Keywords
Dystrophin, Herceptin/Trastuzumab, Duchenne muscular dystrophy, Smooth muscle, Blood vessels, Neuregulin, ERBB2/4, HER2/4
Dystrophin, Herceptin/Trastuzumab, Duchenne muscular dystrophy, Smooth muscle, Blood vessels, Neuregulin, ERBB2/4, HER2/4
In this revised version of the article, the reviewers concerns were addressed and additional results were included (new Figure 2; the old figures are unchanged but have been re-numbered). The new results reflect additional similarities between the dystrophin deficient mdx mouse and the conditional knock-out mouse where neuregulin/ERBB (NRG/ERBB) signalling is specifically abolished in skeletal muscles. The raw data for Figure 2 is provided in Dataset 2. These similarities not only support the in vitro observations that NRG/ERBB signalling is required for dystrophin expression, they also argue against alternative regulatory mechanisms that would compensate in vivo NRG/ERBB signalling in the knock-out mouse. Additional published studies are included and discussed to explain why the NRG/ERBB dKO mice in contrast to the mdx mice do not show a dystrophic pathology and why our results suggest that the loss of dystrophin in vascular smooth muscle, resulting in aberrant vasoregulation of blood and lymph vessels, may be the cause of/be involved in, the onset of Duchenne and Becker muscular dystropy.
See the author's detailed response to the review by Kay E. Davies
See the author's detailed response to the review by Alberto Malerba
See the author's detailed response to the review by Vihang Narkar
Signaling from neuregulin 1 (NRG), through its epidermal growth factor (EGF) family of receptors ERBB1-4, has major functions in several organs such as heart, breast, and nervous system including central and peripheral synapses. The role of NRG signaling in skeletal muscle has been controversial. To investigate signaling events in muscle fibers, myotubes formed by fusion of myoblasts, are routinely used as the in vitro equivalent of muscle fibers. We already reported that in myotubes, formed from C2C12 myoblasts, NRG signaling through ERBB2/4 heterodimeric receptors phosphorylates α-dystrobrevin 1 (α-DB1)1, one of the components of the dystrophin associated protein complex (DAPC). DAPC links the intracellular actin cytoskeleton to the extracellular matrix and is thereby thought to provide structural stability during muscular activity. DAPC, apart from containing dystrophin, a 427 kDa protein, consists of several other proteins such as α- and β-dystrobrevins, dystroglycans, sarcoglycans, sarcospan, syntrophins, and laminins. At the neuromuscular synapse, the DAPC is also formed with utrophin, also a 427 kDa protein, instead of dystrophin. The phosphorylation of α-DB1 through NRG/ERBB signaling stabilized acetylcholine receptors (AChRs) at the neuromuscular synapse1,2.
Duchenne and Becker muscular dystrophy (D/BMD) patients have mutation(s) in the dystrophin gene, resulting in the expression of a truncated dystrophin protein3–6. The main body of research on DMD argues for the lack of dystrophin in skeletal muscle as the cause for DMD. In mice, apart from muscular dystrophy, absence of dystrophin causes neuromuscular junction (NMJ) fragmentation7 similar to the NMJ fragmentation associated with a loss of NRG/ERBB signaling1. Lack of dystrophin, besides causing muscular dystrophies, results in cardiomyopathy8 and is also responsible for several disease states in the brain6. The importance of NRG signaling for normal cardiac development in mice was firmly established by the fact that ablation of NRG, ERBB4, or ERBB2 resulted in premature death during midgestation9–11. In cardiac muscle NRG/ERBB4 signaling is sufficient for cardiomyocyte proliferation and repair of heart injury12, but knowledge of the detailed signaling mechanisms and the target proteins through which this was achieved are lacking. The aim of this investigation was to identify the function of NRG/ERBB signaling in muscle and, as it phosphorylated α-DB1 in the DAPC complex, determine if it had a functional role in muscular dystrophy by identifying downstream signaling targets.
Erbb2/4 dKO and loxP flanked Erbb2/4 myoblasts (a kind gift from M. Courtet, and previously described1) as well as,α-dystrobrevin KO (α-db-/-) myoblasts13 (kind gift from B. Pawlikowski and M. Maimone (Upstate Medical University, State University of New York, Syracuse, NY) and C2C12 cells were cultured on laminin-coated dishes (Roche) and upon reaching 70–80% confluency, were allowed to form myotubes by changing to differentiation media (2% horse serum, 1% penicillin/streptomycin (Sigma-Aldrich), DMEM (Sigma-Aldrich)). Myoblasts were transfected with expression constructs using Fugene HD (Promega, Madison, Wisconsin) according to their protocol when they reached 70% confluency. Expression constructs for GFP-α-DB1 and GFP-α-DB1-P3 were gifts from J.R. Sanes (Harvard University, Cambridge, MA) and C. Mouslim (University of Michigan, Ann Arbor, MI), respectively. Transfected myoblasts were then sorted for EGFP+ cells using the influx cell sorter (Becton Dickinson). To obtain more than 90% positive EGFP+ population, myoblasts were sorted at least twice with a cell culture phase (3–4 passages) between each sort.
Myotubes from 10-cm culture dishes were harvested in 600 μl lysis buffer and protein complexes were immunoprecipitated as described previously1,14 with modifications. In brief, myotubes harvested in ice-cold lysis buffer (10 mM Na3PO4, pH 7.8, 150 mM NaCl, 5 mM EDTA, 1 mM EGTA, 1% Triton X-100, protease inhibitor mixture (Roche), and phosphatase inhibitors Pic1 and Pic2 (Sigma-Aldrich)) were homogenized in a Dounce homogenizer and incubated for 3 h at 4°C with protein G-coupled mouse monoclonal syntrophin antibody 1351 (4 μl/80 μl protein G beads, Abcam, catalog number ab11425). Beads were then washed in lysis buffer containing protease inhibitors, but without Triton X-100, resuspended in 3 x SDS loading buffer (150 mM Tris-HCl [pH 6.8], 300 mM dithiothreitol [added just before use], 6% SDS, 0.3% bromophenol blue, and 30% glycerol), and denatured (94°C, 3 min) before loading on an 8% acrylamide/0.8% bis-acrylamide (Figure 1 A & B) or 6–8% gradient/0.8% bis-acrylamide (Figure 2B) SDS-PAGE gels buffered with Tris-glycine.
