Keywords
Multiple Time-Step Integration, Molecular Dynamics, Resonance Artefacts, RESPA, Twin-range, Liquid Phase Interfaces
This article is included in the Cheminformatics gateway.
This article is included in the Mathematical, Physical, and Computational Sciences collection.
Multiple Time-Step Integration, Molecular Dynamics, Resonance Artefacts, RESPA, Twin-range, Liquid Phase Interfaces
To describe the dynamic processes of biomolecules, such as proteins, DNA, carbohydrates and membranes, molecular dynamics (MD) simulations have been proven to be a powerful technique to provide insights at the atomic level. In MD simulations, physical processes that involve a wide range of time scales have to be considered appropriately. However, the accurate and efficient treatment of various time scales presents a notoriously difficult problem due to resonance artefacts arising from separation into distinct numerical subproblems1,2. Over the past decades, various methods have been proposed to mediate the issue and to speed up the simulation substantially. For example, fast vibrational modes (e.g. covalent bonds) can be removed completely by constraining the motion, e.g. using SHAKE3, SETTLE4, M-SHAKE5, LINCS6, or FLEXSHAKE7. Alternatively, multiple time-step (MTS) integration is an efficient approach to accommodate motions on different time scales. In the twin-range (TR) scheme8–10, the pairwise nonbonded forces acting on particle i are split into a short-range contribution from particles j within the cutoff Rs, and a mid-range contribution from particles j between Rs and the long-range cutoff Rl (see Figure 1),
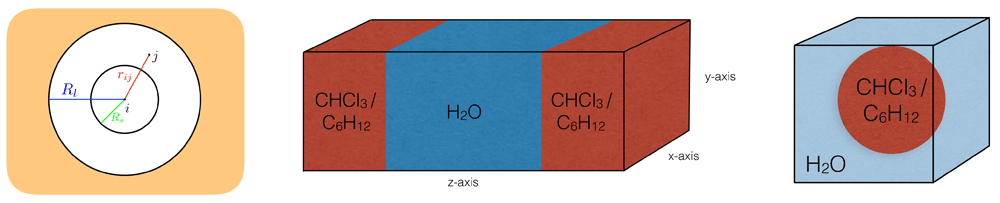
Left: Schematic illustration of the twin-range (TR) setup with the inner cutoff Rs and the outer cutoff Rl. Interactions beyond Rl are not calculated explicitly. Middle: Schematic illustration of the planar layer systems. Right: Schematic illustration of the systems with a droplet in a water box.
with
The short-range forces are updated every time step ∆t (inner time step), while the mid-range forces are updated every nm-th time step, nm∆t with nm ≥ 1 (outer time step). Long-range electrostatic interactions beyond the cutoff radius Rl can be included using the (an)isotropic reaction-field methods (RF or ARF)11,12, or lattice-sum methods13,14. Although theoretically possible, the TR scheme is generally not used together with lattice-sum methods. Simulations with (A)RF typically use larger cutoffs Rl than simulations with PME (e.g. 1.4 nm for the GROMOS force field15 compared to 0.9 nm for the AMBER force field16), which in the past encouraged the use of a TR scheme to reduce the computational cost.
In practice, for each particle i two pairlists are generated, which track the neighbouring short-range and mid-range particles j. To be consistent, the period of updating both pairlists np ∆t should be equal to the period of updating the mid-range forces nm∆t. In the following, the TR scheme is defined by Rs < Rl and nm = np > 1, and the single-range (SR) scheme by Rs = Rl, nm = 1 and np ≥ 1, i.e. there is only one pairlist for SR.
Splitting the forces into different contributions leaves some freedom of how to consider them in the integration. There are two main strategies for the inclusion of the outer contributions: (i) constant mid-range forces application (CFA) at every inner time step ∆t, or (ii) using a reversible reference system propagator algorithm (RESPA)8–10, which is based on the Trotter factorisation of the Liouville propagator17. In the case of RESPA, scaled mid-range forces nm are typically applied impulse-wise, i.e. only at every outer time step nm∆t. Note that in this work the leap-frog scheme18 is used for the integration of the equations of motion, which is similar19 to velocity-Verlet20 as typically employed with RESPA.
