Keywords
Candida albicans, oral candidiasis, seaweed Gracilaria verrucosa
Candida albicans, oral candidiasis, seaweed Gracilaria verrucosa
Smoking is a common problem in most developing countries, including Indonesia. Based on a survey by The Tobacco Atlas in 2015, Indonesia has the highest number of smokers in Asia, with 66% of men in Indonesia being active smokers1. Smoking can lead to addiction owing to the nicotine contents, and harm due to the presence of toxic compounds such as CO, ammonia and tar contents in tobacco1. Besides causing addiction, substances in cigarettes can also cause various diseases, such as oral candidiasis. Oral candidiasis is caused by the infection of the fungus Candida albicans2. This fungus is part of the normal flora of the human mouth, but it can become pathogenic in certain conditions, for example, due to nicotine exposure3.
Infection with C. albicans will increase the formation of a biofilm of the fungus3. The biofilm is an extracellular matrix consisting of C. albicans colonies4. The size of the biofilm increases when exposed to substances in cigarette smoke, as the cigarette has content that can initiate growth and nourish C. albicans5,6.
Currently, fluconazole and nystatin are the most effective drugs for treating oral candidiasis. Unfortunately, these drugs have side effects; for example the prolonged use of fluconazole leads to resistance7, while high dosages of nystatin give gastrointestinal discomfort and increase plaque formation8. Therefore, plant-derived antifungals may be a viable oral treatment option for candidiasis. One of these potential plants is seaweed Gracilaria verrucosa. This seaweed contains several bioactive compounds, including alkaloids, flavonoids, phenolics, saponins, steroids and terpenoids9. Aceh Province, Indonesia, has large G. verrucosa resources, although so far this aquatic plant has not been commonly used for medicinal purposes. Hence, the objective of the present study was to examine the ability of seaweed extract to inhibit the growth of C. albicans obtained from smoker saliva, as indicated by biofilm formation.
The study was conducted in August 2017 at The Laboratory of Microbiology, Veterinary Faculty, Syiah Kuala University. C. albicans was extracted from the saliva of one volunteer active smoker volunteer in Faculty of Dentistry Medicine, Syiah Kuala University. The volunteer was asked directly and accepted, giving written informed consent. The inclusion criteria of the volunteer was an active smoker that smoke at least 20 cigarretes per day. The saliva was collected once the volunter finished smoking. the G. verrucosa seaweed was collected from a farmer in Pulo Aceh, Aceh Province. The subject provided written informed consent to participate in this study. Ethical clearance (No. 1741/UN11.1.21/TU/2017) was obtained from Faculty of Dentistry, Syiah Kuala University, Banda Aceh, Indonesia.
Extraction was performed based on the Maserati method10. A total of 3 kg seaweed was washed with tap water then rewashed using distilled water. The seaweed sample was dried at room temperature 25°C for 24 h, avoiding direct sunlight. The wet seaweed was chopped into small-sized pieces (2 mm), then soaked in 96% ethanol, as a solvent. After 24 h the sample was filtered using Whatman filter paper No. 42 and the resulting residue was soaked again in 96% ethanol. This procedure was repeated until the solvent color which added to sample was not changing the color or limpid. All the filtrate collected in all of the procedures was then evaporated using a vacuum rotary evaporator (Laborta 4003 control, Heildolph) for 15 min at 60°C. The extract was taken and stored in a refrigerator at 4°C.
The saliva was collected from a volunteer active smoker in Faculty of Dentistry Medicine, Syiah Kuala University. Saliva was collected by spitting into a glass jar (15 ml), then 1 ml PBS (0.01 M, pH 7.2) was added to the jar. The jar was centrifuged at 10,000 rpm for 10 min, after which the precipitate was taken and incubated in CHROMagar Candida medium for 2 days to allow for colony development. If the colour of a colony was green, this indicated that the colony was C. albicans.
Following culturing of C. albicans in CHROMagar Candida medium, one colony of cultured C. albicans was mixed with 5 ml peptone in a tube then incubated at 37°C for 24 h. After 24 h, the turbidity of media was compared to a 0.5 McFarland solution standard, equivalent to 1.5 x 108 CFU/ml.
Flavonoid test. A total of 5 ml seaweed extract were mixed with 0.5 cm Mg band and two drops of HCl then heated by passing over a Bunsen flame. The coloration to red or purple after heating indicated the presence of flavonoids11.