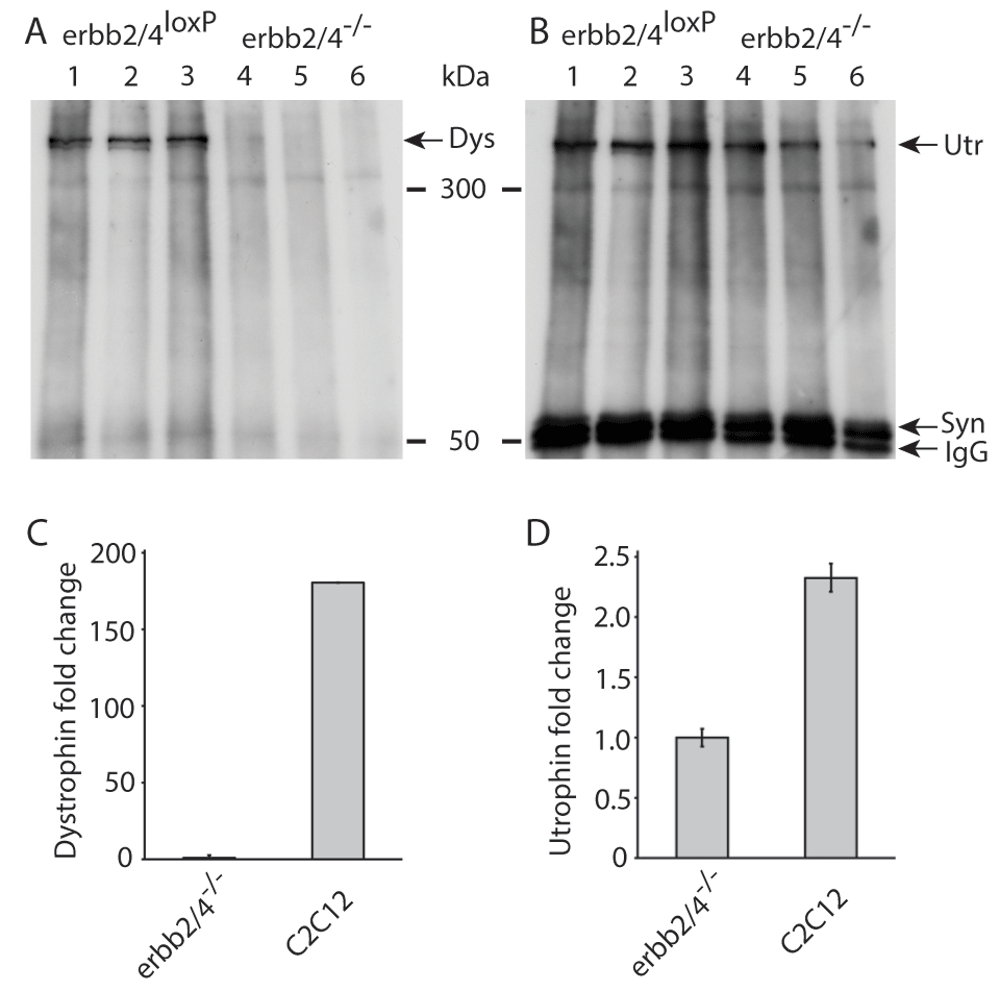
(A and B), Western blots of immunoprecipitated DAPC proteins from myotubes with loxP flanked exons of Erbb2 and Erbb4 gene (lanes 1 to 3) and Cre mediated knock-out of Erbb2 and Erbb4 genes (lanes 4 to 6) detected with dystrophin (A) and utrophin (B) antibodies. Lanes 1 to 3 and 4 to 6 each represent the same experiment performed independently and loaded on the same gel. The lower part of the blot shows detection of syntrophin that served as a loading control. Immunoglobulin G (IgG) detected is the syntrophin antibody used for the immunoprecipitation. As the western blot in (A) was stripped of antibodies and used in (B), the loading control in (B) applies to both A and B. (C and D) qPCR data of dystrophin (C) and utrophin (D) levels in C2C12 myotubes, relative to Erbb2/4 dKO (erbb2/4-/-) myotubes. Expression levels were normalized to ribosomal protein L8 (rL8) expression. This experiment was performed at least twice with similar results. This figure was previously published in a patent (Patent Link: WO 2017/036852 A1), but the copyright is the author’s own.
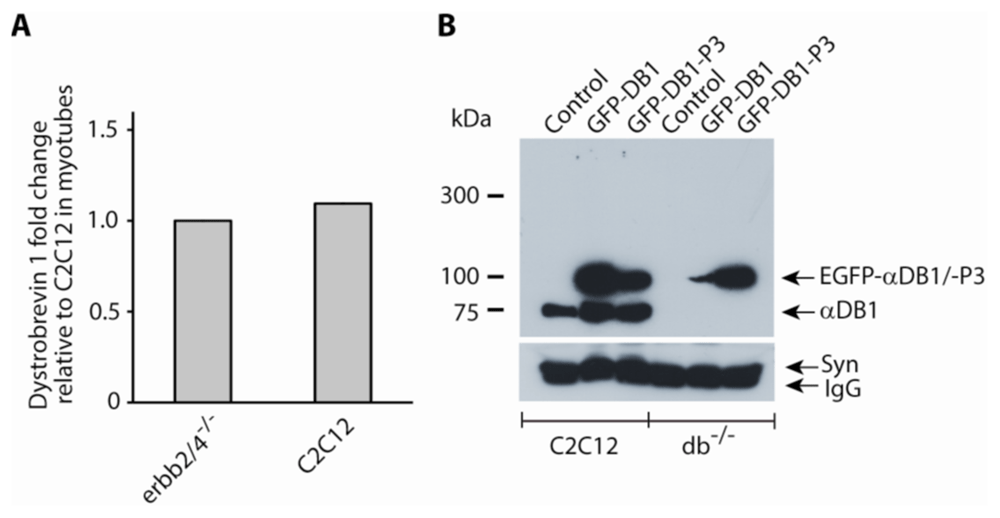
(A) qPCR data of α-dystrobrevin 1 expression in Erbb2/4 dKO (erbb2/4-/-) myotubes relative to C2C12 myotubes. Expression levels were normalized to ribosomal protein L8 (rL8) expression. This experiment was performed at least twice and gave similar results. (B) Western blots of control and transfected C2C12 and dystrobrevin KO (db-/-) myotubes following IP of the DAPC and detection of dystrobrevin and EGFP-dystrobrevins (GFP-DB1 & GFP-DB1-P3 with 3 mutated tyrosine phosphorylation sites) with anti-α-dystrobrevin 1 antibodies. The EGFP-DB1 transfected dystrobrevin KO myotubes appears to have lost the expression construct however a repeat of the experiment showed expression of the transfected construct (see raw data). Following syntrophin detection (lower panel), the blot was stripped and used for dystrobrevin detection (upper panel). The lower panel in figure 2b (syntrophin detection) was previously published in a patent (Patent Link: WO 2017/036852 A1), but the copyright is the author’s own.
