Keywords
Zika, Disease outbreaks, arboviruses, causality, Guillain-Barré syndrome, congenital abnormalities
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Living Evidence collection.
This article is included in the Zika & Arbovirus Outbreaks collection.
Zika, Disease outbreaks, arboviruses, causality, Guillain-Barré syndrome, congenital abnormalities
The Zika virus (ZIKV), a mosquito-borne flavivirus, caused a large outbreak of infection in humans in the Americas between 2015–2017 (WHO Zika -Epidemiological Update). Since then, the circulation of ZIKV has decreased substantially in the Americas1 but ZIKV transmission will likely continue at a lower level2. Smaller outbreaks have been reported from countries in Africa and Asia, including Angola, India3, and Singapore4. Regions with endemic circulation, such as Thailand5, have the potential for new ZIKV outbreaks with adverse outcomes6.
The World Health Organization (WHO) declared ZIKV as a cause of adverse congenital outcomes and Guillain-Barré syndrome (GBS) as early as September 20167, informed by a systematic review of evidence structured according to a framework of ten dimensions of causality, based on Bradford Hill (Table 1)8. The accumulation of evidence on the adverse clinical outcomes of ZIKV has barely slowed down since the WHO declared the Public Health Emergency of International Concern (PHEIC) on February 1st, 2016, with approximately 250 research publications on ZIKV appearing every month (see Zika Open Access Project). We updated the systematic review to January 18, 2017 as a living systematic review by introducing automated search methods to produce a high quality, up to date, online summary of research9 about ZIKV and its clinical consequences, for all the causality dimensions10.
The latest and previous versions of this table are available as extended data16.
| Review | Baseline8 | Update 110 | Update 2 [this review] |
|---|---|---|---|
| Period | <May 30, 2016 | May 30, 2016-January 18, 2017 | >January 18, 2017 -July 01, 2019 |
| Search strategy | “ZIKV” or “Zika” | “ZIKV” or “Zika” | Focussed search strategy (Supplementary File 2) |
| Study design | Epidemiological studies; in vivo/in vitro studies; surveillance reports | Epidemiological studies | |
| Dimensions of the causality framework based on Bradford Hill* | •Temporality (cause precedes effect) | ||
| •Biological plausibility of proposed biological mechanisms | |||
| •Strength of association | •Strength of association | ||
| •Exclusion of alternative explanations | |||
| •Cessation (reversal of an effect by experimental removal of, or observed a decline in, the exposure) | |||
| •Dose-response relationship | •Dose-response relationship | ||
| •Experimental evidence from animal studies | |||
| •Analogous cause-and-effect relationships found in other diseases | |||
| •Specificity of the effect | •Specificity of the effect | ||
| •Consistency of findings across different study types, populations and times | •Consistency of findings across different study types, populations and times | ||
Since 2017, understanding about the pathogenesis of how ZIKV causes congenital abnormalities has evolved11,12. The quality of diagnostic methods, especially for acute ZIKV infection, has also improved13–15. More importantly, understanding of the limitations of diagnostic testing, and the need for interpretation in the context of other flavivirus infections, has developed. Important epidemiological questions about the associations between ZIKV infection and adverse congenital outcomes and GBS remain unanswered, however. Much of the early epidemiological evidence, which relied on surveillance data, was limited in use because of issues with the quality of the reporting and case definitions. The reported strength of association between ZIKV and adverse outcomes has varied in studies of different designs and in different settings. Evidence for a dose-response relationship with higher levels of exposure to ZIKV resulting in more severe outcomes, of clinical findings that are specific to ZIKV infection, or of adverse outcomes caused by different lineages of ZIKV was not found in the earlier systematic reviews.
The objective of this study is to update epidemiological evidence about associations between ZIKV infection and adverse congenital outcomes and between ZIKV and GBS for four dimensions of causality: strength of association, dose-response, specificity, and consistency.
We performed a living systematic review, which we have described previously10. This review updates the findings of the previous reviews8,10 and will be kept up to date, in accordance with the methods described below. Reporting of the results follows the Preferred Reporting Items of Systematic reviews and Meta-Analyses (PRISMA) statement (Extended data, Supplementary File 117)18.
