor variables for mother’s haemoglobin model were; antenatal care attendance, parity, BMI, maternal serum leptin and use of folic acid supplements. Predictor variables for birth weight were; mother’s age, antenatal care attendance, mother’s haemoglobin, placental malaria, mother’s height, delivery gestational age.
The study received ethical clearance from the Research Board at the Faculty of Medicine, University of Khartoum, Sudan. (Approval number: 2-2011).
Pregnant women who delivered at Medani Maternity hospital from August through to October 2014 were recruited for this study, following written informed consent. All participants finally included in the study had to satisfy the selection criteria and have none of the exclusion criteria.
Out of the 175 women enrolled in the study, 77 (44%) were primiparae. The majority of them (105; 60.0%) had rural residency and used bed nets (157; 89.7%) during the index pregnancy (Dataset 1 (Elsheikh et al., 2017)).
In total, 36 (20.6%) had blood group A, 21 (12%) had blood group B, four (2.3%) had blood group AB, and 113 (64.6%) had blood group O. The mean (SD) hemoglobin level was 10.2 (1.1) g/dl, and 129 (73.7%) of the women were anemic (hemoglobin <11 g/dl).Eighteen (10.5%) women delivered low-birth weight neonates (<2500 g)(Dataset 1 (Elsheikh et al., 2017)).
Forty-eight women were infected with placental malaria (PM+), and 127 were free from the disease (PM−). Out of the 48, 2(4%) of PM+ patients had active infection, 3(6%) had chronic and 43 (90%) past infection (Dataset 1 (Elsheikh et al., 2017)).
The mean age (± SD) of PM+ patients was 26± 4.8 years and ranged from 17 to 38 years. The mean age (± SD) of PM−patients was 28± 6 years with a range of 18 to 41 years (Table 1). Younger women (25 – 30 years) were significantly more often infected with PM (P = 0.02)(Dataset 1 (Elsheikh et al., 2017)).
Data are means (SD). Women with placental malaria (PM) infection were on average younger (p= 0.02) and delivered lighter neonates than uninfected women (p= 0.054, not significant).
Moreover, babies born to women with PM tended to be in the LBW (< 2500 g) category more often than those born to non-infected women, but the p-value failed to reach the significance level (p = 0.054). Maternal weight, BMI, gravidity, gestational age at delivery and hemoglobin levels were unchanged significantly between groups (Dataset 1 (Elsheikh et al., 2017)).
Low birth weight (< 2500g) was significantly associated with placental malaria (N = 172, p = 0.006)(Dataset 1 (Elsheikh et al., 2017)).
Univariate and multivariate analysis demonstrated that only the mother’s age and parity were significant risk factors (p-values were 0.008 for mother’s age and 0.009 for parity).
Younger mothers and primigravidae had a higher risk for PM. The risk of infection was lower for older mothers, with an odds ratio (OR) of 0.881 (p = 0.008, 95%CI: 0.802 – 0.968). For each additional year in age, the odds of getting placental malaria lowered by a factor of 0.881. The OR for parity was 4.3 (p = 0.009, 95%CI: 1.45 – 12.998) (Table 2, Dataset 1 (Elsheikh et al., 2017)).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95.0% CI | P-value | OR | 95.0% CI | P-value | |
| Maternal age | 0.934 | 0.879 – 0.993 | 0.028* | 0.881 | 0.802 – 0.968 | 0.008*1 |
| Parity (primigravidae or multigravidae) | 1.23 | 0.621 – 2.44 | 0.553 | 4.345 | 1.452 – 12.998 | 0.009*2 |
| Maternal weight | 0.953 | 0.894 – 1.016 | 0.137 | 0.933 | 0.858 – 1.014 | 0.104 |
| Maternal haemoglobin | 1.011 | 0.753 – 1.357 | 0.944 | 1.257 | 0.827 – 1.911 | 0.284 |
| Education level | 0.000 | 0.00 | 0.999 | 0.000 | 0.000 | 0.999 |
| Residence | 1.26 | 0.63 – 2.51 | 0.517 | 1.342 | 0.536 – 3.359 | 0.529 |
| Use of bed net | 0.905 | 0.301 – 2.7 | 0.859 | 0.795 | 0.139 – 4.560 | 0.797 |
| Antenatal care attendance | 0.859 | 0.433 – 1.702 | 0.662 | 1.581 | 0.551 – 4.539 | 0.394 |
| Use of folic acid supplements | 0.620 | 0.142 – 2.700 | 0.524 | 0.389 | 0.045 – 3.343 | 0.390 |
| Maternal serum leptin | 0.987 | 0.958 – 1.02 | 0.388 | 0.980 | 0.938 – 1.023 | 0.355 |
| Maternal serum IGF-1 | 0.999 | 0.996 – 1.00 | 0.430 | 0.999 | 0.995 – 1.002 | 0.498 |
*: Maternal age showed statistically significant association with placental malaria in univariate and multivariate analysis.
