Keywords
histone modification, H3K27ac, GC-content, CpG dinucleotides
histone modification, H3K27ac, GC-content, CpG dinucleotides
We carefully checked all the names of the genes and plasmids as well as the rest of the text for typos and modified a few statements.
See the authors' detailed response to the review by Vasily N Aushev
See the authors' detailed response to the review by Oleg Gusev
Modification of histone proteins is a key mechanism of epigenetic regulation. Histone modifications vary between cells in some genomic locations but not others. This observation raises the question to what extent histone modifications depend on the underlying nucleotide sequences. It has been reported that the attraction of PRC2 complex and consequent H3K27me3 is positively correlated with the local density of CpG dinucleotides1. More complex sequence patterns, such as transcription factor (TF) binding sites (TFBS), also affect the presence of histone modifications. For example, SUZ12, a member of the PRC2 complex, binds DNA in a sequence-specific manner and NRCF and ZBTB33 recruit histone deacetylase. The ENCODE project demonstrated a specific histone modification profile around binding sites of many TFs2.
Since the same lysine residue cannot be both methylated and acetylated, the presence of H3K27ac is negatively correlated with the presence of H3K27me33. Although there are several pieces of evidence showing that H3K27me3 is established at least partially in a sequence-specific manner, it is unclear if H3K27ac — an antagonistic activator to a repressive H3K27me3 — shares any sequence specific patterns. A computational approach4 predicted some TFBS to be linked to H3K27ac, yet the results were inconsistent and dependent on the a background set. In this work, we perform a direct experiment to test whether a specific genomic sequence is capable of recovering H3K27ac.
We selected a site for Cas9 in the intergenic region of human beta-globin locus using services CCTop5 (hg38) and Off-Spotter6 (hg38). The chosen targeting sequence was CTTGTCCCTGCAGGGTATTA. Then we designed targeting oligonucleotides (Table 2) for pSpCas9(BB)-2A-Puro (pX459 plasmid (Addgene), a gift from Egor Prokhorchouck, E.P.). Oligonucleotides Bgl1Cas_F and Bgl1Cas_R were diluted to 10 µM each with annealing buffer (10 mM Tris, pH 7.5, 100 mM NaCl, 1 mM EDTA), heated for 5 minutes to 95°C and then slowly cooled down to room temperature. 500ng of pX459 was digested with 5U of BpiI (Thermo Scientific) for 1h at 37°C, heat inactivated, run on 1.5% agarose, linearized plasmid was cut out and purified with QIAquick Gel extraction kit (Qiagen, cat. no. 28706) and ligated with Bgl1Cas_F/Bgl1Cas_R duplex. The ligation product was transformed into E. coli Top-10 cells, one of the clones was chosen and the insert was confirmed by Sanger sequencing. Below we refer to this plasmid as pX459-b1.