This review and subsequent updates will focuse on four dimensions of causality that are examined in epidemiological study designs: strength of association, dose-response relationship and specificity of effects and consistency of association (Extended data, Table 1, Supplementary File 217). Evidence for domains of causality that are typically investigated in in vitro and in vivo laboratory studies (Table 1) was not sought. In the absence of licensed vaccines or treatments for ZIKV infection, we did not search for evidence on the effects of experimental removal of ZIKV.
We considered epidemiological studies that reported original data and assessed ZIKV as the exposure and congenital abnormalities or GBS as the outcomes. We based the exposure and outcome assessment on the definitions used in the publications. We applied the following specific inclusion criteria (Extended data, Supplementary File 217):
Strength of association: at the individual level, we selected studies that included participants both with and without exposure to ZIKV (Figure 1), such as cohort studies and case-control studies. At the population level, we included studies that assessed the outcome during the ZIKV outbreak and provided a comparison with pre or post-outbreak incidence of the outcome.
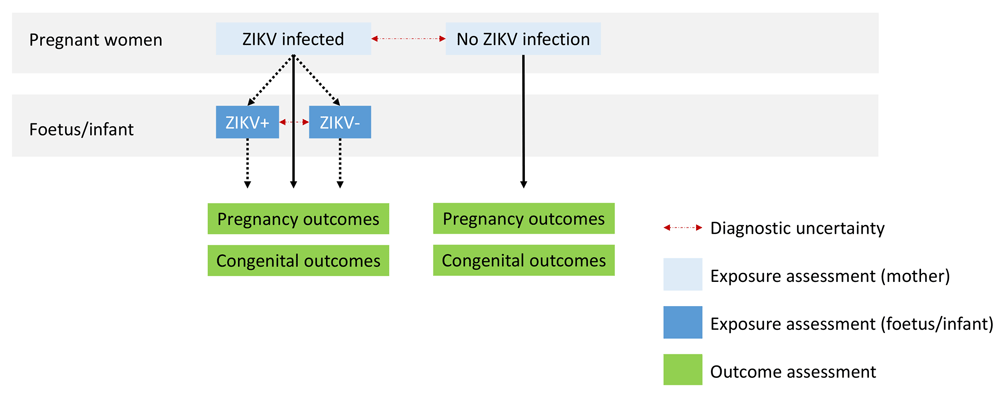
The latest and previous versions of this figure are available as extended data16.
Dose-response relationship: we included studies that assessed the relation between the level of the viral titre or the presence or severity of the symptoms and the occurrence or severity of the outcome.
Specificity of the outcome for ZIKV exposure: we included studies that assessed whether the pathological findings in cases with the outcome are specific for ZIKV infection.
Consistency: we looked at eligible studies to determine the consistency of the relationship between ZIKV exposure and the outcomes across populations, study designs, regions or strains.
We searched PubMed, Embase, LILACS and databases and websites of defined health agencies (Extended data, Supplementary File 216). We included search terms for the exposure, the outcome and specific study designs. We also performed searches of the reference lists of included publications. A detailed search strategy is presented in Supplementary File 2. For this review, the search covered the period from January 19, 2017 to July 1, 2019.
One reviewer screened titles and abstracts of retrieved publications. If retained, the same reviewer screened the full text for inclusion. A second reviewer verified decisions. One reviewer extracted data from included publications into piloted extraction forms in REDCap (version 8.1.8 LTS, Research Electronic Data Capture)19. A second reviewer verified data entry. Conflicts were resolved by consulting a third reviewer.
First, we summarised findings for each dimension of causality and for each outcome descriptively. Where available, we calculated unadjusted odds ratios (OR) or risk ratios (RR) and their 95% confidence interval (CI) from published data for unmatched study designs. For matched study designs, we used the effect measure and 95% CI presented by the authors. For publications that presented results for multiple measures of exposure and/or outcome, we compared these results. We applied the standard continuity correction of 0.5 for zero values in any cell in the two-by-two table20. We used the I² statistic to describe the percentage of variation across studies that is due to heterogeneity for reasons other than chance21. Quantitative synthesis was performed using R 3.5.122. We conducted random effects meta-analyses using the R package metafor (version 2.0-0)20. Finally, we compared descriptive and quantitative findings from this review period with previous versions of the review8,10.