Gels were transferred onto PVDF membranes (Millipore) and subject to ECL (Thermo Fisher Scientific) development after incubation with primary and secondary antibodies. BSA (3%) was used as a blocking reagent. The following primary antibodies were used: rabbit anti-dystrophin (H300) polyclonal (diluted 1:400, catalog number sc-15376) and mouse anti-utrophin (55) monoclonal (diluted 1:400, catalog number sc-136116) were from Santa Cruz Biotechnology, Inc., mouse monoclonal anti-syntrophin 1351 (4 μl antibody/80 μl protein G beads for lysate from a 10 cm culture dish of myotubes, catalog number ab11425) from abcam, rabbit anti-α-dystrobrevin (1:1,500; a kind gift of D.J. Blake and R. Nawrotzki, University of Cardiff, Wales, UK) and rabbit anti-α-syntrophin 258 (5 μg/ml for Westerns; a kind gift from Stanly C. Froehner and Marvin Adams, University of Washington, Seattle, WA). Goat anti-mouse IgG-HRP (catalog number sc-2005) and goat anti-rabbit IgG-HRP (catalog number sc-2004) secondary antibodies (Santa Cruz Biotechnology, Inc.) were used at a 1:5,000 dilution.
RNA isolation and qPCR were performed as previously described15 and the 2−ΔΔCt method was used to analyze relative changes in gene expression. RNA from myotube cultures was isolated with TRIzol (Invitrogen) according to their protocol. DNase I (Promega) treatment and reverse transcription was performed on 1 µg total RNA with random primers and superscript reverse transcriptase from Invitrogen according to their protocol. cDNA was diluted 1:5 before use in qPCR, which was performed with SyBR Green mix (Applied Biosystems) using the Applied Biosystems StepOne machine with two-step PCR (60°C, 1 min and 95°C 15 s) for 40 cycles using the standard program. The quantitative PCR mix was prepared as follows: 12.5 μl SyBR Green mix, 2.5 μl of a 3 µM solution each of forward and reverse primer, 1 μl of diluted cDNA and made up to 25 µl total volume with sterile water. Each sample for real time PCR was done in triplicate and the mean of the resulting three values were taken. The following primers, designed to recognize exons with at least one intron in between for each primer pair, were used for dystrophin, utrophin, dystrobrevin and ribosomal protein L8 (rL8) amplifications: dystrophin forward, 5′-GATGATGAACATTTGTTAATCCAGC-3′ and reverse, 5′-CATATTCTGCTTGCAGATTCCTG-3′; utrophin forward, 5′-CTAAACTCCTGCGGCAGCAC-3′ and reverse, '-GTGTCAAGTGAGTAGCTCAATGC-3′, dystrobrevin (exon 22) forward, 5’-AACCCAACCTTGCTGGCAGAAC-3’ and reverse (exon 26), 5’-AGGCAGATGCTGAACGGATG-3’ and rL8 (normalization gene) forward, 5′-ACTGGACAGTTCGTGTACTG-3′ and reverse, 5′-GCTTCACTCGAGTCTTCTTG-3′.
We previously reported that there were two isoforms of α-DB1 associated with the DAPC, a 75 kDa and an 89 kDa protein1. These two α-DB1 proteins correspond to the previously reported proteins between 66-97 kDa with the smaller protein containing more phosphotyrosine16. We also demonstrated that ablation of ERBB2/4 receptors resulted in a lack of phosphorylation of the 75 kDa protein1 and that lower levels of this α-DB1 isoform associated with the DAPC compared to the 89 kDa isoform. However in myotubes with intact ERBB2/4 receptors, such as in C2C12 myotubes, we did not observe such a difference in the levels of these two isoforms1. In the absence of NRG/ErbB signalling we also observed AChR cluster fragmentation both in vitro in myotubes and in vivo in muscle fibers. Similar observations were made in mdx mice deficient in dystrophin. Mdx mice have a reduction in all the dystrobrevin isoforms at the sarcolemma17, and thus in the DAPC, as well as AChR cluster fragmentation7 in muscle compared to wild-type mice. These observations suggested that a lack or reduced level of dystrophin may have caused the reduction in the 75 kDa α-DB1 protein associated with the DAPC, and AChR cluster fragmentation, in the Erbb2/4 dKO myotubes implying that one of the targets of NRG/ERBB signaling is dystrophin.
To investigate this, Erbb2/4 dKO myotubes derived from immortalized Erbb2/4 dKO myoblasts1 and myotubes formed from myoblasts containing loxP flanked Erbb2/4 genes, before transfection with Cre recombinase, were used. Both, ERBB2 and ERBB4 receptors were ablated in skeletal muscle to eliminate NRG signaling1, because ERBB4 receptors, apart from forming heterodimers, can also form homodimers and ERBB2 can heterodimerize with ERBB318. Furthermore, as cultured myotubes secrete NRG19, the external addition of NRG was not necessary, as demonstrated for phosphorylation of α-dystrobrevin 1 by NRG/ERBB1 signaling.
