Keywords
Candida albicans, oral candidiasis, seaweed Gracilaria verrucosa
Candida albicans, oral candidiasis, seaweed Gracilaria verrucosa
Some revisions and improvements have been done throughout the manuscript as responses to the good comments and suggestions from reviewers. Major revisions performed include grammar check and corrections; sentence improvements; and reorganization and adding appropriate details in each section of the Methods part. Grammar issues, a concern of all reviewers, have been addressed. The ways to present results were also changed as response to comment from Dr Heni Susilowati about the meaning of OD values in presenting the growth of C. albicans and how to interpret them. Restructure of Results and Discussion, therefore, was also performed. These changes became the basis for synthesizing more appropriate Conclusion sentences. One reference was also added to support discussion about the importance of extract concentration for resulting in expected medicinal effects, in this study was growth inhibition that reflected by biofilm formation. Since this study is still in preliminary step, no non-smoker participant was participated. As consequence, no comparison could be made between smoker and non-smoker groups. Yet, the study was still able to confirm potential of seaweed Gracia verrucose extract as anti candidiasis in smokers. Potential of doing further studies was mentioned in relation to this weakness of our study.
See the authors' detailed response to the review by Shahida Mohd-Said
See the authors' detailed response to the review by Heni Susilowati
Smoking is a common problem in both developed and developing countries, including in Indonesia. Based on a survey by the Tobacco Atlas in 2015, Indonesia has the highest number of smokers in Asia, with 66% of men in Indonesia being active smokers1. Smoking can lead to addiction owing to the nicotine contents, and harm due to the presence of toxic compounds such as carbon monoxide, ammonia and tar contents in tobacco1. Substances in cigarettes can also contribute for the occurrence of oral candidiasis, infection of in the mouth cavity caused by the fungus Candida albicans2. This fungus is part of the normal flora of the human mouth, but it can become pathogenic in certain conditions, for example, due to nicotine exposure3.
The C. albicans that infects human tissues generally form biofilm3, an extracellular matrix consisting of C. albicans colonies4. The size of the biofilm increases when the fungus was exposed to substances in cigarette smoke because cigarette contains chemicals that can initiate the growth of and nourish C. albicans5,6.
Currently, fluconazole and nystatin are the most effective drugs for treating oral candidiasis. Unfortunately, these drugs could result in undesired side effects. Prolonged use of fluconazole, for example, can lead to resistance7 whereas high dosages of nystatin give gastrointestinal discomfort and increase plaque formation8. Therefore, plant-derived antifungals may be viable oral treatment options for candidiasis. One of these potential plants is Gracilaria verrucosa. This seaweed contains several bioactive compounds, including alkaloids, flavonoids, phenolics, saponins, steroids and terpenoids9. Aceh Province, Indonesia, has large G. verrucosa resources, although this aquatic plant has not been commonly used for medicinal purposes. Hence, the objective of the present study was to examine the ability of seaweed G. verruosa extract to inhibit the biofilm formation of C. albicans isolated from the saliva of smoker.
The study was conducted in August 2017 at the Laboratory of Microbiology, Veterinary Faculty, Syiah Kuala University. The G. verrucosa seaweeds were collected from a farmer in Pulo Aceh, Aceh Province.
All research protocols used in this study were approved by the Research Ethics Committee of the Dentistry Faculty of Syiah Kuala University No. 1741/UN11.1.21/TU/2017.
The saliva was collected from an active smoker who worked as administrative staff at the Faculty of Dentistry Medicine of Syiah Kuala University and voluntarily participated in this study after providing written informed consent. Inclusion criterion of the volunteer was active smoker who smoked 20 cigarettes per day. Saliva was collected once by spitting into a glass jar (15 ml) right after the subject finished smoking, and added with 10 ml of PBS (0.01 M, pH 7.2). The jar was centrifuged at 10,000 rpm for 10 minutes. The precipitate was stored for the microbiological examination.
Precipitate was cultured in ChromAgar Candida medium and incubated at 37 °C for 2 days. The C. albicans fungus grew as green colonies. One colony of C. albicans was mixed with 5 ml of peptone in a tube and incubated at 37°C for 24 hours. Turbidity of medium was compared to a 0.5 McFarland solution standard, which was equivalent to 1.5 × 108 CFU/ml.
Seaweed extraction was performed by maceration using 96% methanol10. In brief, a total of 500 g of fresh seaweeds were washed with tap water then with distilled water. Seaweed samples were dried at 25°C for 24 hours, chopped into small pieces (2 mm), and soaked in 1.5 liters of 96% methanol. Macerate was filtered using Whatman filter paper No. 42. Filtrate collected was concentrated using a vacuum rotary evaporator (Laborta 4003 control, Heildolph) 60°C and at a speed of 80-90 rpm for 3 days. Concentrated extract was put in a sealed dark bottle and stored at 4 °C.