Daily searches of PubMed, Embase and LILACS are automated and monthly searches are performed manually for other information sources in the first week of the month (Extended data, Supplementary File 217), with screening of all retrieved publications on the same day. The search strategy consisted of a combination of free terms and MESH terms that identified the exposure and outcomes (Extended data, Supplementary File 217). Searches from multiple sources were combined and automatically deduplicated by an algorithm that was tested against manual deduplication. Unique records enter a central database, and reviewers are notified of new content.
The tables and figures presented in this paper will be updated every six months as a new version of this publication. As soon as new studies are included, their basic study characteristics are extracted and provided online https://zika.ispm.unibe.ch/apps/causalitymap/.
We will keep the living systematic review up to date for as long as new relevant data are published and at least until October 31, 2021, the end date of the project funding.
To assess the risk of bias of cohort studies and case-control studies, we compiled a list of questions in the domains of selection bias, information bias, and confounding, based on the quality appraisal checklist of the United Kingdom National Institute for Health and Care Excellence (NICE) and literature23. Two independent reviewers conducted the quality assessment. Disagreements were resolved by a third reviewer.
From January 19, 2017 to July 1, 2019 we screened 1941 publications, of which we included 638 based on title and abstract. After reviewing the full text, 249 publications were included (Table 2, Figure 3). Of these publications, 195 reported on congenital abnormalities linked to ZIKV24–217 and 59 on GBS4,44,118,201,203,206,218–270. Five outbreak reports described both outcomes44,118,201,203,206.
The latest and previous versions of this table are available as extended data16.
| Outcome | Adverse congenital outcomes, number of publications | GBS, number of publications | ||||
|---|---|---|---|---|---|---|
| Review period/version | Baseline* | Update1† | Update2 | Baseline* | Update1† | Update2 |
| Study design | ||||||
| Case report | 9 | 13 | 39 | 9 | 5 | 17 |
| Case series | 22 | 12 | 62 | 5 | 11 | 22 |
| Case-control study | 0 | 2 | 10 | 1 | 1 | 7 |
| Cohort study | 1 | 8 | 35 | 0 | 0 | 0 |
| Cross-sectional study | 2 | 1 | 19 | 0 | 1 | 3 |
| Controlled trials | 0 | 0 | 0 | 0 | 0 | 0 |
| Ecological study/outbreak report | 5 | 4 | 27 | 19 | 7 | 9 |
| Modelling study | 2 | 0 | 3 | 0 | 0 | 1 |
| Total: | 41 | 40 | 195 | 34 | 25 | 59 |
The size of the points correspond with the number of exposed individuals with the adverse outcome, according to the definitions used in the publications. The latest and previous versions of this figure are available as extended data16.
We included 39 case reports24–61, 62 case series62–123, 10 case-control studies124–133, 35 cohort studies134–168, 19 cross-sectional studies169–187, seven ecological studies188–194, three modelling studies195–197 and 20 outbreak reports198–217 that report on congenital abnormalities linked to ZIKV.
Causality dimensions
Strength of association. Individual level: In this review period, five case-control studies reported on strength of association, four in Brazil (n=670 participants)125,126,128,130 and one in French Polynesia (n=123 participants)131. The studies assess adverse pregnancy outcomes including infants born with microcephaly, according to exposure to ZIKV for cases. Of these, all studies matched controls, based on gestational age and/or region. During the review period up to January 18, 2017, we included one case-control study271, which we replaced with a publication reporting the final results of the study126. The meta-analyses incorporate estimates from studies identified in all review periods.
Assessment of exposure status varied between the studies (Extended data, Supplementary File 317). In five case-control studies, exposure to ZIKV was assessed in the mother, based on clinical symptoms of ‘suspected Zika virus infection’125, or presence of maternal antibodies measured by IgM (Kumar et al. (2016)272), PRNT (de Araujo et al. (2018)126, Subissi et al. (2018)131), or both PRNT and IgG (Moreira-Soto et al. (2018)) maternal antibody127.