In three independent experiments (Figure 1A) each using myotubes formed from a different myoblast clone with loxP-flanked Erbb2/4 genes, dystrophin was clearly detected (lanes 1–3). However dystrophin was not detected in Erbb2/4 dKO myotubes (lanes 4–6) where ERBB2/4 receptors were ablated after Cre mediated recombination of loxP flanked Erbb2/4 genes. Utrophin on the other hand was detected in myotubes with and without ERBB2/4 receptors (Figure 1B) demonstrating that NRG/ERBB signaling selectively regulates dystrophin expression (Figure 3).
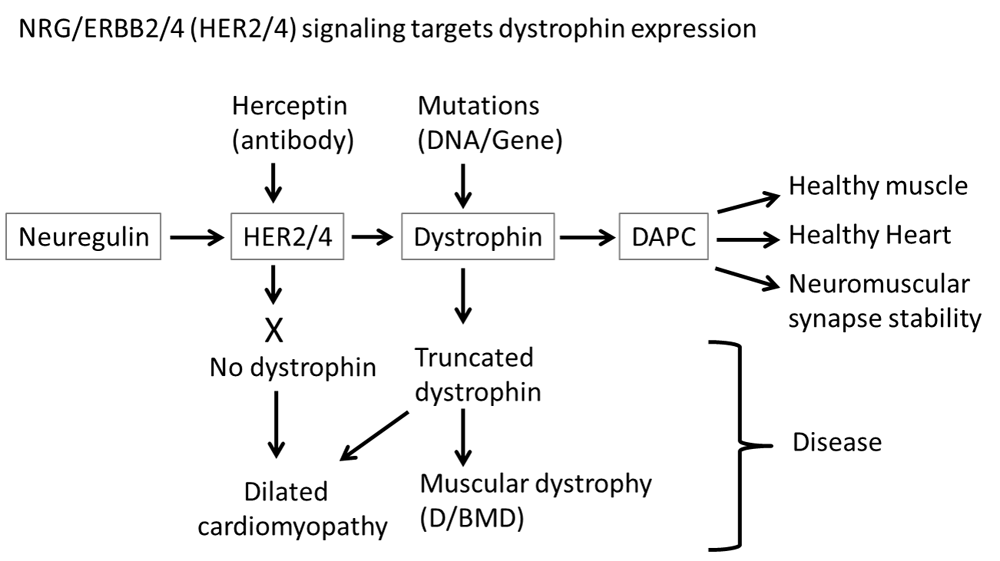
Under normal circumstances, NRG signaling through ERBB2/4 (HER2/4) receptors stimulates dystrophin expression, allowing the formation of a normal dystrophin-associated protein complex (DAPC). If either the dystrophin gene contains mutations or NRG signaling is blocked, then in the absence of functional dystrophin, a normal DAPC is not formed, resulting in various disease states such as dilated cardiomyopathy, Duchenne/Becker muscular dystrophy (D/BMD), and neuromuscular synapse instability.
The estimation by qPCR of dystrophin and utrophin mRNA levels (Figure 1C, D) confirm that not only the protein but also dystrophin mRNA expression is absent in Erbb2/4 dKO (shown in Figure 1 as erbb2/4-/- ) myotubes. Signals in qPCR were only observed above threshold at about 34 cycles which is essentially detection of non-specific amplification or background signal, whereas detection of rL8, used to normalize expression of dystrophin and utrophin, was above threshold at about 22 cycles in Erbb2/4 dKO and C2C12 samples. Detection of dystrophin mRNA in C2C12 cells by qPCR confirmed that the primers used to amplify dystrophin functioned. It is only possible to conclude that dystrophin is present in C2C12 and absent in Erbb2/4 dKO myotubes. The level cannot be estimated. This is because the level of dystrophin in C2C12 was relative to that in Erbb2/4 dKO myotubes, for which essentially background non-specific values were obtained in qPCR due to the absence of dystrophin mRNA. Utrophin detection (Figure 1D), using the same RNA/cDNA preparation used for dystrophin detection, confirmed that the cDNA preparation from Erbb2/4 dKO myotubes was intact. Utrophin expression was reduced to only less than half the amount (Figure 1D) in Erbb2/4 dKO myotubes compared to C2C12 myotubes which may be due to the lack of NRG signaling since NRG was reported to stimulate utrophin expression to some extent20. Myotubes formed from C2C12 myoblasts were used as a control for qPCR instead of myotubes containing loxP flanked Erbb2/4 genes (used for the western blots in Figure 1A, B) as the loxP-flanked Erbb4 gene21 is a hypomorph due to the insertion of the neo selection cassette and therefore levels of dystrophin mRNA may have been affected (NRG signaling through ERBB2/4 stimulate dystrophin expression and reduced Erbb4 expression may have affected this). This is not a problem for dystrophin protein detection (not estimation) in immunoprecipitated samples from myotubes containing loxP flanked Erbb2/4 genes. qPCR on Erbb2/4 dKO myotubes confirmed the absence of dystrophin expression (Figure 1C) as observed on the western blot (Figure 1A). Hence NRG/ERBB signaling is necessary for dystrophin expression. However, the Erbb2/4 dKO mice did not show dystrophic symptoms1.
Due to the presence of tissues and cells other than skeletal muscle and myoblasts, such as vascular smooth muscles (VSM) and satellite cells, in muscle lysates and in primary myoblast purifications from Erbb2/4 dKO mice, it is not possible to resolve the lack of dystrophin in skeletal muscle in these mice. In particular satellite cells were shown to express a high level of dystrophin22 and will still express dystrophin in Erbb2/4 dKO mice since the HSA promoter driving Cre recombinase expression is not active23, and hence ERBB2/4 receptors would not be ablated in these cells. The juxtaposition of dystrophin expression in satellite cells and in myofibers makes it very difficult to distinguish dystrophin expression in satellite cells from that in muscle fibers22. Satellite cells, however, appear to have a limited potential to regenerate skeletal muscle23 and hence it would not fully explain the lack of dystrophic symptoms in Erbb2/4 dKO mice.