*1: Adding one year in age decreases the risk of getting placental malaria by about 11.9%.
*2: The risk for primigravidae to get placental malaria is 4.3 higher than for multigravidae.
The levels of leptin were higher in LBW infants and their mothers, whilst IGF-1levels were higher in normal weight infants and their mothers. However, these differences failed to reach statistical significance (Table 3). Non-infected mothers and their infants showed higher levels of leptin and IGF-1 than infected ones (Figure 1 and Figure 2), but these differences also failed to reach statistical significance (Table 4, Dataset 1 (Elsheikh et al., 2017)).
The data is shown as median (interquartile range).
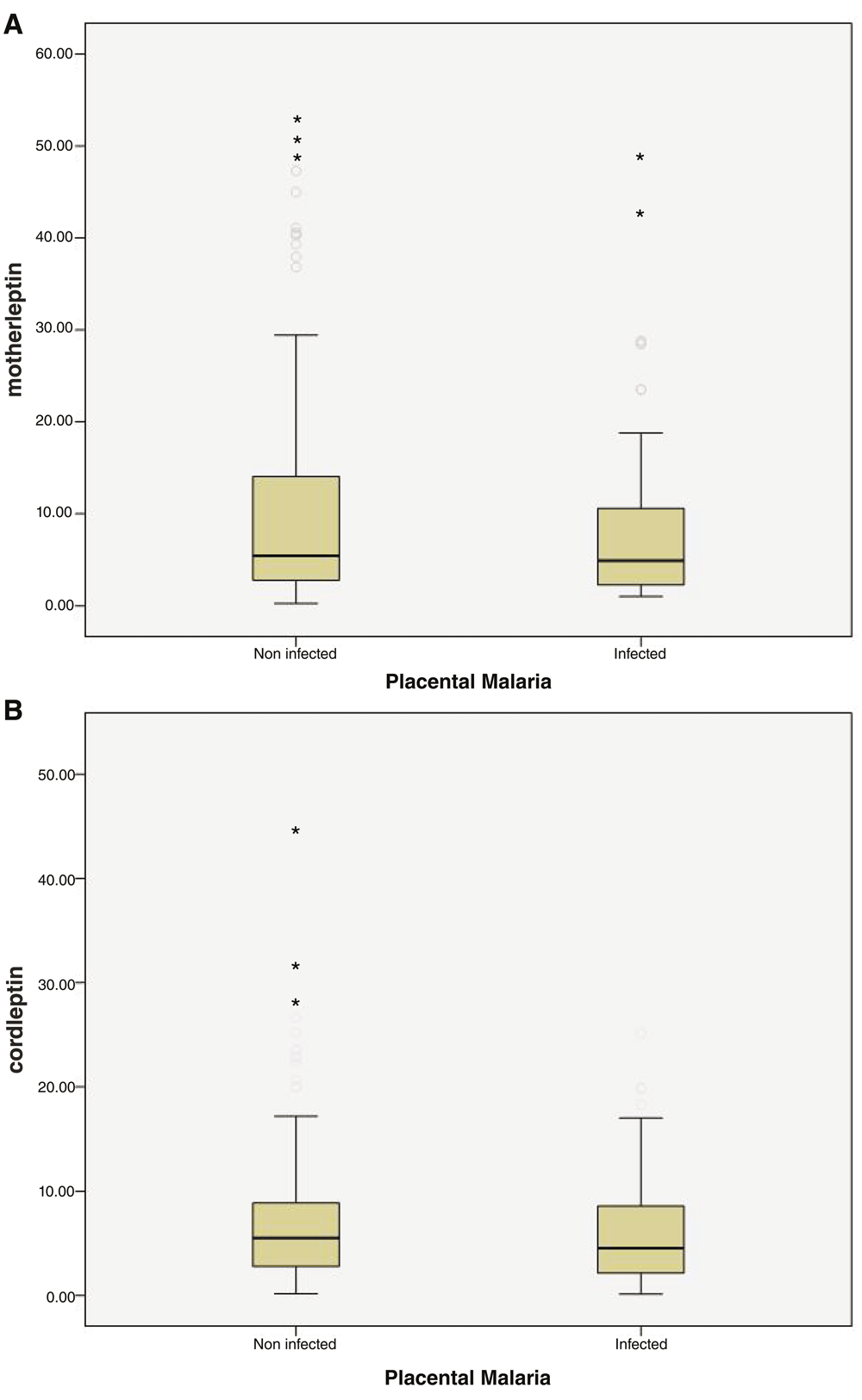
(A) Boxplot of maternal serum leptin concentrations in women with and without placental malaria. (B) Boxplot of umbilical cord serum leptin concentrations in women with and without placental malaria. Maternal and cord leptin levels were measured in serum samples of non- (PM−, n= 122, 5missed samples) and women with placental malaria (PM+, n= 47, 1missed sample). The Mann-Whitney test was used to compare the levels of maternal and umbilical cord leptin between the two groups. PM+ women showed lower levels of maternal and cord leptin but these differences were not statistically significant (Dataset 1).
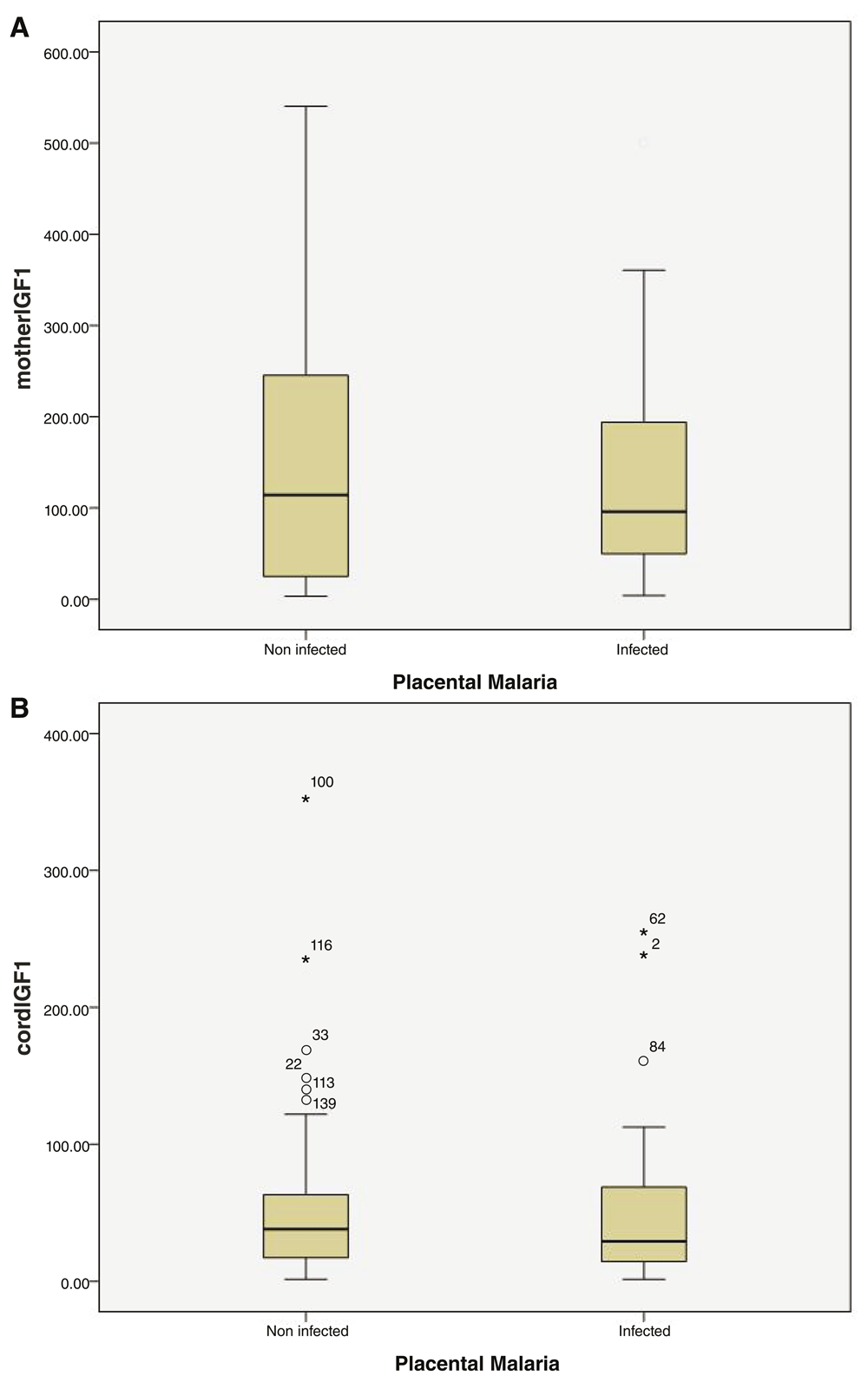