In meta-analysis, we found that the odds of adverse congenital outcomes (microcephaly or congenital abnormalities) were 3.8 times higher in ZIKV-infected mothers (95% CI: 1.7-8.7, tau2=0.18, I2=19.8%, Figure 4). Moreira-Soto et al. (2018) found that in Bahia, Brazil, Chikungunya infection was also associated with being a case127.
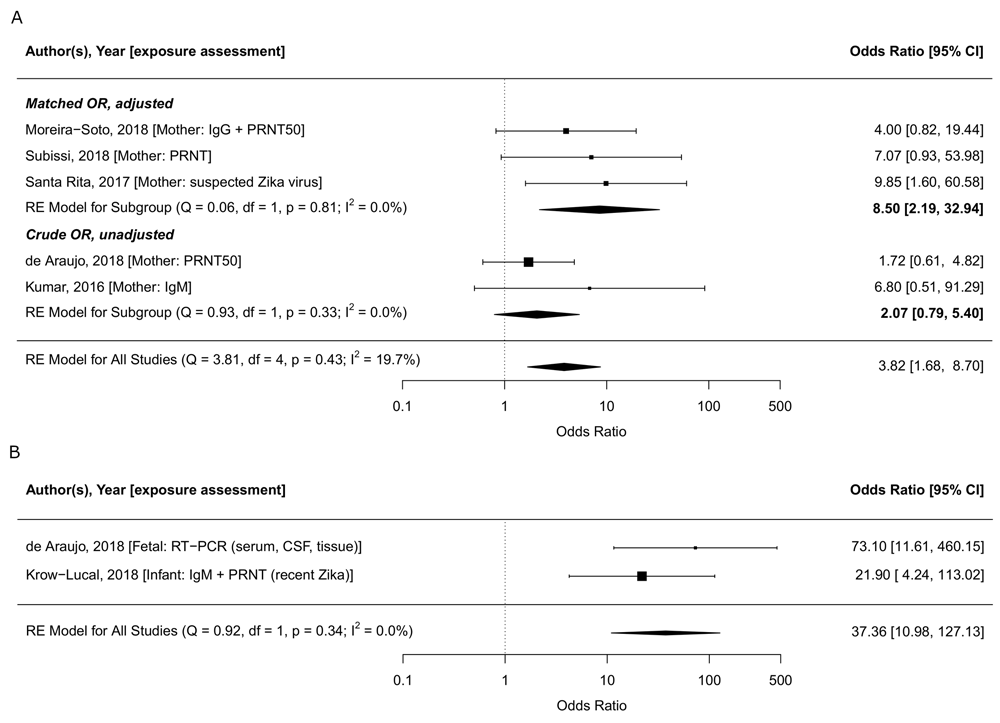
Forest plot and meta-analysis of case-control studies reporting on ZIKV infection assessed in mothers (A) and in infants (B) and adverse congenital outcomes (microcephaly, congenital malformations, central nervous system abnormalities). The odds ratio from the five case-control studies that assess exposure in mothers combined is 3.8 (95% CI: 1.7-8.7, tau2=2.37, I2=19.8%); the odds ratio for the studies that assess exposure in infants is 37.4 (95% CI: 11.0-127.1, tau2=0, I2=0%). The odds ratios are plotted on the log scale. Abbreviations: CSF, cerebrospinal fluid, PRNT, plaque reduction neutralisation test; RE, random effects; RT-PCR, reverse transcription polymerase chain reaction. The latest and previous versions of this figure are available as extended data16.
In two matched case-control studies, exposure to ZIKV was assessed in infants; Araujo et al. found a 73.1 (95% CI 13·0–Inf) times higher odds was reported for microcephaly when ZIKV infection was assessed by reverse transcription polymerase chain reaction (RT-PCR) in the neonate126. Krow-Lucal et al. (2018) found an OR of 21.9 (95% CI: 7.0-109.3) based on evidence of recent Zika infection assessed using IgM followed by PRNT in infants in Paraiba, Brazil128. When exposure was assessed at the infant-level, the combined odds of adverse congenital outcomes was 37.4 times higher (95% CI: 11.0-127.1, tau2=0, I2=0%, Figure 4).