We previously demonstrated that phosphorylation of the 75 kDa α-DB1 by NRG/ErbB signaling stabilized AChR at the NMJ1 and that in Erbb2/4 dKO myotubes we detected on western blots, after immunoprecipitation (IP) of the DAPC, lower amounts of the 75 kDa α-DB1 protein compared to the 89 kDa protein. The 89 kDa protein was also recognized by the anti-α-DB1 antibody but did not show phosphorylation. However in IP performed on C2C12 myotubes, we detected similar amounts of the 75 kDa and 89 kDa proteins on western blots1. Hence this raises the possibility that the AChR cluster fragmentation observed in vitro and in vivo when ERBB2/4 receptors are lacking was due not only to a lack of phosphorylation of α-DB1 but also due to the low amounts of the 75 kDa α-DB1 associated with the DAPC. Since Erbb2/4 deletion causes a lack of dystrophin (Figure 1), this low amount of α-DB1 protein associated with the DAPC could be due to: i) the lack of dystrophin as levels of α-DB1 is reduced at the sarcolemma in mdx mice17; 2) due to downregulation of the 75 kDa α-DB1 expression, if NRG/ErbB signaling normally increased its expression; 3) due to the lack of phosphorylation of the 75 kDa α-DB1 since phosphorylation may have stabilized its association with the DAPC.
To resolve this question, qPCR was performed on cDNA from Erbb2/4 dKO myotubes and control C2C12 myotubes using primers that would detect α-DB1 message based on an mRNA sequence (NCBI Ref. NM_207650.3) that would express a dystrobrevin protein with a calculated molecular weight of 76.817 kDa (NCBI Ref. NP_997533.1). Both, Erbb2/4 dKO and C2C12 myotubes expressed similar amounts of α-DB1 (Figure 2A). For both Erbb2/4 dKO and C2C12 samples α-DB1 expression was above threshold at about 28 cycles whereas detection of rL8, used to normalize expression of α-DB1, was above threshold at about 22 cycles. As in Figure 1, myotubes formed from C2C12 myoblasts were used as a control instead of myotubes containing loxP flanked Erbb2/4 genes as the loxP-flanked Erbb4 gene21 is a hypomorph. Hence the lower amounts of the 75 kDa α-DB1 associated with the DAPC in Erbb2/4 dKO myotubes is not due to lower expression levels of α-DB1.
To find out if phosphorylation of the 75 kDa α-DB1 stabilized its association with the DAPC when dystrophin was present, GFP-α-DB1 and GFP-α-DB1-P3, where the three tyrosine phosphorylation sites were mutated2, were expressed in dystrobrevin KO (lacking dystrobrevin)13 and in C2C12 (expressing dystrobrevin) myotubes. Both, dystrobrevin with (GFP-α-DB1) or without (GFP-α-DB1-P3) tyrosine phosphorylation sites associated with the DAPC irrespective of whether endogenous dystrobrevin was present as in C2C12 myotubes or absent as in dystrobrevin KO myotubes (Figure 2B) as they could be pulled down with IP of the DAPC. Hence association of the 75 kDa α-DB1 to the DAPC is independent of its phosphorylation state. Interestingly, the 89 kDa α-DB1 isoform was not observed in these cultures but the 75 kDa α-DB1 isoform was consistently observed. In this particular experiment, the dystrobrevin KO (db-/- in Figure 2B) myotubes transfected with GFP-α-DB1 appears to have lost its expression construct during culture (Figure 2B) and the band observed is most probably from spill over from the adjacent well. However, in a repeat of this experiment (see raw data), expression of the transfected constructs was observed in all transfected myotubes. Control myotubes were not transfected and hence GFP-α-DB1 expression was not detected, and as expected in the dystrobrevin KO myotubes, endogenous dystrobrevin expression was not detected, on western blots.
This implies that the AChR cluster fragmentation observed in vitro in Erbb2/4 dKO myotubes and in vivo in skeletal muscle1 may be due to a combination of the reduced amounts of the 75 kDa α-DB1 associating with the DAPC and due to a lack of phosphorylation (of the reduced amount of the 75 kDa α-DB1) as NRG/ErbB signaling, that stimulates dystrophin expression and phosphorylates α-DB1, is lacking. Hence the lack of NRG/ErbB signaling causing AChR cluster fragmentation as observed in vitro in myotubes, is not compensated for by another signaling pathway in vivo as this AChR cluster fragmentation is also observed in vivo1. The reduced association of the 75 kDa α-DB1 to the DAPC is most likely due to a lack of dystrophin since in mdx mice, where NRG/ERBB signaling is present, and therefore α-DB1 associated with the DAPC would be phosphorylated, less dystrobrevin is found at the sarcolemma in muscle and AChR cluster fragmentation was observed17. Therefore abolishing NRG/ErbB signaling in myotubes and skeletal muscle results in fragmentation of AChR clusters, most likely due to a lack of dystrophin and as a consequence reduced association of the 75 kDa α-DB1 (which would not be phosphorylated), with the DAPC.
We previously reported that in HSA-Cre/Erbb2/4 dKO mice, loss of NRG signaling in skeletal muscles led to a lack of α-DB1 phosphorylation and its reduced association with the DAPC, resulting in AChR cluster fragmentation1. The current study shows abolishing NRG/ErbB signaling in myotubes results in the loss of dystrophin expression and that the reduced association of α-DB1 with the DAPC is independent of its phosphorylation state.