Although computationally efficient, MTS algorithms have the disadvantage of introducing numerical artefacts. Due to nonlinearity, it is notoriously difficult to formulate general physical or chemical conditions, which favour or suppress the build-up of these undesired resonances. Instead, specialised thermostats have been proposed as a remedy, which suppress resonance artefacts from MTS integration, for example by imposing isokinetic constraints on every degree of freedom21–23, or by selectively targeting the high-frequency modes using a colored noise thermostat24. In practice, however, standard thermostats like weak-coupling25, Nosé-Hoover (chain)26–28, or stochastic thermostats29–31 remain widely used in combination with a moderate integration time-step difference between fast and slow motions, as this should limit resonance artefacts to an acceptable value.
In this work, artefacts due to MTS integration are investigated with the main focus on a water-chloroform layer system using different thermostats (Figure 1). This system represents a simplified version of a biological membrane, for which an accurate simulation is of high interest in biology. The impact of splitting the nonbonded interactions into a short-range and mid-range contribution on the density profile normal to the interface and the kinetic energy distribution is investigated. In addition, a planar system consisting of cyclohexane and water as well as corresponding droplet systems are investigated. These additional setups allow to assess the impact of different solvents as well as different interface geometries on the formation of MTS artefacts. Overall, the artefacts are larger than those observed for more well-behaved, homogeneous systems32,33.
For all simulations, the GROMOS software package34 version 1.4.0 was used with the GROMOS 53A6 force field15. Periodic boundary conditions were applied. Newton’s equations of motion were integrated using the leap-frog scheme18 with an inner time step of 2 fs. Different outer time steps for the pairlist update or mid-range force evaluation were used (i.e. nm, np ∈ [1, 20]). If not indicated otherwise, the SR scheme corresponds to a midrange force evaluation every 2 fs (nm = 1) and a pairlist update every 10 fs (np = 5). The TR scheme typically corresponds to a mid-range force evaluation in combination with a pairlist update every 10 fs (nm = np = 5). If not stated otherwise, the CFA method was used for the mid-range force contributions. The inner cutoff radius Rs was set to 0.8 nm and the outer cutoff radius Rl to 1.4 nm. For all simulations with RF, a charge-group based pairlist algorithm and cutoff were used (each solvent molecule is a single charge group), whereas an atomic cutoff was used for the PME simulations. The center of mass motion was stopped every 1 ps. The coordinates were saved every 0.2 ps and the energies every 0.1 ps. Bond lengths were constrained using the SHAKE algorithm with a geometric tolerance of 10−4. Three different thermostats were tested, i.e. weak coupling25 (WC), Nosé-Hoover26,27 (NH,) and Nosé-Hoover chain28 (NHchain). Different temperature baths were used for different solvents. The coupling time τ was set to 0.1 ps for all three thermostatting methods. A chain length of three was employed for the NHchain thermostat. A standard homogeneous RF approach11 was used to mimic long-range electrostatic interactions beyond a charge-group based cutoff Rl . The dielectric permittivity was set to 78.5, which corresponds to the experimentally measured value of water35.
The MTS investigation was performed mainly using a water-chloroform layer, in combination with three auxiliary systems consisting of a water-cyclohexane layer, a chloroform sphere in a water box, and a cyclohexane sphere in a water box. An illustration of the setups is shown in Figure 1. With these additional systems, different geometries of the interface as well as different chemical compositions were tested. An overview of the simulated systems can be found in Table 1. Corresponding snapshots are provided in Figure S1 (Supplementary File 1).
The main system consisted of 2002 chloroform (CHCl3)36 molecules and 8129 SPC37 water molecules within an elongated rectangular box of 30.2 nm length in z-direction. Identical initial coordinates were used for all simulations, which were equilibrated for 1 ns using the SR scheme under NVT conditions. The simulation length of the production simulations was 1–4 ns, performed under NVT conditions. For the water-cyclohexane layer system, 6750 cyclohexane (C6H12) and 39’366 water molecules were used in an elongated rectangular box of 33.85 nm length in z-direction. This system was simulated under NVT as well as NPT conditions (using a Berendsen barostat25 with a coupling time τb = 0.5 ps). The droplet systems consisted of a droplet of about 3 nm radius, solvated in a box of 12.2 nm length. This setup led to a droplet with 847 CHCl3 molecules in 59’484 SPC water molecules, or a droplet with 619 C6H12 molecules solvated in 59’018 SPC water molecules, respectively. As the convergence towards the numerical artefacts was found to be very fast, all auxiliary systems were simulated without initial equilibration for 1 ns.
