Keywords
Pegasparginase Activity, Premedication, Silent Inactivation, Allergy, Anaphylaxis
This article is included in the Oncology gateway.
Pegasparginase Activity, Premedication, Silent Inactivation, Allergy, Anaphylaxis
1) Reanalyzing the data showed a new finding that was previously missed and not previous report in any publication to our knowledge. Specifically we found the day 6-8 asparagase activity was significantly greater in in samples from patients where pre-medications were given.
2) We added power calculations to show that our study was underpowered to show a significant decrease in allergic reactions with the use of pre-medications
3) Citations of two recently published studies showing that pre-medications reduce allergic reactions were added.
See the authors' detailed response to the review by Vinod Pullarkat
See the authors' detailed response to the review by Bernard L. Marini
See the authors' detailed response to the review by David J. Young
PEG-L-asparaginase (pegaspargase) is a critical component of therapy for children and adults with acute lymphoblastic leukemia (ALL). Its use is hampered by many issues including allergic reactions, silent inactivation, thrombosis, hyperbilirubinemia and pancreatitis1. Other common toxicities, such as hyperglycemia and hypertriglyceridemia, may be mitigated with the use of metformin2 and omega-33 and fenofibrate4. There is also ongoing interest in the use of carnitine to treat, and possibly prevent, hepatic toxicity, manifested by a severe increase in direct bilirubin, among other findings5.
The optimal dose, dose interval and target asparaginase level for pegaspargase is not completely established6. In pediatrics, a dose of 2500 units/m2 is the norm, whereas for adult patients, doses are often reduced due to increased toxicity at the pediatric dose. Some investigators have suggested using a pharmacokinetic driven model to individualize pegaspargase dosing7.
The use of premedication (acetaminophen, diphenhydramine and a corticosteroid) has been suggested as a possible means of reducing allergic reactions. In a multi-center study testing the use of pediatric-based regimens in young adults, the rate of grade 3 or 4 allergic reactions was reduced from 10% to 4% after premedication was mandated8. A study in adults with ALL reported allergic reactions in 7.2% of patients when pegaspargase was given concurrently with, or followed by, one week of prednisone9. Using a novel mouse model of asparaginase hypersensitivity, pretreatment with seven days of oral dexamethasone was the only agent capable of mitigating the severity of hypersensitivity and partially restoring asparaginase activity10. Dexamethasone given at the time of, or for one week following, asparaginase was not as effective.
The presence of antibodies against asparaginase may be found, from as early as the end of induction therapy11,12. The presence of asparaginase antibodies had sensitivity of 87–88% and specificity of 68–69% for clinical reactions11. The presence of asparaginase antibodies at end of induction did not appear to alter prognosis in a large multi-center study12. This suggests that measuring asparaginase activity is more useful than looking for the presence of antibodies.
Silent inactivation of pegaspargase activity by anti-asparaginase antibodies or other immune-mediated mechanisms are potentially of greater concern than bone-fide allergic reactions, as patients with grade 3–4 allergic reactions to pegaspargase will be switched to erwinase, which theoretically will improve outcome. The true incidence of silent inactivation is unknown, as there are no reports of a comprehensive screening program for silent inactivation in a large multi-institutional trial. The largest published study found silent inactivation in 7/89 (8%) of patients13. However, these patients received induction with native Escherichia coli asparaginase before switching to pegaspargase, which is not current practice. The authors also report in the same group of patients that silent antibodies may spontaneously resolve with continued pegaspargase14. Notably, lower silent inactivation with pegaspargase than native Escherichia coli asparaginase have been reported15–17 Prudence suggests that patients who receive premedications should have pegaspargase activity monitored after every dose, due to the possible but unproven concern that premedication will mask allergic reactions and silent inactivation. In fact, a consensus panel of experts recommends screening for silent inactivation in all patients undergoing therapy for ALL with asparaginase18. Additionally, the low grade 1–2 allergic reactions that are more common than silent inactivation do require pegaspargase activity monitoring to ensure a switch to erwinase if confirmed as true inactivation.