Selection cassette design (HSV thymidine kinase, T2A peptide, NeoR in one frame) was performed with Ugene7. HSVtk and NeoR sequences were PCR amplified with Phusion polymerase (Thermo Scientific) from pBS246-neo/Tk (a gift from E.P., construction of this plasmid is described elsewhere8) with primers HSVtk_F, HSVtk_R, and G418_F, G418_R, correspondingly. T2A peptide coding sequence, corresponding to amino acid sequence GSGEGRGSLLTCGDVEENPGP, was synthesized by hybridization of oligonucleotides T2A(+) and T2A(-) in annealing buffer and consequent treatment of hybridized duplex with T4 polymerase (Thermo Scientific) and gel purified. The NeoR fragment was digested with XbaI (Thermo Scientific), the T2A fragment was digested with NheI (Thermo Scientific), then these fragments were ligated and the fragment of expected size (881bp) was gel purified. This T2A-NeoR fragment was digested with XhoI (Thermo Scientific), HSVtk fragment was digested with SalI (Thermo Scientific), fragments were ligated and the fragment of predicted length was gel purified again. This HSVtk-T2A-NeoR fragment was double digested with EcoRI (Thermo Scientific) and BshTI (Thermo Scientific) and ligated with pX459-b1 double-digested with EcoRI and BshTI. This step gave the plasmid with full-length selection cassette. Below we refer to this plasmid as pHSVtk-T2A-NeoR. Then the pHSVtk-T2A-NeoR plasmid was double-digested with XbaI and Acc65I (Thermo Scientific), gel purified and ligated with hybridized AdaptUp(+)-AdaptUp(-) duplex. A successful insert was verified by digestion of newly introduced restriction sites: SalI and BamHI. We refer to this plasmid as pAdaptUp-HSVtk-T2A-NeoR. This plasmid was digested with NotI (Thermo Scientific), treated with FastAP (Thermo Scientific), gel purified and ligated with AdaptDown(+)-AdaptDown(-) duplex to introduce a 2xBpiI site that generates half-sites for BclI and XhoI after cleavage. The resulting plasmid was transformed into E. coli Top-10 cells. We refer to this plasmid as pAdaptUp-HSVtk-T2A-NeoR-AdaptDown. To obtain LoxP-flanked sequences, we used a pBK-CMV-derived plasmid with a modified multiple cloning site containing restriction sites for BclI, NheI, and XhoI. This plasmid was transformed into E. coli JM110 to eradicate Dam methylation, double digested with BclI and NheI, dephosphorylated, gel purified and ligated with LoxP(+)- LoxP(-) duplex, treated with T4 PNK. This plasmid was transformed into E. coli Top10 cells and referred to as pLoxP. To obtain homology regions that flank Cas9 cleavage site we PCR amplified them from Caki1 gDNA, using primer pairs Bgl1Up_F and Bgl1Up_R for an upstream fragment and Bgl1 Down_F and Bgl1Down_R for a downstream fragment. These PCR fragments were double digested with NheI and XhoI and ligated with pLoxP, double digested by the same sites and dephosphorylated. Ligation products were transformed into E. coli JM110, and purified plasmids were digested with BclI and XhoI to yield upstream and downstream fragments of DNA bearing LoxP site on its end. The upstream fragment was ligated with pAdaptUp-HSVtk-T2A-NeoR-AdaptDown double digested with SalI and BamHI to give pUp1L-HSVTK-T2A-NeoR-AdaptDown. The downstream fragment was ligated with pUp1L-HSVTK-T2A-NeoR-AdaptDown treated with BpiI and dephosphorylated. We refer to this plasmid as pUp1L-HSVTK-T2A-NeoR-L1Down.
We co-transfected Caki1 cells with the plasmids pX459-b1 and pUp1L-HSVTK-T2A-NeoR-L1Down using Lipofectamine 3000 (Thermo Scientific) in 2cm2 wells following manufacturers instructions. After one week of Puromycin (3 µg/ml) selection, cells were split into 96-well plates and selected with 1mg/ml G418 for two weeks. Cells from successfully growing clones were split into two equal aliquots, one for growth and another for genomic DNA isolation. Clones were checked for the presence of the insert with a primer pair BGL1pcr_F - BGL1pcr_R surrounding the insert. One clone (referred to as Caki1-GcvS-G418R) with a homozygous insert was selected for the further experiments.