Abolishment of NRG/ERBB signaling in skeletal muscle results in the specific loss of dystrophin and this is supported by: 1) Erbb2/4 dKO myotubes fail to express dystrophin whereas utrophin is still expressed; 2) Loss of NRG/ERBB signaling abolishes phosphorylation of the 75 kDa α-DB1 and results in lower amounts of the 75 kDa α-DB1 associated with the DAPC1. Similarly in dystrophin deficient mdx mice less dystrobrevin isoforms are detected at the sarcolemma17; 3) The reduced association of α-DB1 with the DAPC is not dependent on the phosphorylation state of α-DB1 (Figure 2B) and hence is not due to a lack of NRG/ErbB mediated phosphorylation of α-DB1 in Erbb2/4 dKO myotubes, but more likely due to the loss of dystrophin; 4) The reduced association of α-DB1 with the DAPC is not due to downregulation of α-DB1 expression (Figure 2A) as a consequence of the loss of NRG/ErbB signaling; 5) Loss of NRG/ERBB signaling in vitro and in vivo results in AChR cluster fragmentation1, implying that the loss of NRG/ERBB signaling is not compensated for in vivo by another signaling pathway.
Reduced localization of dystrobrevin at the sarcolemma and AChR cluster fragmentation due to a lack of dystrophin are also seen in dystrophin deficient mdx mice. However in contrast to mdx mice, Erbb2/4 dKO mice do not show dystrophic symptoms24. In these mice24, muscle fibers were examined extensively and centralized nuclei, a hallmark of DMD25,26 were not observed. Also atrophic fibers could not be observed in these mice (supplementary information of reference 24)24. Finally, sustained muscle strength was also not affected in the Erbb2/4 dKO mice and neither did they have a myasthenic condition24. However these mice showed a hind limb extension reflex (personal observation) which was also reported for the HSA-CRE/Erbb2 KO mouse27. The loxP flanked Erbb2 mouse is the same mouse that was used in breeding to finally generate the HSA-Cre/Erbb2/4 dKO mouse24. The abnormal muscle spindle formation reported for the HSA-Cre/Erbb2 mouse was not looked at in the HSA-Cre/Erbb2/4 dKO mouse probably because it had already been described for the HSA-Cre/Erbb2 KO mouse.
The reason why Erbb2/4 dKO mice do not show a dystrophic muscle phenotype as seen in mdx mice, despite the absence of dystrophin, could be due to the specificity of the promoter that drives the expression of Cre recombinase in these conditional KO mice. In both, HSA-Cre/Erbb2 mice27 and HSA-Cre/Erbb2/4 dKO mice24 used in this study, Cre recombinase expression is driven by the HSA promoter that is active in the striated muscles, skeletal and heart muscle but not in vascular smooth muscle (VSM)17,28,29. Hence, ERBB2 receptors would not be ablated and dystrophin will still be expressed in VSM, in contrast to skeletal muscles in these mice.
Interestingly an Erbb2 conditional knock-out mouse with Cre expression driven by the muscle creatine kinase (MCK) promoter, MCK-Cre/Erbb2 did show impaired muscle regeneration and a requirement of ERBB2 for survival of muscle spindles and myoblasts30. As MCK is expressed in both, skeletal muscle and VSM31, the MCK promoter would be active in these tissues. Hence in mice where Cre recombinase expression is driven by the MCK promoter, ERBB2 would be ablated in both skeletal muscle and VSM, resulting in a loss of dystrophin expression in both muscle types, explaining the different histopathology with HSA-Cre/Erbb2/4 dKO mice.
In the HSA-Cre/Erbb2/4 dKO mice, ERBB2/4 receptors and dystrophin in smooth muscle of blood vessels would still be expressed, as Cre recombinase would not be expressed in VSM, allowing the formation of a normal functional DAPC. Hence VSM in these mice still allows for increased blood flow to skeletal and cardiac muscle during exercise. In healthy VSM, neuronal nitric oxide synthase (nNOS) associates with dystrophin and generates nitric oxide (NO) that signals to soluble guanylate cyclase, generating cyclic guanosine 3’,5’-monophosphate (cGMP) in VSM, causing vasodilation enabling exercise-induced increase of blood flow and thereby preventing muscle ischemia32. It cannot be excluded from the results presented here, that dystrophin expression in endothelial cells also plays a role in vasodilation during exercise, assuming that the HSA promoter used to drive Cre recombinase expression to ablate ERBB2/4 receptors is not active in those cells in HSA-Cre/Erbb2/4 dKO mice.
The absence of obvious dystrophic pathology in HSA-Cre/Erbb2/4 dKO mice despite the lack of dystrophin in skeletal muscle strongly suggests that the main cause of muscular dystrophy is not the lack of dystrophin in skeletal muscle per se but systemic lack of functional dystrophin, especially in smooth muscle of blood vessels, resulting in impaired sympatholysis and muscle ischemia during exercise32. This hypothesis is consistent with published data describing the effects of phosphodiesterase type 5 (PDE5) inhibitors, which interfere with breakdown of NO by PDE5 and thereby prolong the half-life of cGMP, the target of NO33. Treatment with PDE5 inhibitors alleviated the dystrophic phenotype in mdx mice32 and also in DMD patients. PDE5 inhibition with either tadalafil or sildenafil treatment in Duchenne muscular dystrophy boys restored normal blood vessel function and blood flow during exercise33. However, a phase 3 randomized trial of tadalafil for DMD did not slow down the decline in ambulatory ability in boys with DMD34. The authors suggested that the boys in the trial may not have engaged in sufficient daily ambulation, something they could not keep track of due to the large number of patients enrolled and geographical locations of the study. Tadalafil regulates blood flow through its target NO-cGMP signaling in skeletal muscle, only when muscles are active. Since smooth muscle cells are also lining the lymph vessels, ambulation would also help lymphatic drainage and in patients where ambulation is limited, lymph drainage massage may be beneficial for skeletal muscle health.