(A) Boxplot of maternal insulin-like growth factor-I (IGF-I) concentrations in women with and without placental malaria. (B) Boxplot of umbilical cord IGF-I concentrations in women with and without placental malaria. Maternal and cord IGF-1 levels were measured in serum samples of non-infected women (PM−, n= 122, 5missed samples) and women with placental malaria (PM+, n= 47, 1missed sample). The Mann-Whitney test was used to compare the levels of maternal and umbilical cordIGF-1 between the two groups. PM+ women showed lower levels of maternal and cord IGF-I but these differences were not statistically significant (Dataset 1).
The data is shown as median (interquartile range).
Linear regression analysis showed that gestational age had the strongest positive effect on birth weight (β = 0.191, p = 0.01), followed by antenatal care attendance (p= 0.043) and mother’s age (p= 0.85, not significant) (Table 5, Dataset 1 (Elsheikh et al., 2017)).
| Birth Weight | Maternal Haemoglobin | |||||
|---|---|---|---|---|---|---|
| B Coefficients | Std. Error | P | B Coefficients | Std. Error | P | |
| Maternal age | 0.139 | 0.007 | 0.085 | |||
| Antenatal care attendance | -0.176 | 0.092 | 0.043* | -0.365 | 0.162 | 0.000 |
| Parity | 0.172 | 0.153 | 0.015* | |||
| BMI | 0.090 | 0.036 | 0.193 | |||
| Maternal haemoglobin | 0.098 | 0.039 | 0.255 | |||
| Placental malaria | -0.099 | 0.089 | 0.196 | |||
| Maternal serum leptin | -0.221 | 0.069 | 0.002* | |||
| Use of folic acid supplements | -0.123 | 0.703 | 0.080 | |||
| Maternal height | 0.078 | 0.006 | 0.331 | |||
| Delivery gestational age | 0.191 | 0.026 | 0.018* | |||