For the spatially resolved density analysis, a program was developed using the data structures provided by the GROMOS++ collection of programs38. The energy distributions were calculated by post-processing the output of the GROMOS++ program ene_ana accordingly. For the density calculations of the spherical interfaces, a slightly more sophisticated analysis approach was necessary. In order to obtain a radial density profile, the molecules were assigned to equidistant concentric spherical shells with respect to the center of geometry of the droplet. However, for the droplet it was difficult to define consistently a distance to the surface, because the interface may deviate substantially from its ideal spherical shape during simulation. In our analysis, the center of mass was chosen as an approximation of the center of geometry, which was then shifted (if necessary) in order to account for periodic boundary conditions, i.e. to ensure that the droplet remained at the center of the periodic box. Note that the volume of the spherical shells scales cubically with the radius, which increases the uncertainty of the estimator substantially close to the center of the droplet.
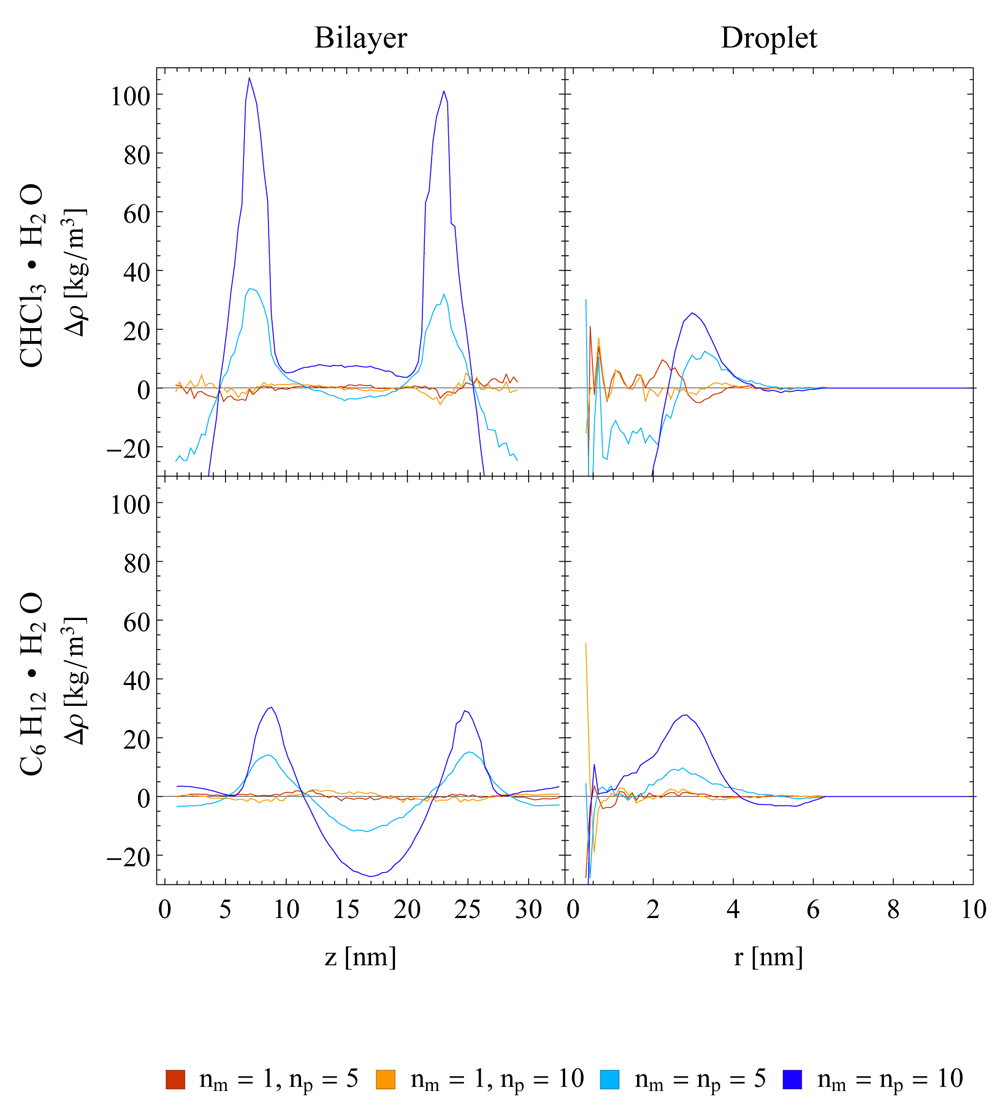