Plasmid pBK-CMV was digested with SacI and HpaI, blunted with T4 polymerase, self-ligated, transformed into E. coli Top10 and called pBCK-CMVdHpaI-SacI. LoxP(+) oligo was PNK treated and annealed with LoxP(-) to form a duplex. Bait(+) and Bait(-) oligos were annealed and ligated with hemi-phosphorylated LoxP duplex. Ligation products were resolved on 3% agarose gel, the longest fragment was excised and purified with QIAquick gel extraction kit, treated with T4 PNK and ligated to NheI treated and dephosphorylated pBCK-CMVdHpaI-SacI. The ligation product was transformed into E. coli Top10, we refer to the resulting plasmid as p1L-bait-L1. Ten sequences chosen to be inserted (Table 1) were PCR amplified from genomic DNA of Caki1 cells with primers (Table 2, rows 25 to 44). Amplicons were gel purified, diluted to a concentration of 100nM, treated with T4 PNK and ligated with a plasmid p1L-bait-L1 treated with Ecl136II and dephosphorylated. Then the library of ligation products was transformed into E. coli Top10, the plasmid library was purified using a plasmid mini kit (Evrogen, cat. no. BC021) and co-transfected with pBS598 EF1alpha-EGFPcre (Addgene) to Caki1-GcvS-G418R cells for Cre-mediated recombination exchange of the insert and the HSVTK-T2A-NeoR cassette. This step was performed in two independent replicates. After 3-day growth Ganciclovir (2 µM) was added to eliminate cells that did not undergo recombination. After selection for 10–14 days survived cells were grown to a subconfluent monolayer in 10cm dishes and then ChIP on H3K27Ac was performed.
ChIP was performed according to Abcam X-chip protocol with the following modification: we increased the number of washes in High Salt buffer from one to three times. Sonication was performed in PCR tubes (SSIbio, cat. no. 3245-00) on ice using Sonics Vibra-Cell VCX 130 with an eight-element probe (cat. no. 630-0602). Sonication setup was: 10s pulse, 20s pause, 75% power, total sonication time is 30min. We used 2 µg of Anti-H3K27Ac antibody (Abcam, ab4729) per ChIP. Biological replicates of targeted inserts of DNA sequences were processed independently. Control ChIP was performed under the same conditions with Caki1 cells.
After ChIP, the DNA was pooled and 1ng of DNA was PCR amplified with a primer pair Bait-seq_F – Bait-seq_R. At this step we amplified all DNA sequences inserted between this primer pair. Then 10ng of PCR amplicons from the first step was used as a template for a PCR with a primers (Table 2, rows 25 to 44), each pair in an individual reaction tube.
None of the GC- and CpG rich promoter regions, that were acetylated in their original genomic loci (rows 1–3 in Table 1 and lanes 1–3 in Figure 1) recovered H3K27ac after relocation to a foreign genomic context in the beta globin locus, suggesting that H3K27ac may not depend directly on such features. An alternative explanation is that some of the CpG dinucleotides became methylated in a foreign genomic environment preventing chromatin from gaining H3K27ac - an active chromantin mark. Surprisingly, two extremely GC-rich but CpG poor (and, therefore, unmethylated) sequences (rows 5, 6 in Table 1 and lanes 5, 7 in Figure 1) gained H3K27ac in the foreign environment, while in their native environment (in their original genomic location) they had no H3K27ac. Sequences 5 and 6 are located far from promoters of known genes. The sequence 5 contains a lowly expressed CAGE cluster, representing a weak alternative promoter of the KMT2D gene (ZENBU). Therefore, we conclude that the gain of H3K27ac in these regions is unlikely to be explained by transcriptional activity. The lack of H3K27ac in the sequence 6 in the native location may be due to the presence of the antagonistic mark H3K27me3 in Caki1 cells, which is lost after translocation to a foreign environment. Yet, for the sequence 5, H3K27me3 is absent in all cell types reported in ENCODE. Our results suggest that in contrast to H2K27me3, H3K27ac gain is unlikely affected by the CpG content of the underlying DNA sequence, while extremely high GC-content might contribute to the gain of the H3K27ac.
