Keywords
Zika virus; ZIKV; birth defects; microcephaly; congenital Zika syndrome; West Africa; seasonality
This article is included in the Emerging Diseases and Outbreaks gateway.
Zika virus; ZIKV; birth defects; microcephaly; congenital Zika syndrome; West Africa; seasonality
In this revision, we have clarified some characteristics of the hospital from which the birth registry data were obtained, including the requirement that the name of the hospital and the country be withheld as a condition of accessing the data. We added the emphasis to the statement about surveillance needs, in particular, that these should encompass both the infectious agents surveillance as well as birth defects surveillance. We also updated the Figure 1 and its legend for more clear presentation, and have added references to seroprevalence studies of ZIKV in West Africa.
See the authors' detailed response to the review by Matthew T. Aliota
See the authors' detailed response to the review by Diana Valencia
See the authors' detailed response to the review by Catherine M. Brown
Since 2015, when an initial link between Zika Virus (ZIKV) and microcephaly was discovered in Brazil1,2, the term congenital Zika syndrome (CZS) has been coined to reflect a broad range of Zika-linked neurodevelopmental damages3 beyond microcephaly3–5, including ocular6,7 and auditory defects8,9. The overall risk of Zika-linked birth defects has been estimated at ~10% and 15% for infections during the 1st trimester10, potentially impacting thousands of infants in the US and US territories alone. Concerns exist that ZIKV-related outcomes are underreported, particularly when ZIKV infections result in neurodevelopmental abnormalities without visible microcephaly (e.g., developmental delays and learning disabilities that would not be immediately noticeable11–13). Such outcomes are likely not recorded, particularly if the causative infection was asymptomatic14. Further, a recent CDC report that examined birth defect records from 15 US jurisdictions showed a statistically significant increase in prevalence of birth defects potentially consistent with CZS in areas with documented local ZIKV transmission in the second half of 201615. Notably, the majority of infants and fetuses with birth defects potentially related to ZIKV infection in this report lacked ZIKV infection testing, which may be in part attributed to lack of known maternal exposure or other such indicators16. Nonetheless, these findings are alarming and underscore not only the need for continued monitoring and surveillance, but also the need to better understand the full extent – as well as mechanisms – of neurodevelopmental defects associated with CZS.
Currently our understanding of the mechanisms that underlie CZS remains limited, including the possibility that ZIKV infection is a necessary but insufficient condition for CZS17. One key question is whether ZIKV-mediated birth defects are associated with a specific strain of ZIKV, which, for example, could have evolved after ZIKV migrated from South East Asia to French Polynesia and Brazil18 and now spread to North America, including the US10,15. However, other studies suggest that African strains are likely to be as pathogenic as the Asiatic strains (e.g., 19, 20). Thus, the lack of connection between ZIKV infection in pregnancy and birth defects prior to the 2015–16 Brazilian outbreak may instead be attributable to the benign nature of ZIKV infection in adults21 and lack of surveillance, including that infectious agents as well as surveillance of birth defects, among other factors. This can be illustrated by a report of a handful of birth defects in Hawaii in 2009–2012, which now has been shown to be associated with ZIVK infection, but was undetected as such until the Brazilian microcephaly epidemic brought ZIKV into the spotlight22.
Earlier studies from the African Continent – where ZIKV is endemic – have documented a relatively high prevalence of ZIKV antibodies in human populations, including in West Africa (e.g., Nigeria23,24, Sierra-Leone25, Senegal26, Burkina Faso, Cote D'Ivoire, Guinea-Bissau, Cameroon, Mali, Niger, Benin, Gabon27, supporting the premise that ZIKV is present across multiple countries in West Africa region (reviewed in Kindhauser et al.28)). Despite this, there exists negligible data regarding CZS across the African Continent; nevertheless, lack of evidence should not be taken as definitive proof of absence19. Thus, here we examine birth registries from one particular hospital in West Africa from a country considered by the WHO to be at medium risk of a ZIKV outbreak29. The study was approved by the Committee on Administration of the hospital where the data was collected in lieu of a functioning Ethics Committee. But as there are ongoing security concerns in this location, that committee required that the hospital name and location be kept anonymous to ensure the safety of the hospital and staff. The chosen hospital has a full service maternity/OB department, staffed by well-trained qualified obstetricians, midwives, and obstetric nurses. It handled about 2000 births per year during the studied period; reports compiled from the hospital data are routinely used by the country’s government.