The main findings of the current study are that placental malaria is significantly associated with LBW. Younger mothers and primigravidae had a higher risk for PM infection. There was no significant difference in leptin and IGF-1 levels between PM+ and PM− women and their infants, as well as between LBW infants and their mothers and normal weight infants and their mothers. Maternal and umbilical cord leptin and IGF-1 levels were not associated with birth weight.
Our results coincide with what has been reported previously about LBW being significantly associated with placental malaria (Albitiet et al., 2010; Aribodor et al., 2009; Menendez et al., 2000). Although some studies conducted in different areas in Sudan did not report this association. A study conducted in Gadarif hospital in an area characterized by unstable malaria transmission in eastern Sudan found that placental malaria affects pregnant women regardless of their parity and had no effects on birth weight (Salih et al., 2011). Another study showed that, while placental malaria infections that were positive by histology were not associated with LBW, submicroscopic malaria infections (diagnosed by PCR) were (Mohammed et al., 2013). Moreover, a study conducted by Batran et al. (Batran et al., 2013) found that placental infections had no effect on LBW or anemia.
Many other studies also observed that the mother’s age and parity are risk factors for placental malaria (Falade et al., 2010; Ndeserua et al., 2015; Ojurongbe et al., 2010; Tako et al., 2005; Walker et al., 2013), which is in contrast with our previously published results (Adam et al., 2005a; Adam et al., 2005b; Adam et al., 2007; Adam et al., 2009; Adam et al., 2011; Albiti et al., 2010).