Alkaloid tests. A total of 5 ml seaweed extract and 8 ml HCl were mixed to homogeneity then filtered. The filtrate was then subjected to Mayer, Wagner and Dragendroff tests for alkaloids to ensure detection of any alkaloids, based on those described by Vimalkumar et al.11. For the Mayer test, approximately 2 ml filtrate was mixed with 5 g potassium mercuric iodide. The formation of white or pale precipitates indicates the presence of alkaloids. For the Wagner test, a total of 2 ml filtrate was mixed with 2 ml Wagner reagent. The formation of brown or reddish-brown precipitates indicates the presence of alkaloids. For the Dragendroff test, 2 ml of filtrate was mixed homogenously with bismuth potassium iodide solution, the red precipitates indicate the presence of alkaloid.
Tannin/phenolic test. Two drops of 1% FeCl3 was added to 1 ml seaweed extract. The change in the color to a blackish green indicates the presence of tannin/phenolic content12.
Saponin test. A total of 1 ml seaweed extract was mixed with distilled water to 20 ml then shaken vertically for 15 s. Persistent foaming is indicative of saponin content.
Steroid test. Approximately 2 ml seaweed extract was diluted in 2 ml CHCl3, a few drops of H2S and 1 ml of CH3COOH. The formation of green or blue precipitates indicates the presence of steroid11.
Terpenoid test. A total of 5 ml seaweed extract was mixed in 2 ml of chloroform followed by the careful addition of 3 ml concentrated H2SO4. A layer of the reddish brown coloration was formed at the interface thus indicating a positive result for the presence of terpenoids13.
A total of 100 µl casein-peptone lecithin polysorbate broth (Merck-1117230500) was prepared in each well of a 96-well plates for 5 min then the peptone was removed from the wells. A total of 50 µl cultured C. albicans, which diluted to a 0.5 McFarland standard turbidity, were added into 96-well plates and left in wells for 5 min. Next, the seaweed extracts were added at decreasing concentrations test (100, 75, 50, 25, 12.5 and 6.25%), with fluconazole 0.31 µg/ml as a control. The plates were incubated for 24, 48 or 72 h at 37°C, then approximately 200 µl of 0.1% violet crystal were added into the plates and incubated for 15 min at room temperature.
After 15 min, each well was washed three times with 200 µl PBS. The crystal violet in each well was then dissolved in 100 µl 96% ethanol for 2 min. The biofilm formation was analyzed using an ELISA reader at 620 nm wavelength14,15.
The results of phytochemical tests, showed that seaweed G. verrucosa extract had the positive reaction to a steroid, terpenoid, and tanin indicates these substances are present in the seaweed (Table 1).
In general, the inhibitory effect was increased as seaweed concentration increased. Results of Kruskal-Wallis analysis (P<0.05) showed that seaweed extract significantly inhibited formation of the biofilm of C. albicans. However, a higher optical density was recorded for fluconazole (control), followed by 100% seaweed extracts in all exposure times; there were no significant differences between these treatments. The results showed that the best inhibition effect was recorded with fluconzole followed by 100% seaweed extract 48 h after exposure (Figure 1).
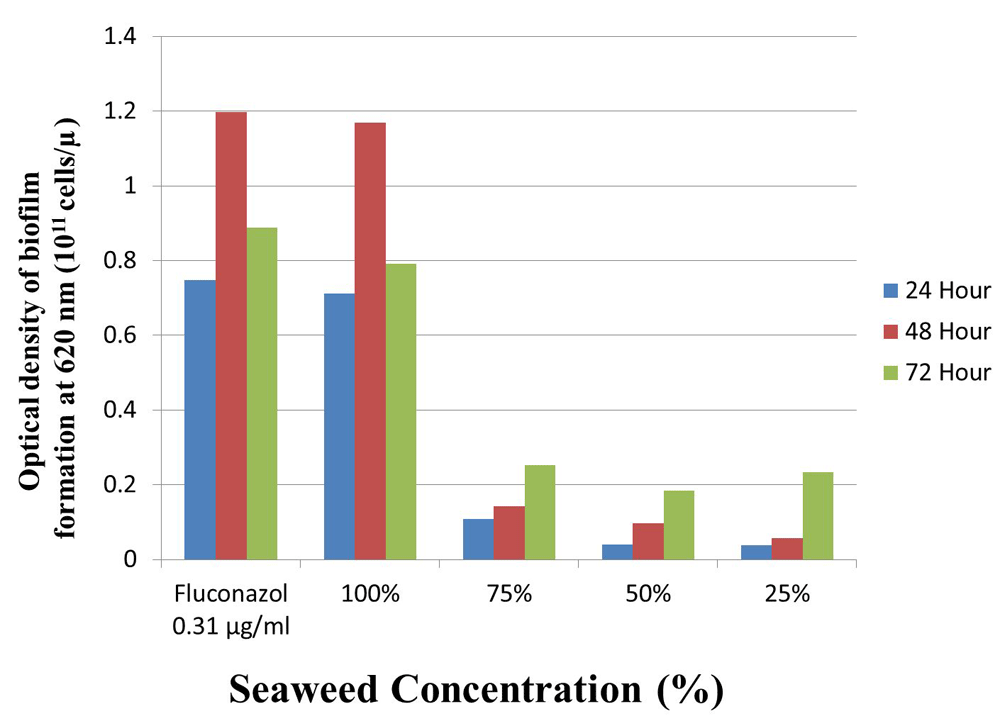
The study showed that 100% seaweed extract is promising for inhibiting the growth of C. albicans, indicating that it has the potential to be used as an anti-fungus C. albicans to treat oral cardiosis in smokers. C. albicans is a normal micro-organism in the human mouth; however, this fungus can be pathogenic in certain circumstances3, such as in the mouth of smokers2. Smoking can increase the protein levels of HWP1, EAP1 and SAP2 in C. albicans. Higher levels of these proteins increase the virulence of C. albicans. This can then increase biofilm formation and cause oral candidiasis6. In addition, smoking can also cause a decrease in immune function, making individuals more susceptible to oral candidiasis4,6.