The density profile of the water-chloroform system was determined along the z-axis normal to the layer. As can be seen in Figure 2, the splitting of the forces into a short-range and a less frequently updated mid-range contribution (given in Equation (1)) using the TR CFA scheme with nm = np = 5 led to a significant density increase for chloroform close to the interface. The density increase was accompanied by a density decrease further away from the interface due to the constant volume conditions. The presence of an increase was found to be independent of the chosen thermostatting algorithm. An analysis of the density resonance pattern with respect to the outer time step nm∆t is shown in Figure 3. Note that the density artefacts are not perfectly symmetric with respect to the layer norm due to finite simulation time. It can be seen that the TR CFA scheme leads to large resonance artefacts beyond nm∆t ≈ 20 fs independent of the thermostatting method. On the other hand, evaluating the mid-range forces every step while keeping the pairlist update every fifth step (i.e. SR scheme with nm = 1 and np = 5) resolved the issue almost entirely for all three investigated thermostats (Figure 2 and Figure 3, data not shown for NH and NHchain). The minor differences between SR schemes with different update frequencies can only be seen in density difference plots (Figure 4) with respect to the SR reference run (nm = np = 1).
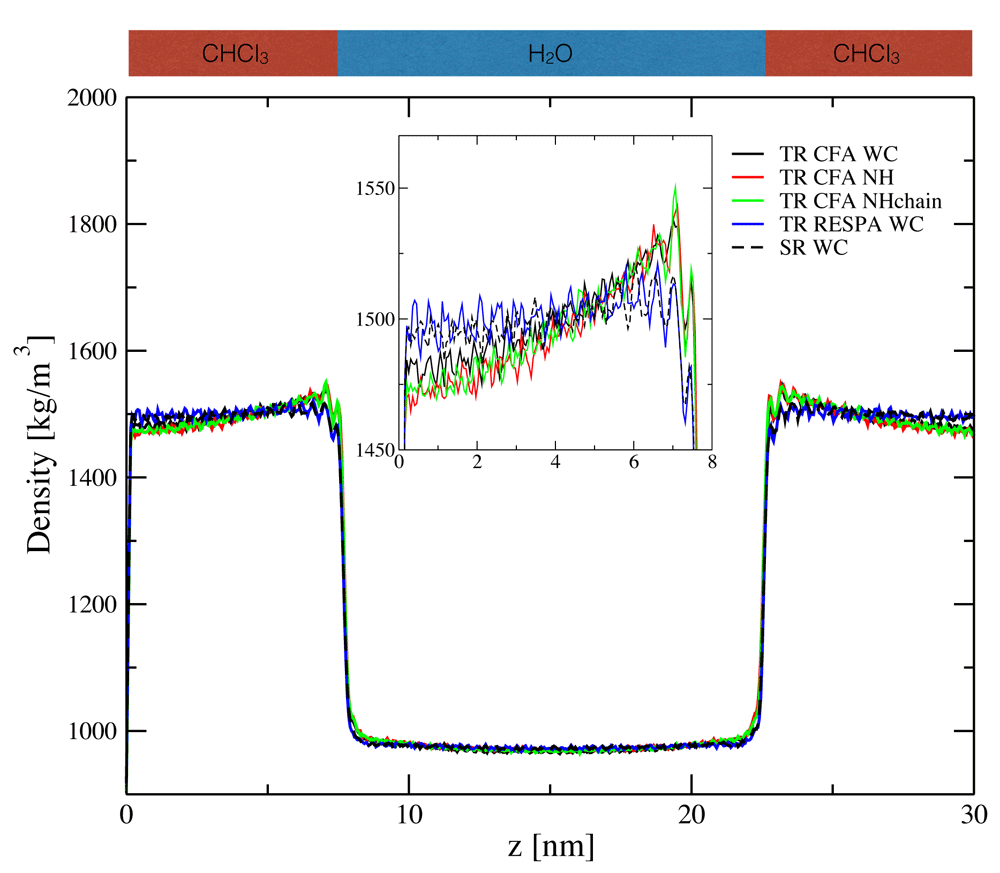
For the four setups with the twin-range (TR) scheme, the mid-range forces were updated with the same frequency as the pairlist (i.e. nm = np = 5). For the single-range (SR) scheme, the mid-range forces were evaluated every time step ∆t (i.e. nm = 1), whereas the pairlist was updated every 5th step (i.e. np = 5). The weak-coupling (WC), Nosé-Hoover (NH) or Nosé-Hoover chain (NHchain) thermostat was used.
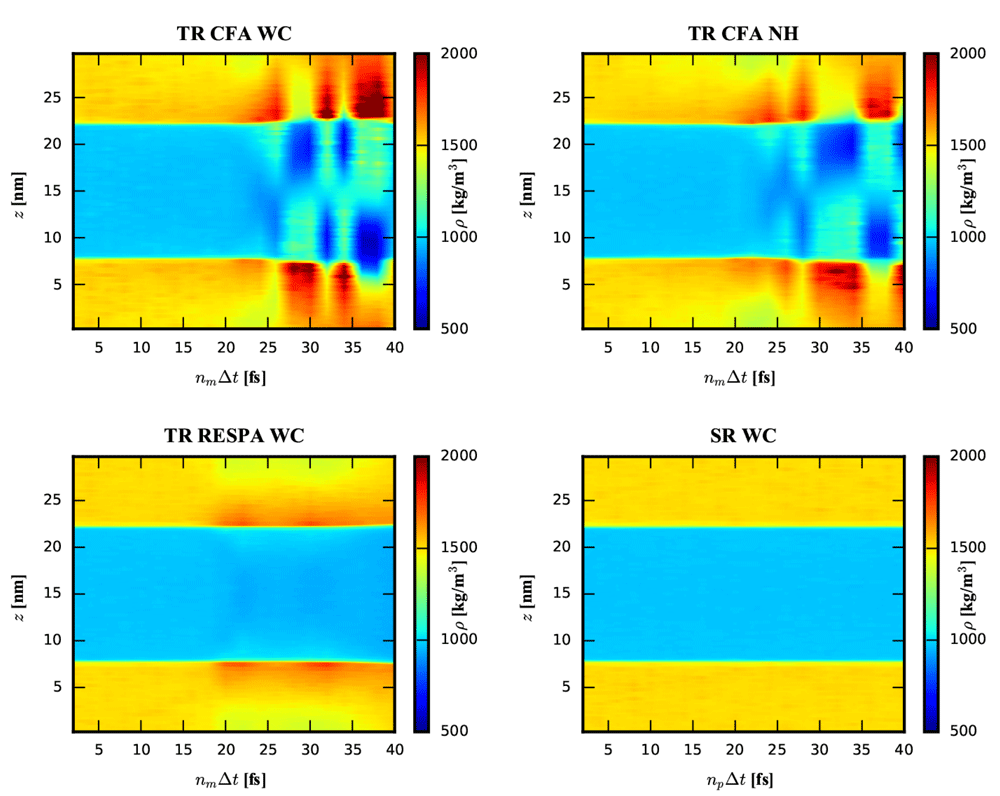
For the single-range (SR) scheme, the mid-range forces were evaluated every time stepc ∆t (i.e. nm = 1). For the three setups with the twin-range (TR) scheme, the mid-range forces were updated with the same period as the pairlist (i.e. np = n). The TR scheme was applied in a constant (CFA) or impulse-wise (RESPA) manner. The weak-coupling (WC) or Nosé-Hoover (NH) thermostat was used. Note that the density range is chosen to visualize the major artefacts occurring for nm∆t > 15 fs. A visualisation of the minor artefacts around nm∆t ≈ 10 fs can be seen in Figure 4.