In this review period, one cohort study reported on strength of association, in 610 pregnant women returning from ZIKV-affected areas in Central and South America to the USA138. Maternal ZIKV exposure was measured using RT-PCR or IgM followed by plaque reduction neutralisation test (PRNT). Among the 28 infants born to ZIKV-infected mothers, none was diagnosed with microcephaly and, one was born with a major malformation. In the ZIKV-unexposed group, eight out of 306 had major malformations. A complete overview of different outcomes assessed is presented in the extended data, Supplementary File 317. During the review period up to January 18, 2017, we included two cohort studies, one in women with rash and fever (Brasil et al. (2016)) and one in unselected pregnant women (Pomar et al. (2017))273,274. In meta-analysis of all three studies, we found that the risk of microcephaly was 3.5 times higher in ZIKV-infected mothers of babies (95% CI: 0.90-13.51, tau2=0, I2=0%, Figure 5).
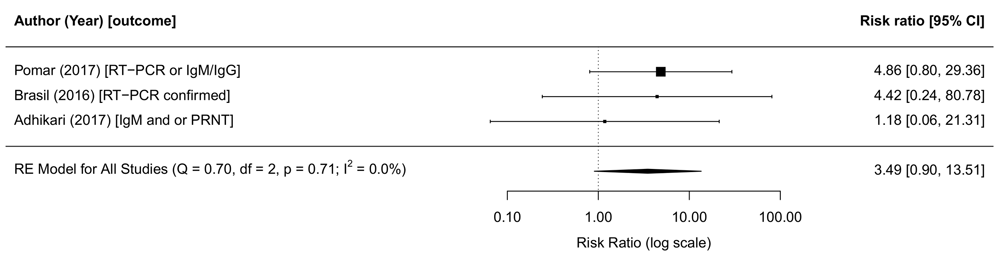
The risk ratio from the random effects model is 3.5 (95% CI: 0.9-13.5, tau2=0,I2=0%). The risk ratios are provided on the log scale. Abbreviations: ZIKV, Zika virus; PRNT, plaque reduction neutralisation test; RE, random effects; RT-PCR, Reverse transcription polymerase chain reaction. The latest and previous versions of this figure are available as extended data.
Population level: At a population level, data from Mexico collected at different altitudes during the ZIKV outbreak, showed that the risk of microcephaly was increased in regions at altitudes below 2200m, in which ZIKV can circulate196. Hay et al. (2018) reanalysed surveillance data from Colombia and northeast Brazil and concluded that time-dependent reporting changes might have caused apparent inconsistencies in the proportion of congenital abnormalities as a result of maternal ZIKV infection197.
Dose response. Halai et al. (2017)120 examined the severity of congenital outcomes according to measures of the severity of maternal ZIKV infection in a subset of mothers in the cohort presented by Brasil et al. (2016)274. They evaluated ZIKV load, assessed by RT-PCR using the cycle threshold (CT) as a measure of number of RNA copies, and a severity score of symptoms in 131 pregnant women. They concluded that neither higher viral load nor more severe symptoms was associated with more severe congenital abnormalities136. Moreira-Soto et al. found higher maternal antibody titers in microcephaly cases compared with controls127. In previous review periods, Honein et al. (2016) compared outcomes in neonates born to symptomatic and asymptomatic infected pregnant women returning to the USA with possible ZIKV infection and found no differences275.
Specificity. Although some outcomes, such as lingual phenotype177 or neurogenic bladder276, have been hypothesised as a specific phenotype for congenital ZIKV infection, no additional evidence was identified that certain congenital adverse findings are specific for congenital ZIKV infection.