Risk of major neurodevelopmental defects, such as microcephaly, appears to be particularly high if vertical transmission occurs during the first trimester, especially within a “vulnerability window” around 12 weeks (10 to 14 weeks) post-conception10,30. Thus, we hypothesized that – similar to seasonal malaria infections, which peak a few weeks following abundant rainfalls during the “rainy” season, typically from August through October in the study region31,32 – seasonal variations in the number of CZS-type birth defects would be detectable from the aforementioned hospital data. Such expectations are consistent with prior findings from Senegal (West Africa)33 and Kenya (East Africa)34, where Rift Valley Fever epizootics were associated with heavy rainfalls. Our hypothesis was further informed by the temporal relationships between the number of ZIKV infections and microcephaly cases reported in Brazil35. We expected that the peak of CZS-type birth defects (such as documented cases of microcephaly and/or stillbirth) would coincide with vertical ZIKV transmission at around 12-weeks post-conception during the peak of the rainy season, assuming an approximate 3-week lag between maternal infection and vertical transmission36, as suggested by data from Brazil in 201537.
A total of 13445 birth registries (2009–2015) from a non-governmental hospital in West Africa were examined to determine whether we could identify a seasonal pattern of birth defects potentially attributable to ZIKV infection (i.e., CZS-type birth defects). The number of births and respective outcomes (i.e., live birth versus stillbirth) and reported complications (i.e., fetal malformation, breech, etc.) were collated by month/year. Reporting standards for birth complications varied between years; thus, we focused exclusively on visible neurodevelopmental complications (such as microcephaly) and pregnancy losses (such as stillbirth) that could be attributed to potential CZS3,4 (Supplementary Table 1 and Supplementary Table 2). To infer the “vulnerability window” of 12 weeks (spanning 10 to 14 weeks) post-conception, we assumed that births that were not reported as premature in the records were full-term, thus enabling us to infer the likely month of conception35.
We also considered national average monthly temperature and rainfall data for the study years, collected from the World Bank Climate Change Knowledge Portal database. These values were treated as proxy indicators for mosquito activity in the hospital catchment area at time of maternal infection. To visualize any relevant trends, we plotted these data, as well as the average percentage of birth defects consistent with potential CZS by month.
As shown in Figure 1, the average percentage of births consistent with potential CZS demonstrates a marked peak between March and July, which places maternal month of infection between August and December of the previous year. These months encompass the peak and latter half of the warm, rainy season (August–October) as well as the first half of the cool, dry season that immediately follows (October–December) in the study region, which likely represent months with considerable mosquito activity. Notably, the hospital from which these birth defects data were acquired also generally experiences a peak in childhood malaria cases every October, which falls squarely in the middle of the August to December range of presumed maternal month of ZIKV infection determined here.