The Nosé-Hoover (NH) thermostat was used in all setups. The single-range (SR) scheme (red and blue lines) and the twin-range scheme with constant-force application (TR CFA, orange and green lines) and two different update frequencies (np = 5, 10) are compared. Running averages with a window of seven data points are shown.
These findings indicate that the observed density increase is mainly caused by resonance artefacts due to the CFA MTS integration. This argumentation is supported by the pairlist error analysis shown in Figure S2, Supplementary File 1. The change in the pairlist after 10 fs is only on the order of 1%, and, even more importantly, it scales sublinearly with nm. Thus, the error from the pairlist cannot explain the substantial density increases seen in Figure 3. Instead, resonance effects lead to the build-up of the observed density artefacts over time, until a local equilibrium configuration is reached at the interface. This was found to be accompanied by a local change in the diffusion of the molecules at the interface. The mean residence time with respect to the layer normal z shows that regions of high density imply a locally reduced diffusivity of the molecules and vice versa (Figure S3, Supplementary File 1).
The numerical artefacts stem likely from an increasing mismatch between the forces and the position of the atoms the forces are applied to. In line with this interpretation, the density artefacts were suppressed for a typical value of the outer time step nm∆t = 10 fs when the mid-range forces were applied impulse-wise (TR RESPA scheme, blue line in Figure 2), i.e. the direction of the forces and the atom positions matched at the time point when the forces were applied. However, the TR RESPA scheme does not eliminate the resonance artefacts but only shifts them to longer outer time steps in this context (Figure 3). Thus, the partitioning of the system into different time scales together with the choice of the time steps in the MTS integration remain delicate aspects of current MD implementations39, also for impulse-wise MTS algorithms1,2,40.
On the contrary, only small effects on the density profile could be observed for the SR scheme with pairlist update periods up to np∆t = 40 fs (Figure 3 and Figure 4). Due to the large cutoff Rl used here (i.e. 1.4 nm), the changes in the pairlist are small between updates and scale sub-linearly with nm (Figure S2, Supplementary File 1). This observation is in line with the study of Krieger et al.41, where they found a negligible energy drift when using a similarly low pair-list update frequency in a homogeneous system. A rough performance analysis of the run time showed that for a realistic setup approximately half of the computational performance loss due to evaluating the mid-range forces at every time step could be compensated by a less frequent update of the pairlist (Table 2).
All systems were simulated for 0.4 ns on 1 CPU with 4 cores. A time step of 2 fs was used. The pairlist was generated using a charge-group based algorithm.
| Force splitting | nm | np | Run time [h] |
|---|---|---|---|
| TR CFA | 5 | 5 | 6.1 |
| SR | 1 | 5 | 11.4 |
| SR | 1 | 20 | 8.8 |
In order to exclude that the observed artefacts are specific to the water-chloroform system, the densities at a water-cyclohexane interface in a layer system were investigated. The corresponding density difference plots with respect to a SR reference are given in Figure 4. They show a similar behaviour of the TR scheme compared to the one observed for the water-chloroform systems, with slightly less pronounced artefacts. In addition, imposing constant pressure (NPT) conditions in the simulation setup does not reduce the observed density artefacts (Figure 5).