Consistency. Geographical region: All four WHO geographic regions (the Africa region [AFRO], the American region [AMRO], the South-East Asian region [SEARO] and the Western Pacific region [WPRO]) with past or active ZIKV transmission have now reported congenital abnormalities due to ZIKV infection. During this review period, the first congenital abnormality due to infection with the Asian lineage of the virus on the African mainland occurred in a traveller returning from Angola47. Possible cases of congenital abnormalities have occurred in Guinea-Bissau96. In the most recent WHO situation report from March 2017, two cases of microcephaly are documented in Thailand and one in Vietnam, which were also described in detail in other works24,54,107. We identified another publication on congenital abnormalities due to endemic ZIKV in Cambodia110. The occurrence of congenital adverse outcomes in AFRO, SEARO, and WPRO seems sporadic, despite the endemic circulation of ZIKV. As noted above, the observed complication rate varied strongly between regions. Extended data, Supplementary File 3 provides a full overview of the published studies on congenital abnormalities per region and country17.
Traveller/non-traveller populations: In this update, we found further evidence that congenital abnormalities occurred in infants born to women travellers returning from ZIKV-affected areas and women remaining in those areas. In total, 25 publications report on 272 congenital abnormalities due to ZIKV infection in travellers27,29,30,32,34,36,38,42,45,56,58,61,89,94,98,109,117,122,123,137,140,151,153,166,173, with 109 publications reporting congenital abnormalities due to ZIKV in 2652 non travellers24–26,28,31,33,35,37,39–41,43,44,46–48,50,51,54,57,60,62–79,81,83–85,87,88,90–93,95–97,100,101,104–106,110–113,115,116,118,119,121,124–135,142–147,149,152,154,156–164,167,168,171,172,175–180,182,186
In this review period, evidence emerged that transmission through sexual contact with infected travellers also resulted in foetal infection58,59.
Study designs: The association between ZIKV infection and congenital abnormalities was consistent across different study designs (Table 2).
Lineages: We found no new evidence of consistency across different lineages from observational studies. The currently observed adverse congenital outcomes are linked to the ZIKV of the Asian lineage.
In all case-control studies, uncertainty about the exposure status due to imperfect tests could result in a bias towards the null. Some studies might suffer from recall bias where exposure was assessed by retrospectively asking about symptoms125,131. For the cohort studies138,274, the enrolment criteria were based on symptomatology. As a result, even in the absence of evidence of ZIKV, the unexposed groups might have had conditions that were unfavourable to their pregnancy. We expect this to bias the results towards the null or underestimate the true effect. Owing to imperfect diagnostic techniques, both false positives (IgM, cross reactivity) and false negatives (due to the limited detection window for RT-PCR) might occur, potentially resulting in bias; the direction of this bias would often be towards the null. None of the studies controlled for potential confounding. Extended data, Supplementary File 4 provides the full risk of bias assessment of the studies included in the meta-analysis17.
During this review period, we included 17 case reports44,218–233, 22 case series118,234–254, seven case-control studies4,255–260, one ecological study264, one modelling study265 and eight outbreak reports201,203,206,266–270 that reported on ZIKV infection and GBS.
During this review period, we included 17 case reports46,220–235, 22 case series120,236–256, seven case-control studies4,257–262, one ecological study266, one modelling study265 and eight outbreak reports201,203,206,266–270 that reported on ZIKV infection and GBS.
Causality dimensions
Strength of association. Individual level. The number of studies reporting on the strength of association between ZIKV infection and GBS at an individual level increased substantially. We identified five case-control studies255–259 published since the previous update, which included one case-control study from French Polynesia277. All studies were matched for age and place of residence. In the studies from Brazil, Colombia, Puerto Rico and New Caledonia, temporal clustering of cases in association with ZIKV circulation was documented255–258. In Bangladesh, ZIKV transmission was endemic259. Exposure assessment was based on serology255,256 or a combination of RT-PCR and serology257–259. Extended data, Supplementary File 3 shows the variability in ORs according to criteria for ZIKV exposure assessment, based on unmatched crude data extracted from each case-control study17.
Figure 6 shows the association between GBS and ZIKV infection, using the diagnostic criteria that were most similar across studies. Heterogeneity was considerable (I2=78.3%), but was reduced slightly after stratification based on the method of selection of controls. The summary OR was higher in studies that enrolled controls from hospital (OR: 55.8, 95% CI: 17.2-181.7, tau2=0, I2=0%)258,277 than in studies that enrolled controls at random from within the same community255–257 or from the same household259 (OR: 2.0, 95% CI: 0.8-5.4, tau2=0.46, I2=74.6%). Amongst studies with community controls, ORs were lower when enrolment and assessment took place several months after onset of symptoms255,256 than in studies with contemporaneous enrolment257,259. To further illustrate the heterogeneity in exposure assessment between and within the studies, we provide additional aggregations of the data in Extended data, Supplementary File 317.