As the use of premedications and measurement of pegaspargase activity was considered by the leukemia provider group at Children’s Minnesota to be necessary for optimal care, no informed consent was obtained. Parents/adult patients were not informed of results unless intervention was indicated, which did not occur. This retrospective review study was approved by the institutional review board of Children’s Minnesota (IRB# 1606-062).
This retrospective study occurred in a large pediatric oncology center that diagnoses and treats approximately 40 new cases of ALL yearly in children and young adults up to age 30. If there are open studies, the patients are enrolled on Children’s Oncology Group protocols. Otherwise, patients are treated according to the most recent risk adapted protocols for standard risk B, high risk B and T-ALL. In order to reduce acquisition bias, charts of every patient in first remission who received pegaspargase from December 2013 to September 2016 were abstracted (N=99). As this was a pilot study and the expected reduction of grade 3 or 4 allergic reactions with premedications was unknown at the time, sample sizes calculations could not be calculated. Data from all 99 patients were used to estimate the incidence of grade 3 or 4 allergic reactions by patient and by dose. For the detailed pharmacokinetic analysis, we used a subgroup of all patients from May 2014 to September 2016 (N=46) who had pegaspargase levels drawn. This number was sufficient to define the confidence intervals of the pegaspargase activity.
A total of 112 blood samples from these 46 patients were collected from a central venous portacath in conjunction with scheduled clinical visits from 3 to 12 days following pegaspargase administration at the standard dose of 2500 mg/m2. Pegaspargase was given by intramuscular injection or intravenously per Children’s Oncology Group protocols on an intermittent schedule starting with induction and completed prior to starting maintenance therapy. Because the distribution of the collection days clustered in ranges from day 3–5, 6–8 and 10–12, for analyses, pegaspargase activity was grouped in these categories. One data point was omitted from analysis in this version of the manuscript because it was and extreme outlier that was not congruent with other values from the patient or group (UPN 11; day 7 after 3rd dose; value 2.86; greater than 99.9th percentile). Two data points were removed because they were drawn after anaphylaxis and as expected undetectable (UPN 38 and 42 after 2nd dose). These values have been retained in the online dataset. To better estimate the incidence of silent inactivation, pegaspargase levels lower than 0.01 units/ml were looked for in the data from an additional 13 patients making a total of 59 evaluated. No evidence of silent inactivation was found in these 13 patients.
These patients were all treated according to Children’s Oncology Group protocols, using either intramuscular or intravenous pegaspargase as the only form of asparaginase. Intramuscular asparaginase was the standard of care until 2010 when intravenous administration became the new standard of care based on the Children’s Oncology Group AALL0932 protocol19. A comprehensive review of published studies concluded that the risk of grade 3 or 4 allergic reactions is independent of the pegaspargase route of administration19.
We became aware of an abstract showing a decrease in grade 3 or 4 allergic reactions in a multi-institutional study employing pegaspargase in young adults with ALL20. This prompted us to institute in May 2015 strict manditory premedication with acetaminophen (10–15 mg/kg orally), diphenhydramine (1 mg/kg orally or intravenously), and methylprednisolone (1 mg/kg intravenously), within the hour prior to administering pegaspargase. Every subsequent patient was to receive with all three of the premedication drugs without exception. The numbers with and without premedication are listed in Table 2 (per pegaspargase dose) and Table 3 (per patient).
Allergic reactions to were graded per CTC 4.0 toxicity scales. We compared the incidence of grade 3 or 4 allergic reaction in patients with and without premedication, both per pegaspargase dose and per patient.
Routine monitoring of pegaspargase activity in patients with ALL was initiated in 2013 after the ‘asparaginase activity analysis’ test approved by Clinical Laboratory Improvement Amendments was introduced by AIBioTech, Richmond, VA 23225 US. Subsequent to the introduction in 2015 of a quantitatively identical test by Next Molecular Analytics, Chester, VA, samples were exclusively sent there.