The study showed that leptin levels were higher in non-infected mothers and their infants than in infected ones, however these differences failed to reach statistical significance. This concurs with a study conducted in Malawi which found a significant reduction of leptin levels in mothers infected with PM, and according to this the authors suggested leptin to be an informative biomarker for diagnosis of PM (Conroy et al., 2011). Another study in Tanzania also reported the same finding (Kabyemela et al., 2008a). Kabyemela and his colleges (Kabyemela et al., 2008b) also investigated the effect of PM in the relationship between cord leptin levels and birth weight. They found that cord leptin had a strong positive relationship with birth weight in offspring of PM− women (P = 0.02 to P < 0.0001) but not in offspring of PM+ women, however the differences did not reach significance level.
Although the current study failed to detect a significant differences in IGF-1 levels between PM+ and PM− women, one study have shown that placental malaria-associated inflammation disturbs maternal and fetal levels of IGFs, which regulate fetal growth (Umbers et al., 2011). This may be one mechanism by which placental malaria leads to fetal growth restriction, but this study did not report the effect size of PM on IGF-1 levels.
It is worth mentioning that the differences between the current study and later ones that reported low leptin (Conroy et al., 2011; Kabyemela et al., 2008a; Kabyemela et al., 2008b) and IGF-1 levels in maternal and umbilical cord serum (Umbers et al., 2011) might be due to the duration of malaria infection itself. While the majority of malaria infections in the current study were past placental infection (4% of PM+ patients had active infection, 6% had chronic and 90% past infection), the later studies reported results from active placental infections. Furthermore, submicroscopic placental malaria infection using PCR (Polymerase Chain Reaction) was not investigated in the current study.
We have recently reported that women with submicroscopic malaria were at higher risk to have LBW (Mohammed et al., 2013). Likewise Adegnika et al. have reported that microscopic and submicroscopic P. falciparum infection, but not inflammation (C-reactive protein) caused by infection, is associated with low birth weight (Adegnika et al., 2006).
The main limitation of this study was that we relied on a single measure of leptin and IGF-1 levels at delivery; however it was not feasible to obtain the levels of these infants before birth. Most studies relating umbilical cord blood IGF-1levels and birth weight reported single measurements at birth (Ong et al., 2000; Yang & Yu, 2000), and findings are similar. Another limitation was that we did not measure the concentrations of any other components of the IGF axis, including growth hormone, insulin, IGF binding proteins (IGFBP1-5), and receptors (IGF-1R and 2R) to further establish the potential implication of the IGF system in fetal growth. More limitation is that although we are interested only in the biologically active IGF-1 (free form), the ELISA technique used in this study measures the total amount of IGF-1 (free and protein-bound) in serum.
The current study shows that there is no statistically significant difference in the levels of maternal and cord leptin and in the levels of IGF-1 between PM+ women and PM – women, and between women who delivered LBW infants and those who delivered normal weight ones. The main finding is that placental malaria is significantly associated with LBW. Neither maternal and umbilical cord leptin levels nor IGF-1 levels were associated with birth weight.
Dataset 1. The file contains data on socio-demographics (age, parity, residence and gestational age), obstetric and medical history, bed net use, maternal weight, height and BMI, maternal hemoglobin, infant birth weights and maternal and umbilical cord leptin and IGF-1 levels for each participant. HME has confirmed that all raw data provided with this manuscript has been de-identified. DOI, 10.5256/f1000research.10641.d158697 (Elsheikh et al., 2017)
HME and IA designed the experiments. IA conceived the study and participated in study coordination. EME conducted the clinical work. AAM performed the pathological analysis. HME carried out the laboratory work, statistical analysis and study coordination. HME, IA and MIE prepared the first draft of the manuscript. MIE contributed to the experimental design. AHK contributed in statistical analysis. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
This work was partially funded by the Ministry of Higher Education (Khartoum, Sudan).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We are grateful to the staff of the Department Of Immunology of the National Laboratory of Public Health (Khartoum, Sudan), Dr. Kawther Abdel Galeil, Mohammed Salih, Mohammed Karrar Abdalla, Sayed Mutasim and Omer Mahjoob, who participated in laboratory analysis. We are also grateful to Ms. Azza Osman Mohamed Osman for statistical consultation.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology, Chemical Pathology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 22 May 17 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)