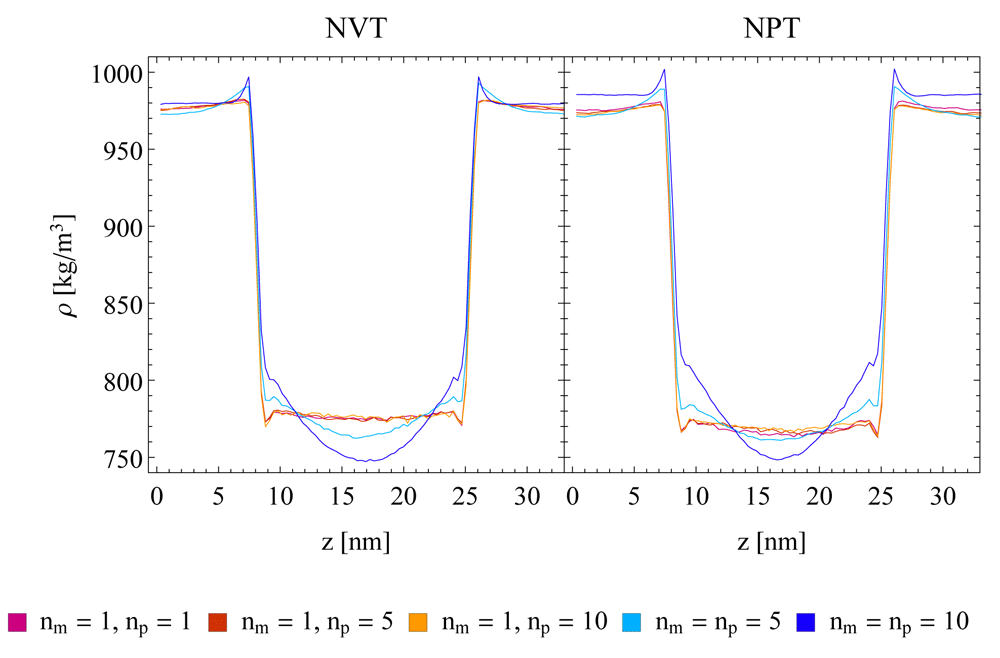
A semi-aniostropic pressure coupling was applied in x/y-direction using a Berendsen barostat, while the box length in z-direction remained fixed. A Nosé-Hoover (NH) thermostat was used in all setups. The single-range (SR) scheme (red, blue and orange lines) and the twin-range scheme with constant-force application (TR CFA, green and purple lines) and three different update frequencies (np = 1, 5, 10) are compared. Running averages with a window of seven data points are shown.
In addition to planar interfaces, systems with a droplet of chloroform or cyclohexane in a water box were investigated. As can be seen in the right panels of Figure 4, density artefacts occur also at curved interfaces. Note that the corresponding plots contain more noise at small radii compared to the planar setups due to surface fluctuations, which influence the position of the centre of mass used as a reference. In addition, the surface fluctuations also give rise to a broadening of interface in terms of densities compared to the planar setup as can be seen in Figure S4 (Supplementary File 1).
The impact of the chosen thermostatting algorithm as well as the MTS integrator, and the energy distributions was investigated for the water-chloroform layer system. The WC thermostat does not give a canonical distribution of the kinetic energy by construction42, whereas the NH and NHchain thermostats do. In Figure 6, the kinetic energy distributions within water and chloroform are shown for the different setups. For chloroform, the difference in the width of the distribution between WC and NH(chain) thermostats is clearly visible, whereas the average kinetic energy remains the same, despite the significant density gradient in this solvent. In water, on the other hand, a clear shift in the kinetic energy distribution towards a higher average value was observed for the TR CFA scheme compared to the SR scheme with the WC thermostat. This shift was less pronounced with the NHchain thermostat, and absent with the NH thermostat. These observations indicate that a temperature shift may occur due to MTS resonances. However, as the density increase occurred with all three thermostatting methods, but the temperature shift only with two of them, the latter cannot be the cause for the former. Therefore, one can conclude that the resonance artefacts caused by the TR scheme cannot be removed with thermostats that control the velocities globally. For this, one would have to control the velocities selectively, e.g. by imposing isokinetic constraints or restraints on each degree of freedom21–23.