SPSS version 23 was used for graphing and analyses. Grouped data were displayed with box graphs depicting the ~1st, 25th, 50th (median), 75th and ~99th percentiles. The data was normally distributed by visual inspection of the normal curve and Kolmogorov-Smirnov test, with the exception of the day 6–8 pegaspargase activity level. Removal of one extreme outlier as described above rendered the day 6–8 pegaspargase activity level normally distributed. The comparison of pegaspargase activity with and without premedication was done by independent sample t-test. The comparison of grade 3 or 4 allergic reactions by patient and pegaspargase dose with and without the use of premedication was done by chi-squared analysis. As some premedication doses were missed due to omission by the treating physician, an additional analysis of the incidences of those who received premedication after every dose or most doses were compared to those who received no premedication before any dose. Missed pegaspargase activity samples were omitted from analysis (Table I).
| One | Two | Three | Four | Five | Six | Seven | Eight | Nine | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Collected | 8 | 33 | 18 | 19 | 18 | 6 | 7 | 2 | 1 | 112 |
| Missed | 38 | 12 | 16 | 10 | 7 | 6 | 3 | 3 | 1 | 96 |
| Total | 46 | 45 | 34 | 29 | 25 | 12 | 10 | 5 | 2 | 208 |
| % Collected | 17% | 73% | 53% | 66% | 72% | 50% | 70% | 40% | 50% | 54% |
| Premeds used with the dose | Total doses | Doses with allergic reaction | Percent of doses with grade 3–4 allergic reaction |
|---|---|---|---|
| no | 155 | 8 | 5.2% |
| yes | 185 | 7 | 3.7% |
Pharmacokinetic analyses were done on 100 specimens from 46 patients. The 46 patients included 12 standard risk B-cell patients, 21 high risk B-cell ALL patients, and 13 T-cell ALL patients. There were 25 males and 21 females. The ages ranged from one to 29 years with a median of 8.3 years. The number of specimens and missed specimens per pegaspargase dose number are shown in Table 1. These include all patients who had pegaspargase activity collected which included those eliminated from other analyses as they were not in first remission. First dose specimens were frequently missed, whereas specimens on doses two to seven were collected at least half the time.
Figure 1 is a box and whisker graph of pegaspargase activity on days 3–5, 6–8 and 10–12. The mean, standard error of the mean, standard deviation and number of data points are: 1.37, 0.21 0.76 units/mL and 13, respectively, for day 3–5; 0.88, 0.04, 0.31 units/mL and 61 for day 6–8; 0.89, 0.06, 0.28 units/mL and 26 for day 10–12. These values are similar to those previously reported in pediatric patients with ALL21,22.
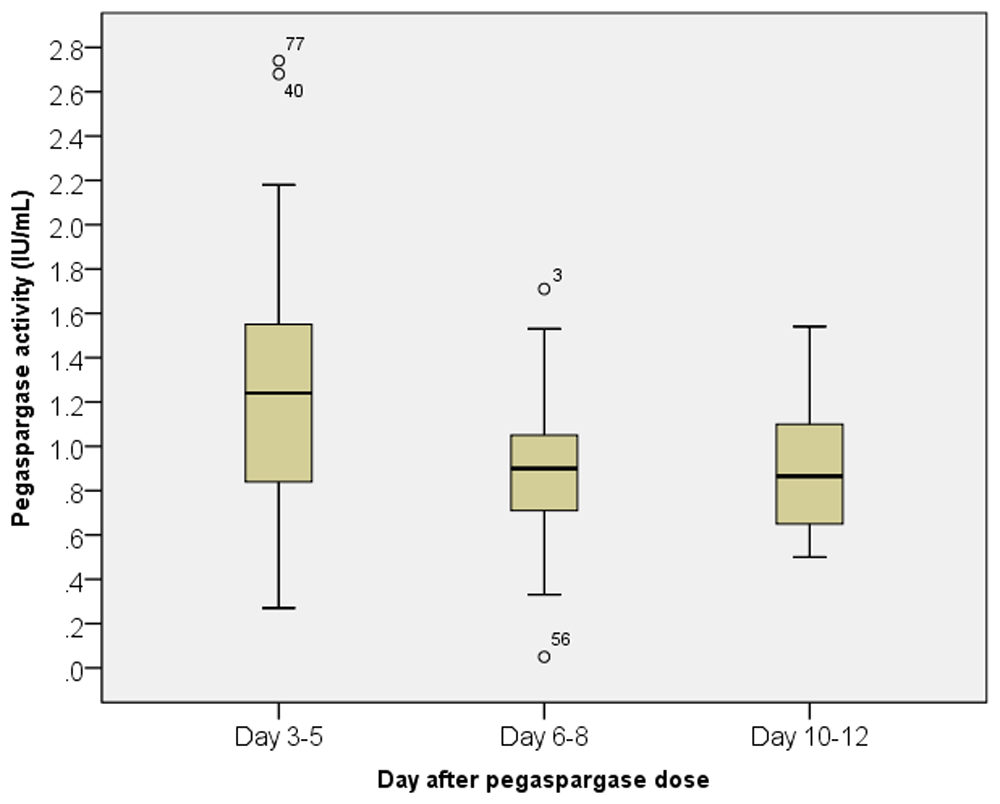
The data point below the line is from the patient with silent inactivation. Data points outside of the whiskers of the ~1st and ~99th percentiles are represented by a circle (outlier more than 1.5 times the interquartile range). The attached number is a data point and not a value.