The observation that absence of dystrophin in skeletal muscle does not result in a dystrophic phenotype is supported by a study where siRNA mediated silencing of dystrophin expression in the muscles of adult mice resulted in a clear absence of dystrophin in skeletal muscle without any of the histopathology characteristics observed in mdx mice35. Because in this study adult mice were used, the authors suggested that the dystrophic pathology normally observed in dystrophin deficiency may be developmentally regulated. However in the Erbb2/4 dKO mice, dystrophin would be ablated early during development in skeletal muscles since the HSA promoter driving Cre recombinase expression is active on embryonic day 9.5 (E9.5), when dystrophin would also start to be expressed36, which would argue against the dystrophic pathology being developmentally regulated.
Taken together, the following studies support the conclusion that the lack of dystrophin in VSM is the root cause of DMD: 1) Skeletal muscle specific HSA-Cre/Erbb2/4 dKO mice, based on the results described here, lack dystrophin in skeletal muscle but would still express dystrophin in VSM and do not show a dystrophic pathology24; 2) HSA-Cre/Erbb2 KO27 mice show a similar pathology to the HSA-Cre/Erbb2/4 dKO mice; 3) MCK-Cre/Erbb2 mice30 where NRG/ErbB signaling and hence dystrophin expression is ablated in both, skeletal muscle and VSM (as MCK promoter is active in both), suffered from impaired muscle regeneration; 4) Loss of dystrophin in skeletal muscle fibers through RNAi knock down did not result in an overt dystrophic pathology35; 5) expression of dystrophin in VSM, even at significantly lower levels compared to wild type (wt) controls, improved aberrant vasoregulation in mdx mice37. Taken together, all these studies support the notion that aberrant vasoregulation, when dystrophin is lacking in VSM, is likely the cause of the onset of DMD and that lack of dystrophin in skeletal muscle exacerbates the dystrophic pathology.
There have been numerous attempts to restore dystrophin in skeletal muscle that ameliorated the dystrophic pathology to some extent, but none have led to an applicable therapy. As the focus of most therapies is to restore skeletal, and sometimes also cardiac muscle, it was not of concern whether VSM would or would not be targeted during therapy. In a study where dystrophin overexpression in transgenic mdx mice eliminated dystrophic symptoms38, the MCK promoter was used to drive dystrophin expression and hence dystrophin would have been expressed in both, skeletal muscle and VSM. Gene therapy using Rous sarcoma virus (RSV) or cytomegalovirus (CMV) promoters39 would also result in the expression of a functional dystrophin in surrounding VSM after intra-muscular (IM) injection or particle bombardment40. In a Phase I study of gene therapy for Duchenne/Becker muscular dystrophy (D/BDM) the CMV promoter was also used41 which would also restore dystrophin expression in VSM. Systemic delivery of antisense oligoribonucleotides42, or restoration of dystrophin expression by (intravenous) stem cell transplantation43, would also result in dystrophin restoration in VSM.
Similarly, even though in vivo rescue of the dystrophic phenotype in dystrophin deficient mice and dogs focused on delivery of the therapeutic to skeletal muscle (see example below), these therapies would also target VSM. Furthermore, simply expressing dystrophin in skeletal muscle fibers lacking dystrophin would restore the dystrophin associated protein complex (DAPC) but it does not imply impaired skeletal muscle regeneration and function is restored. The VSM should be analysed to determine whether dystrophin may also have been expressed through leaking of the therapeutic into VSM, sometimes through the circulation especially when using viral based therapies as described below.
Gene editing of mutated dystrophin using adeno-associated viruses (AAV) to deliver CRISPR components is a promising therapeutic strategy and has recently been demonstrated to function in a canine model of Duchenne muscular dystrophy44. However, it still needs to be determined if the gene editing will be sustainable over long term. In this study an AAV serotype 9 that shows tropism for heart and skeletal muscle was used to deliver the CRISPR components. To drive the expression of Cas9 (one of the gene editing components), a muscle specific creatine kinase regulatory cassette was used that should also result in gene editing of dystrophin in VSM especially when the viruses are injected intravenously for systemic delivery. In a second experiment, 6 weeks following delivery through direct injection into muscles, dystrophin expression and assembly of the DAPC in dystrophic muscles was observed. Dystrophin expression in contralateral muscles that were not injected was also observed and it was attributed to leakage of AAV9 into circulation indicating a broad distribution. Therefore, from what is known so far, it is neither possible to exclude the role of VSM in the onset of DMD, nor to conclude that expression of dystrophin in skeletal muscle only, is sufficient for the rescue of the dystrophic phenotype in DMD.
Published data on DMD, together with the data presented in this article, suggests that the lack of dystrophin in skeletal muscle exacerbates the onset of dystrophy caused by a lack of increased blood flow during exercise. This is also supported by an observation in mdx muscles that only some fibers, and not all, in skeletal muscle initially show dystrophic symptoms45 and also by the fact that expressing the full length dystrophin in smooth muscles in mdx mice, even though it was expressed at significantly lower levels than the level of dystrophin found in wt mice, improved aberrant vasoregulation37. The fact that skeletal muscle in those mice still showed some atrophy could be due to insufficient dystrophin expression in the smooth muscle causing dystrophic symptoms as a consequence of reduced blood flow during exercise and exacerbated due to the lack of dystrophin in skeletal muscle (Reviewed in Ennen JP et al.)46. The exacerbation of dystrophic symptoms in skeletal muscle, apart from structural defects, may also be as a result of disruption of signaling events from the DAPC due to the absence of dystrophin.
We previously reported that the blockade of NRG signaling through ERBB2/4 receptors prevented phosphorylation of α-dystrobrevin1 and hence affected NMJ stability1. In the current study it is shown that ablation of ERBB2/4 receptors, and thus elimination of NRG signaling, results in a lack of dystrophin expression (Figure 1). Thus NRG/ERBB signaling maintains NMJ stability through at least two pathways, one where it phosphorylates α-dystrobrevin 11,2 and the other where it stimulates dystrophin expression thereby allowing the formation of a functional DAPC that stabilizes acetylcholine receptors (Figure 3).