The results showed that treatment with 100% seaweed extract can inhibit the formation of C. albicans biofilm to an almost equivalent degree as the fluconazole (control), This activity is presumably caused by the bioactive compounds in the extract of seaweed, such as the steroids, terpenoids, and tannins that were detected in this study. According to Sampaio et al.17, the anti-fungal activity of a substance strongly depends on the composition of its bio-active compounds; these bio-active compounds have the potential to cause destruction to the biofilm and affect the viability of C. albicans; for example, steroids can kill C. albicans through their lipophilic properties, interfering with the formation of fungal spores and mycelium18. This activity weakens C. albicans, inhibiting the formation of the biofilm. The activity of the steroids requires oligosaccharides that are also present in the seaweed content to function optimally19.
Terpenoids are derivatives of saponins. Terpenoids act as an antifungals by damaging the organelles of the fungi and inhibiting the secretion of enzymes, leading to inhibition of the growth of C. albicans fungal cells. Terpenoids can also damage the morphology of C. albicans20. Tannins may inhibit chitin synthesis in C. albicans cell walls; as a result, there is no protection of the C. albicans cell membrane and can cause inhibit cellular metabolism. In addition, tannin can inhibit ergosteron activity of Candida albicans21.
The effectiveness of the extracts in inhibiting fungi is influenced by at least three factors, namely the concentration, exposure time, and contact surface media22. The present study showed that the inhibitory effect of seaweed extract increased as seaweed extract concentration increased, with the best effect recorded at 48 h of exposure, this is probably because the farnesol works effectively after 48-72 h of exposure. Farnesol is a quorum-sensing molecule that has the potency to inhibit C. albicans growth23.
Further studies should be conducted to extract the individual bioactive compounds in seaweed then test their action on C. albicans at different dosages. The purpose of these further studies will be to assess which bioactive compound, and at which dosages, are playing a vital role in inhibiting the growth of C. Albicans.
Gracilaria verrucosa seaweed extract inhibited the growth of the biofilm of C. albicans isolated from the saliva of a smoker, with the inhibitory effect increasing with the concentration, up to an optimal concentration of 100% at 48 h of exposure.
Dataset 1. The raw data of the Triplo anti-Biofilm seaweed to C. albicans for 24, 48 and 72 h at a wavelength 620 nm. DOI: 10.5256/f1000research.14879.d20427016.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microbiology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Periodontology, natural product drug discovery
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
References
1. Sebaa S, Hizette N, Boucherit-Otmani Z, Courtois P: Dose‑dependent effect of lysozyme upon Candida albicans biofilm.Mol Med Rep. 2017; 15 (3): 1135-1142 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 18 Sep 18 |
read | read | |
|
Version 2 (revision) 16 Aug 18 |
read | read | |
|
Version 1 31 May 18 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (1)