Figure 2 is box and whisker graph of the pegaspargase activity on day 6–8, following doses with or without premedication. This time ranged was used for the comparison as it is the most common time for checking asparaginase activity. The mean and standard deviation for the no premedication group is 0.69 and 0.21 units/mL (N=12 samples), respectively, and for the premedication group is 0.93 and 0.32 units/mL (N=49 samples). These were significantly different by independent samples t-tests for equal variances not assumed (p = 0.003). The day 3–5 data had only one patient in the no premedication group so was not analyzed. The day 10–12 data showed higher median value in the group that received premedications (0.92 vs 0.83) which was not statistically significant due to insufficient power.
Only one patient had silent inactivation with the following activity levels by dose number and day following pegaspargase activity was checked: dose 1, day 24 - 0.11 units/mL; dose 2, day 8 - 0.05 units/mL; dose 3 day 6 - 0.01 units/mL; dose 4 day 8 - 0.33 units/mL; dose 5, day 8 - 0.82 units/mL; and dose 6, day 10 - 0.62 units/mL The low values after doses 2 and 3 were not reviewed due to a clerical error until after dose 4, which showed adequate activity, so pegaspargase was continued until the end of treatment.

Patients who received premedication has a significantly greater value (p=0.003). The mean and standard deviation for the premedication group is 0.93 and 0.32 units/mL and for the no premedication group is 0.69 and 0.21 units/mL. Data points outside of the whiskers of the ~1st and ~99th percentiles are represented by a circle (outlier more than 1.5 times the interquartile range). The attached number is a data point and not a value.
For the analysis of the role of premedication in preventing grade 3–4 allergic reactions, the data was analyzed per pegaspargase dose and per patient. In the analysis per pegaspargase dose, premedication did not significantly reduce grade 3–4 allergic reactions. However due to insufficient numbers the power was only 0.24. With premedication, 7/185 (3.7%) had grade 3–4 allergic reactions compared to 8/155 (5.2%) without premedication (Table 2).
Table 3 shows the incidence of grade 3–4 allergic reactions per patient. Without premedication, 7/42 (17%) had grade 3–4 allergic reactions. When premedication was given most of the time (usually the first dose was missed), 4/62 (6%) had grade 3–4 allergic reactions. When premedication was given for every dose, 4/34 (12%) had allergic reactions. There was no significant effect of premedication on grade 3–4 allergic reactions by dose when the premedication group (8/96; 8.3%) was compared to the no premedication group (7/42; 17%) (chi square = 2.09; p = 0.15) (Table 3). However due to insufficient numbers the power was only 0.54. There was no difference in the distribution of patients who did or did not receive premedication by risk group.