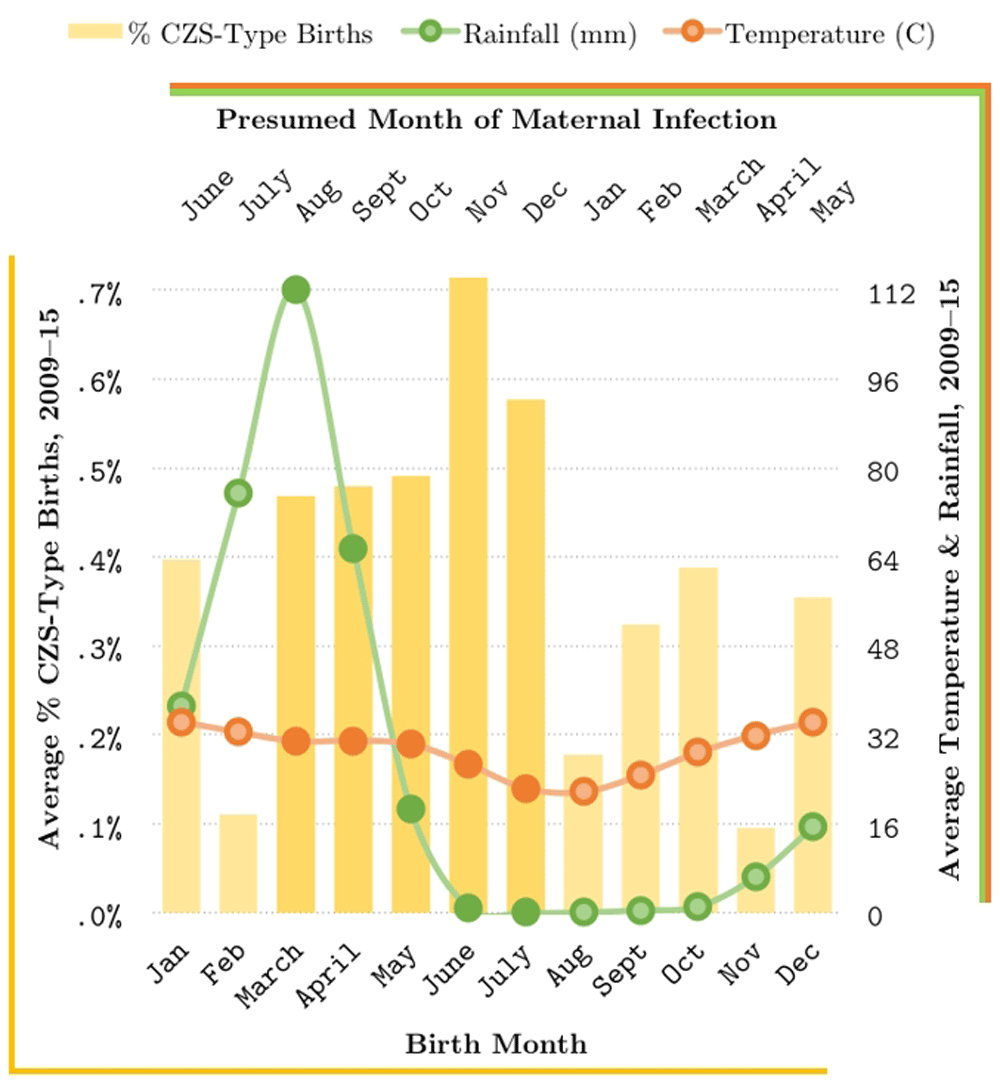
Bottom axis corresponds with the birth month depicted with yellow bars (i.e., with the left scale that shows % CZS defects), while the top axis corresponds with the month of maternal infection (and thus, with the rainfall and temperature shown on the left, depicted in green and red, respectively).
With this in mind, the early months of the cool, dry season (October–December) are likely hospitable enough for mosquito vectors to thrive and spread pathogens, including ZIKV; this may explain why the range of our presumed maternal month of infection (August–December) extends past the rainy season, given that mosquitoes require a balanced environment for survival, including both moderate rainfall and optimal temperatures38.