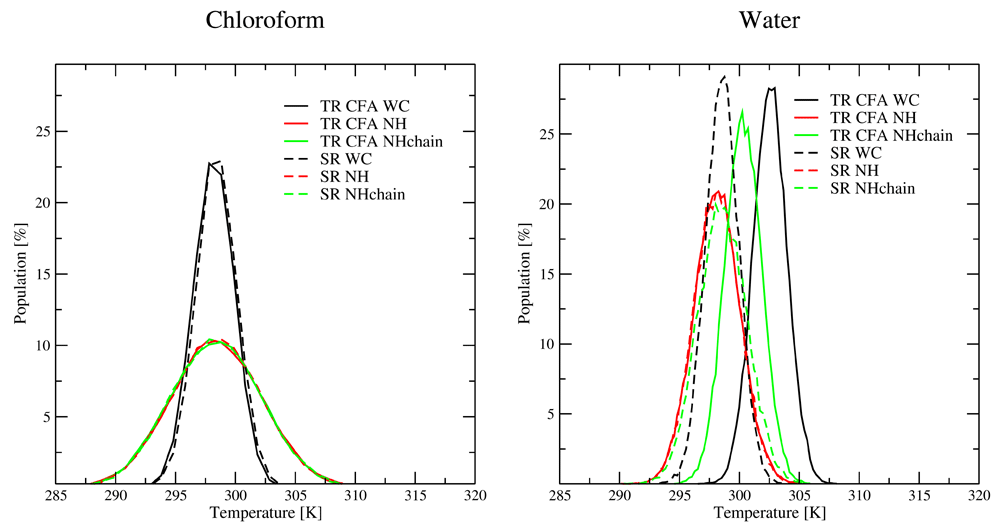
Note that a different number of molecules is simulated for chloroform (left) and water (right), which leads to a different width of the canonical distribution functions. For the twin-range (TR) scheme (solid lines), the mid-range forces were updated with the same period as the pairlist (i.e. nm = np = 5). For the single-range (SR) scheme (dashed lines), the mid-range forces were evaluated every time step Δt (i.e. nm = 1), whereas the pairlist was updated every 5th step (i.e. np = 5).
The effect of using the TR scheme with a constant force application (CFA) at solvent-solvent interfaces was investigated with layer and droplet systems of different composition (water-chloroform and water-cyclohexane). These systems represent simplified versions of a biological membrane and a protein in solution. The force splitting was found to lead to substantial MTS resonance artefacts at the interfaces. It could be shown that the presence of the artificial density increase at the interfaces is independent of the thermostatting method, the interface geometry as well as the chemical composition of the system although the magnitude could vary. In addition, the diffusivity of the solvent molecules at the interface changed due to the density artefacts.
The resonance effects likely stem from a mismatch between the forces and the position of the atoms the forces are applied to. This hypothesis is supported by the fact that the pairlist changes only in the order of 1 % between updates and that the TR scheme with RESPA (where the forces are applied impulse-wise) does not eliminate the numerical artefacts but shifts them to longer time steps. Based on these findings, the use of TR CFA is not recommended for heterogeneous systems. Instead, the SR scheme should be used, which did not show any significant density errors in the entire range of pairlist updates (analysed up to np∆t = 40 fs). Thus, the increase in computational cost for SR can be partially compensated by updating the pairlist less frequently (i.e. np∆t > 10 fs). In addition, using only a single pairlist reduces the complexity with respect to implementation, as the additional difficulties arising from efficient memory management of two (or more) pairlists can be avoided.
We are unable to provide the output files from the simulations directly due to file size. We instead provide the input files that when used as input with the GROMOS software package will generate the same results -
Dataset 1: Zip containing simulation input files 10.5256/f1000research.16715.d22294943
The authors gratefully acknowledge financial support by the Swiss National Science Foundation [200021-178762] and by Eidgenössische Technische Hochschule (ETH) Zürich [ETH-34 17-2].
The authors thank Philippe Hünenberger and Wilfred van Gunsteren for helpful discussions.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: molecular simulation, physical chemistry
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Hantal G, Sega M, Kantorovich S, Schröder C, et al.: Intrinsic Structure of the Interface of Partially Miscible Fluids: An Application to Ionic Liquids. The Journal of Physical Chemistry C. 2015; 119 (51): 28448-28461 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 08 Mar 19 |
read | |
|
Version 2 (revision) 18 Jan 19 |
read | |
|
Version 1 05 Nov 18 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)