Flavonoid test. A 0.5 cm magnesium plate was rinsed in 5 ml of seaweed extract, mixed with two drops of HCl, and heated by passing it over a Bunsen flame. Red or purple coloration formed on the heating indicated the presence of flavonoids11.
Alkaloid test. Seaweed extract (5 ml) was mixed with 8 ml of HCl and filtered. Filtrate was subjected to Mayer, Wagner and Dragendroff tests for alkaloids11. This was done by mixing 2 ml of filtrate with 5 g potassium mercuric iodide (Mayer test), 2 ml of Wagner reagent, or 2 ml of bismuth potassium iodide solution (Dragendroff test). The formation of white or pale precipitates (Mayer test), brown or reddish-brown precipitates (Wagner test) and red precipitates (Dragendroff test) indicated the presence of alkaloids.
Tannin/phenolic test. Two drops of 1% FeCl3 was added to 1 ml seaweed extract. The change in the color to a blackish green indicated the presence of tannin/phenolic12.
Saponin test. Seaweed extract, 1 ml, was diluted in 20 ml of distilled water and shaken vertically for 15 seconds. Persistent foaming indicated the presence of saponin content.
Steroid test. Two milliliters of seaweed extract was added with 2 ml of CHCl3, 2 drops of H2S and 1 ml of CH3COOH. The formation of green or blue precipitates indicated the presence of steroid11.
Terpenoid test. Seaweed extracts (5 ml) was mixed with 2 ml of chloroform. Concentrated H2SO4, 3 ml, were carefully added. The formation of reddish brown layer at the interface of extract and chloroform solution indicated the presence of terpenoids13.
Casein-peptone lecithin polysorbate broths (Merck-1117230500), 100 µl, were poured in each well of a 96-well plate, incubated at room temperature for 5 minutes and discarded. Fifty microliter C. albicans culture had turbidity was equal to 0.5 McFarland standard were added to each well and incubated for 5 minutes. In triplicate, decreased concentrations of seaweed extracts (100, 75, 50, 25, 12.5 and 6.25%) were added. The same reaction using fluconazole 0.31 µg/ml was prepared as positive control. The plate was then incubated at 37 °C for 24, 48 or 72 hours. Each well was added with 200 µl of 0.1% violet crystal, incubated at 25 °C for 15 min, and washed three times with 200 µl of 0.01 M PBS. The crystal violet in each well was then removed by adding 100 µl of 96% ethanol for 2 min. The biofilm formation was analyzed by reading optical density of mixture using an ELISA reader at 620 nm14,15.
The results of phytochemical tests in Table 1 show that methanol extract of seaweed G. verrucosa contained bioactive compounds belonged to steroids, terpenoids, and tannins/polyphenols.
In general, the inhibitory effect was reduced as seaweed concentration increased (Figure1). The highest inhibition after 24 and 48 hours time exposures was recorded from C. albicans culture treated with 25% seaweed extract, followed by those with 50% and 75%. The best inhibition after 75 hours time exposure, however, was found in C. albicans culture treated with 25% seaweed extract. In all time exposures, the inhibitory effect caused by fluconazole (control) was the worst, followed by those caused by 100% seaweed extracts, but there was no difference between these two groups. Results of Kruskal-Wallis analysis showed that seaweed extract significantly (p<0.05) inhibited the formation of biofilm by C. albicans, indicated potential of the extract to inhibit the growth of the fungus.
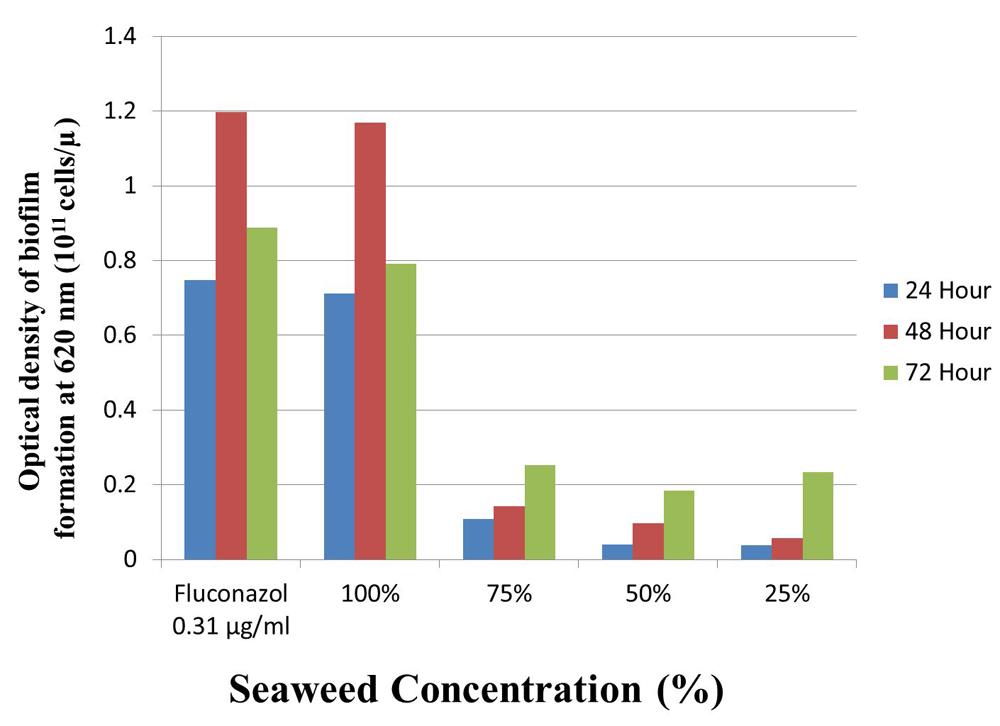
The study showed that 25–75% seaweed extracts are promising for inhibiting the growth of C. albicans as shown by much lower biofilm formation after 24, 48 and 72 hours exposure compared to those caused by positive control (fluconazole) and 100% seaweed extract. This indicated potential of seaweed extract as natural anti-fungus to treat oral candidiosis caused by against C. albicans in smokers.