Our results suggest that a seasonal pattern exists with respect to CZS-type birth defects reported in the study region (Figure 1), where the largest fraction of said defects appear to occur in the months of March through July. Furthermore, this pattern can be linked to ecological evidence, such as rainfall and temperature trends that likely facilitate maternal ZIKV infection. While consistent with the expectation that some of these defects might be attributable to (unreported) ZIKV infections that occurred early in pregnancy (and indeed resemble temporal patterns from studies in Brazil35), our findings stop short of definitively linking ZIKV infection and birth defects in the study region, in part due to scant data. Instead, by reviewing the potential limitations of the data analyzed here, we hope that this report will initiate broader surveillance efforts – of both the infectious agents, including ZIKV, as well as that of birth defects - that may help shed light onto mechanisms that underlie CZS, including utilization of data that might already be available across various African countries where ZIKV is endemic and/or competent vectors exist (e.g., Gabon39, Central African Republic40). For example, a recent WHO Bulletin on Outbreaks and Other Emergencies41 reported a number of microcephaly cases from Angola (a country listed in the High risk category29) that appear to be linked to ZIKV infection, despite the lack of direct PCR confirmation from the specimens. Due to only recent implementation of active surveillance in the country, the true magnitude of the event is not yet clearly understood. Nonetheless, it is important for insights into a broader pattern of potential CZS defects, despite the lack of experimental ZIKV infection confirmation or lack of evidence of ongoing active ZIKV transmission in Angola (i.e. only two ZIKV cases were reported from Angola in early 201742).
Several conservative assumptions made in this analysis, such as assuming a gestation period of ~9 months, or not classifying “low birth weights” (which may represent full-term births of ZIKV-infected fetuses) as a CZS-type birth defect, would likely lead to underestimation of potential trends, if any. We also assumed that the available birth records were representative of the pregnancy/birth patterns that occur across the entire region. Other limitations of the available data are related to the standard of care that is feasible in much of West Africa, including (i) lack of family history and/or genetic testing for mutations in loci responsible for primary microcephaly; (ii) lack of laboratory evidence or testing for ZIKV and/or other infections, including TORCH agents43,44, often due to inability to pay for testing (e.g., 45); and (iii) lack of detailed clinical prenatal history, including whether rash and/or other symptoms of Zika infection were present at any point during pregnancy. This final limitation may be considered minor, given that the majority of ZIKV infections are asymptomatic21,30. Additionally, no data were available regarding other clinically relevant factors that are also associated with microcephaly46, such as history of excessive alcohol consumption or recreational drug use, and/or prolonged exposure to pesticides, such as pyriproxyfen. However, the former life-style factors are unlikely to have a seasonal effect spanning several years, and the role of the latter factor as a causative agent of microcephaly remains unclear47. There is also a lack of precise ecological data, including estimates of rainfalls in the hospital catchment area, the distribution and feeding habits of mosquitoes, and whether or not said mosquitoes carry ZIKV, as well as data regarding ZIKV prevalence in the human population.
Despite these limitations, our findings suggest that using the data we already have – even in the absence of formal surveillance systems for CZS – can provide compelling, introductory insights. In the future, work that employs existing data from hospitals across the African continent – which encompasses countries with a variety of climates, dry and rainy seasons, and suitability for widespread mosquito habitats – should be pursued.
Figshare : Data for Figure 1. Average birth defects consistent with congenital Zika syndrome per month, with corresponding average daily temperatures and rainfall during presumed maternal month of infection. doi: 10.6084/m9.figshare.5387029. Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
This work was partially supported by the Research Seed Award from Kent State University (to HP) and by Research For Health, Inc. (to RH).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplemental Table 1. A list of birth complications (as recorded in the health records) consistent with potential congenital Zika syndrome.
Click here to access the data.
Supplemental Table 2. A list of birth complications (as recorded in the health records) that are not consistent with potential congenital Zika syndrome and/or missing.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Birth defects surveillance in low and middle income countries
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR: Zika Virus and Birth Defects--Reviewing the Evidence for Causality.N Engl J Med. 2016; 374 (20): 1981-7 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Birth defects surveillance in low and middle income countries
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Virology, medical entomology, evolution and transmission dynamics of arthropod-borne pathogens
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 19 Jul 18 |
read | read | |
|
Version 1 07 Feb 18 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)