Compared with historical controls that received similar therapy, premedication did reduce the incidence of grade 3 or 4 allergic reactions when measured per patient or per dose of pegaspargase. The power calculation was only 0.54 per patient and 0.24 per pegaspargase dose, thus our study was underpowered to show statistical significance due to insufficient numbers. As premedication does not negatively affect pegaspargase activity levels, and other studies using historical comparisons have suggested premedication may reduce allergic reactions, we are continuing the practice8,9.
The interesting observation by Tong et al. that asparaginase antibodies generated after native E. coli asparaginase may resolve while on pegaspargase continuation therapy needs to be confirmed in patients who receive only pegaspargase during induction and beyond14,19. We noted a transient decrease in pegaspargase activity, likely due to silent inactivating antibodies in 1/59 patients (1.7%). This decrease of pegaspargase activity occurred after the second dose in a high-risk B-cell ALL patient and resolved with continuation of pegaspargase dosing. No decrease in pegaspargase activity was seen in standard risk patients who received only two doses of pegaspargase in combination with oral dexamethasone. This observation is limited by small numbers of patients and multiple missed levels after the first pegaspargase dose.
Limitations of the study include multiple missed activity levels that may have found additional patients with silent inactivation. The sample size also makes it difficult to estimate the true incidence of silent inactivation and if premedication reduces the incidence of grade 3 or 4 allergic reactions. Additional studies are needed to clarify this. Another limitation is the patients had only one activity level per pegaspargase dose, limiting evaluation of true pharmacokinetics especially in patients with higher or lower than expected levels.
We found low incidence of silent inactivation with intravenous pegaspargase. Our study suggests that patients treated with regimens that include only two doses of pegaspargase, given with dexamethasone, may not need asparaginase levels due to even lower silent inactivation, but this would need confirmation by larger studies. We do suggest that patients who have questionable allergic reactions (grade 1–2) would benefit from asparaginase levels that will direct switch to erwinase if confirmed as true inactivation.
Our data showed a statistically significantly greater pegaspargase activity of the day 6–8 pegaspargase level with the use of premedication. Analysis of the day 3–5 and 10–12 pegaspargase levels did not show a significant difference likely due to the small sample sizes.
The finding of difference in the pegaspargase level with premedication has not been previously described to our knowledge. This may be of clinical significance and should, if possible confirmed in other retrospective studies. Given the increased acceptance of premedication for pegaspargase it is unlikely and perhaps unethical to confirm in a prospective randomized trial, in our opinion.
A recent publications by 2 other groups also reported reduction of infusion reactions and need for erwinase using premedication. They also found a low incidence of silent inactivation with intravenous pegaspargase of 1.5% in one report and < 1% in another report similar to our finding of one in 59 patients (1.7%)23,24.
The authors would like to thank the Children’s Minnesota leukemia nurse case managers who did and continue to do an outstanding job to ensure PEG activity is collected on as many patients as possible.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pediatrics, pediatric hematology/oncology, drug development, clinical trials, leukemia
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pediatrics, pediatric hematology/oncology, drug development, clinical trials, leukemia
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: Speakers Bureau and Advisory board for Servier and Jazz pharmaceuticals
Reviewer Expertise: Treatment of acute lymphoblastic leukemia
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Cooper SL, Young DJ, Bowen CJ, Arwood NM, et al.: Universal premedication and therapeutic drug monitoring for asparaginase-based therapy prevents infusion-associated acute adverse events and drug substitutions.Pediatr Blood Cancer. 2019; 66 (8): e27797 PubMed Abstract | Publisher Full TextCompeting Interests: Servier (consultant)
Reviewer Expertise: Asparaginase, leukemia treatment, therapeutic drug monitoring
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 17 Jan 20 |
read | ||
|
Version 1 04 Jul 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)