If the absence of NRG/ERBB signaling, and as a consequence absence of dystrophin in skeletal muscle and lack of phosphorylation of α-DB1, could be compensated in vivo by another mechanism, even possibly through another ligand other than NRG1, then this compensation mechanism would be independent of ERBB2/4 receptors as they are ablated in skeletal muscles of Erbb2/4 dKO mice. However there is probably no compensatory signal for NRG/ERBB in vivo as AChR cluster fragmentation is observed in vivo as well. As far as could be determined from the literature, there are no compensatory signals in vivo that can ameliorate the effects caused by deficient ERBB2 or ERBB4 signalling such as defective muscle spindle formation27, dilated cardiomyopathy (DCM) following Herceptin (antibody against HER2/ERBB2) treatment47, DCM in conditional Erbb228,48 or Erbb449 KO mice, AChR cluster fragmentation (also observed in mdx mice) in muscle specific Erbb2/4 dKO mice1, and the embryonic lethality observed in Erbb211 or Erbb410 KO mice. Based on what is known so far, NRG and its ERBB receptors appear to exert their function in a spatio-temporal fashion in development and maintenance (in adults).
NRG/ErbB signaling also induces cardiomyocyte proliferation and repairs heart injury12 and is essential for normal cardiac development9–11, whereas dystrophin deficiency does not impair cardiac development but does result in dilated cardiomyopathy (DCM)8. ERBB4 can heterodimerize with ERBB2 and a function blocking ERBB2/HER2 antibody treatment results in DCM in mice28 and in cancer patients47, consistent with our observations that NRG/ERBB signaling is required for dystrophin expression since DMD patients and mdx mice lacking functional dystrophin, develop DCM (Figure 3). Hence the data presented here suggests that a target protein for the reported beneficial effects of signalling through ERBB4 in repairing heart injury is dystrophin whose expression is regulated through NRG/ERBB2/4 signaling. Thus NRG/ERBB signaling carries out different functions, through different signaling targets, in cardiac development and maintenance, with the latter being carried out through regulating dystrophin expression.
Although normal blood flow is important for prevention of the onset of dystrophic symptoms, the general consensus is that exercise would cause damage to skeletal muscle and in the absence of dystrophin, muscle regeneration would be impaired. However, the Erbb2/4 dKO mice lacking dystrophin in skeletal muscle only, do not show any obvious dystrophic pathology which challenges this general consensus.
There are two studies that may give an explanation as to why a dystrophic pathology is not observed in skeletal muscles when dystrophin is lacking as in HSA-Cre/Erbb2/4 dKO mice. It has been demonstrated that the host environment is critical for controlling the function of satellite cells50. Hence normal dystrophin expression in VSM may prevent or delay the onset of dystrophy in skeletal muscle and thereby maintain a host environment that is permissive to muscle regeneration by satellite cells. Alternatively muscle regeneration in response to injury could be mediated by myofibers derived from circulating mature myeloid cells (blood cells) that migrate to skeletal muscle in response to inflammatory cues51. Hence, upon muscle damage in HSA-Cre/Erbb2/4 dKO mice where dystrophin is absent only in skeletal muscle, satellite cells and/or circulating myeloid cells could help regenerate mature myofibers underscoring the importance of the vasculature and increased blood flow during exercise.
Although current therapy for Duchenne patients is largely focused on skeletal muscle, the present study suggests that development of therapeutics should (also) focus on VSM (and possibly also vascular endothelial cells) for any long term therapeutic benefit for patients with Duchenne and Becker muscular dystrophy. Dystrophin deficient mdx mice show a milder dystrophic pathology and have a shorter life span compared to humans. It thus needs to be determined whether in humans, restoring dystrophin in VSM (and endothelial cells) and thereby a functional DAPC, is sufficient to ameliorate the dystrophic pathology.
The current study also suggests that increasing NRG signaling through ERBB2/4 receptors, especially in the smooth muscle of blood vessels, could be a way to increase truncated dystrophin expression in D/BMD patients. This would ameliorate dystrophic symptoms in those patients where the mutation in dystrophin does not affect association of nNOS52 and thereby enabling normal blood flow during exercise. Apart from its implications for muscular dystrophy, the demonstration that NRG/ERBB signaling stimulates dystrophin expression should help to improve treatment regimens with the anti-HER2 antibody, Herceptin/Trastuzumab, to prevent cardiomyopathy. Furthermore, as dystrophin is also present in all regions of the brain, being most abundant in the cerebellum53, and since NRG/ERBB signaling regulates dystrophin expression, changes in functional dystrophin levels could be the underlying cause of some of the disease states such as schizophrenia, associated with abnormal NRG/ERBB signaling in the brain.
NRG signaling through ERBB2/4 receptors is necessary for stimulation of dystrophin expression. However, when ERBB2/4 receptors are lacking in skeletal muscle only, mice do not exhibit dystrophic symptoms supporting the notion that lack of dystrophin expression in smooth muscle of blood vessels is the root cause of the onset of D/BMD.
F1000Research: Dataset 1. Uncropped western blot images and raw Ct values from qPCR. DOI: https://dx.doi.org/10.5256/f1000research.15889.d21462854.
F1000Research: Dataset 2. Raw data for figure 2. DOI: https://doi.org/10.5256/f1000research.15889.d22679755
This work was supported by funds from the University of Basel, Basel, Switzerland.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
I am grateful to Janine F. Zankl, Emmanuel Traunecker, and Toni Krebs for performing the FACS sort.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Skeletal muscle, signaling,. transcription, nuclear receptor signaling and muscle-linked diseases such as DMD, diabetes, vascular regression/PVD.
References
1. Wasala N, Lai Y, Shin J, Zhao J, et al.: Genomic removal of a therapeutic mini-dystrophin gene from adult mice elicits a Duchenne muscular dystrophy-like phenotype. Human Molecular Genetics. 2016. Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Muscular dystrophy, gene therapy
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Muscular dystrophy, gene therapy
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
References
1. Escher P, Lacazette E, Courtet M, Blindenbacher A, et al.: Synapses form in skeletal muscles lacking neuregulin receptors.Science. 2005; 308 (5730): 1920-3 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: muscular dystrophy
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 04 Dec 18 |
read | read | |
|
Version 1 20 Aug 18 |
read | read | |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)