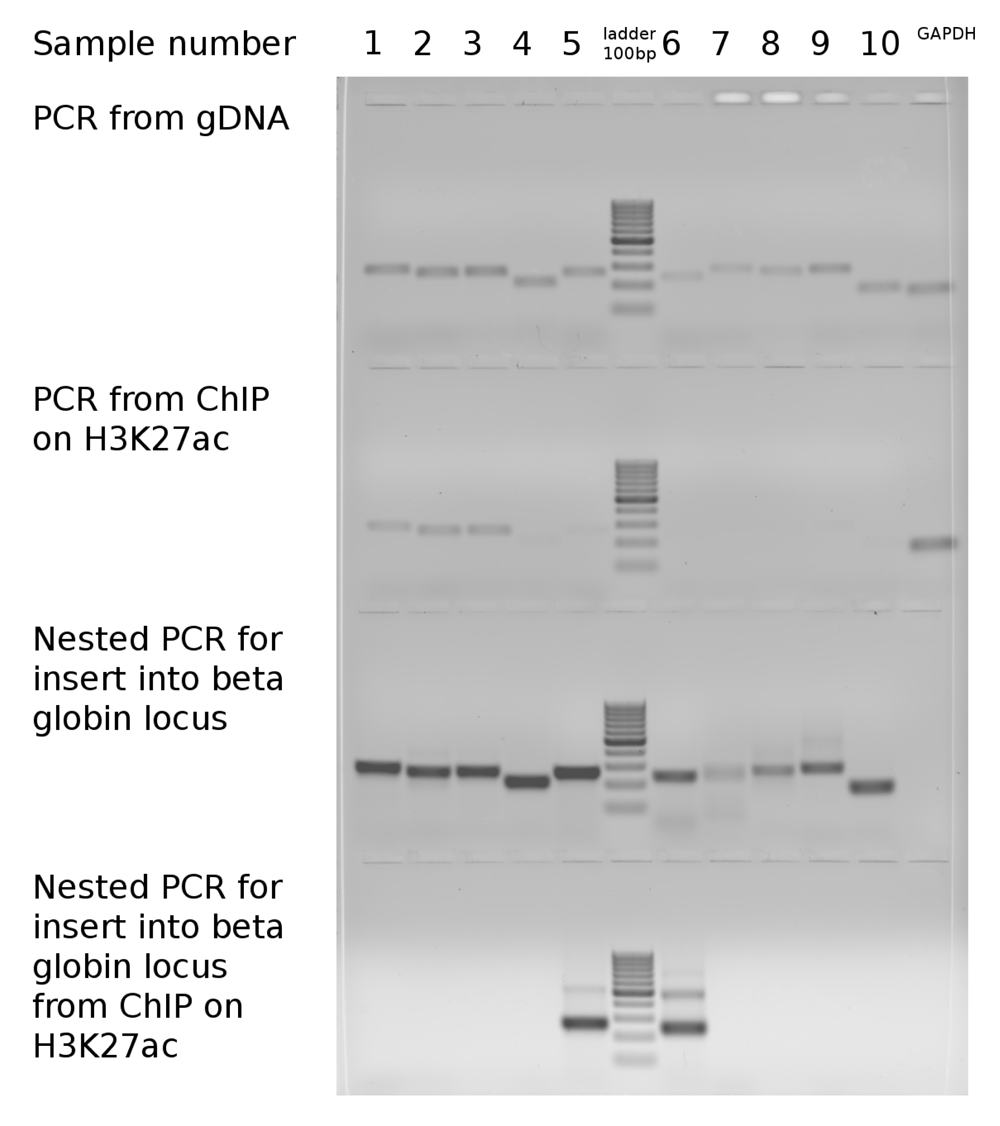
Dataset 1: Gel image of amplification of target sequences from native genomic loci compared with amplification from beta-globin locus with or without ChIP on H3K27ac. DOI, 10.5256/f1000research.13441.d1927749
This work was supported by the Russian Science Foundation [15-14-30002].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We are thankful to Egor Prokhorchouck for providing access to the laboratory equipment, cell lines, plasmid library and help with the experimental setup.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cancer biomarkers, oncogenesis, signal transduction, miRNA regulation
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 09 Aug 18 |
||
|
Version 1 08 Feb 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)