C. albicans is a normal micro-organism in the human mouth. This fungus, however, can be pathogenic in certain circumstances3 such as in the mouth of smokers2. Smoking can stimulate synthesis of HWP1, EAP1 and SAP2 proteins in C. albicans, causing higher virulence of the fungus. This can lead to increased formation of biofilm and finally cause oral candidiasis6. Smoking also cause a decreased immune function, making individuals more susceptible to oral infections, including candidiasis4,6.
The ability of 25–75% seaweed extracts to inhibit the formation of C. albicans biofilm significantly stronger than those of fluconazole (positive control) was probably caused by bioactive compounds presence in the extracts. These included steroids, terpenoids, and tannins/polyphenols, all of which have been known their benefits for human health. According to Sampaio et al.16, antifungal activity of a substance strongly depends on the composition of its bioactive compounds. Steroids can kill C. albicans through their lypophilic properties, interfering with the formation of fungal spores and mycelium17. This activity weakens C. albicans, inhibiting the formation of the biofilm. To function optimally, steroids require oligosaccharides that are also present in the seaweed content18.
Terpenoids are derivatives of saponins that may act as an antifungals by damaging organelles of the fungus and by inhibiting secretion of enzymes, leading to the inhibition of C. albicans cell growth. Terpenoids can also damage the morphology of C. albicans19. Tannins may inhibit chitin synthesis in C. albicans cell walls, leading to lost of membrane cell protection and disrupted cellular metabolism. Tannins also can inhibit ergosteron activity of C. albicans20.
The effectiveness of seaweed extracts in inhibiting fungal growth is influenced by at least three factors, namely concentration, exposure time, and contact surface media21. The present study showed that diluted concentration (25–75%) of extracts showed better inhibitory effect on the growth of C. albicans than the more concentrated 100% extract. Concentrated extract usually has lesser effectiveness in vitro due to solubility and import problems. In more aqueous condition plant extracts generally show better medicinal properties with increased concentration as shown by a study testing antifungal activity of ethanol extracts of Syzygium jombolanum, Cassia siamea, Ordina wodier, Momodica charantia and Melia azedarach as well as two algal species Sargassum wightii and Saulerpa scalpellformis against 25 C. albicans isolates in vitro22.
This study also showed the best inhibitory effect of seaweed extracts was recorded at 24 hour of exposure. This is probably because the farnesol, a quorum-sensing molecule that has the potency to inhibit C. albicans growth23, works effectively after 48–72 hours of exposure.
This study, however, was still preliminary due to limited number of subject and unavailability of non-smoker, control. Virulence factor of C. albicans analyzed was also only biofilm formation. Since more virulence factors are possibly synthesized by the fungus under certain environmental condition, further studies are need to be conducted to investigate effect of individual bioactive compound contained in the seaweeds on the formation of biofilm and on the expression of other virulence factors of C. albicans isolated form both smoker and non-smoker individuals.
Extract of Gracilaria verrucosa seaweed could inhibit the growth of C. albicans isolated from the saliva of a smoker. The inhibitory effect decreased with the increase of concentration, and reached the highest at concentration of 25% and time exposure of 24 hours.
Dataset 1. The raw data of the Triplo anti-Biofilm seaweed to C. albicans for 24, 48 and 72 h at a wavelength 620 nm. DOI: 10.5256/f1000research.14879.d20427024.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Periodontology, natural product drug discovery
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular Biology and Oral Microbiology
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microbiology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Periodontology, natural product drug discovery
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
References
1. Sebaa S, Hizette N, Boucherit-Otmani Z, Courtois P: Dose‑dependent effect of lysozyme upon Candida albicans biofilm.Mol Med Rep. 2017; 15 (3): 1135-1142 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 18 Sep 18 |
read | read | |
|
Version 2 (revision) 16 Aug 18 |
read | read | |
|
Version 1 31 May 18 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (1)