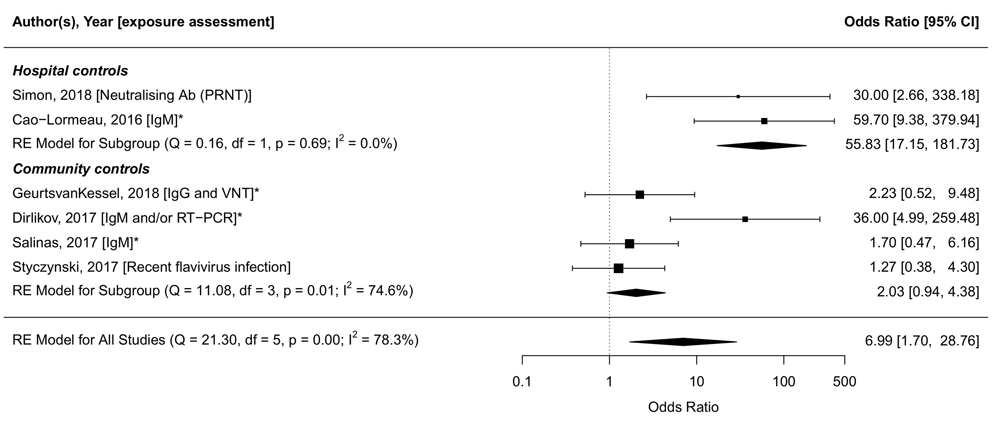
Odds ratios (ORs) are shown on the log-scale. The meta-analysis is stratified by the selection of controls: Hospital controls, or community/household controls. Most similar exposure assessment measures are compared (IgM256,258,259,277, recent flavivirus infection255, or IgM and/or RT-PCR257). OR: 7.0 [95% CI: 1.7-28.8, tau2=2.78, I2=78.3%]. ORs from studies marked with an asterisk (*) are matched ORs, unmarked studies provided crude ORs. The latest and previous versions of this figure are available as extended data16.
Population level. At a population level, Mier-Y-Teran-Romero et al. (2018) found that the estimated incidence of GBS ranged between 1.4 (0.4–2.5) and 2.2 (0.8–5.0) per 10,000 ZIKV infections comparing surveillance/reported cases from Brazil, Colombia, Dominican Republic, El Salvador, French, Honduras, Puerto Rico, Suriname, Venezuela, and Micronesia. The across-location minimum and maximum estimates were used to estimate an average risk of having GBS and being reported after ZIKV infection across locations of approximately 2.0 GBS cases per 10,000 infections (95% credible interval 0.5–4.5 per 10,000 ZIKV infections)265.
Dose response. In a case-control study, Lynch et al. (2018) found higher titres of neutralising antibodies in ZIKV-infected GBS cases than in patients with symptomatic ZIKV infection but without GBS260.
Specificity. Dirlikov et al. (2018) compared Puerto Rican GBS cases reported through public health surveillance that were preceded by ZIKV and cases that were not preceded by ZIKV infection249. Clinical features involving cranial nerves were observed more frequently in ZIKV-related cases and, at a six-month follow-up visit, residual cranial neuropathy was noted more often in this group. However, clinical symptoms did not allow a distinction to be made between ZIKV and non-ZIKV related GBS.
Consistency. Geographical region: During this review period, GBS likely due to ZIKV infection was reported in Asia; including Thailand, Bangladesh, Singapore and India4,48,252,259. Publication in the WHO Region of the Americas followed the pattern as observed before and no GBS linked to ZIKV infection was reported in Africa. Extended data, Supplementary File 3 provides a full overview of the published studies on congenital abnormalities by region and country17. In a reanalysis of surveillance data from the Region of the Americas, Ikejezie et al. (2016) found consistent time trends between GBS incidence and ZIKV incidence264.
Traveller/non-traveller populations: In studies included in this update, we found additional evidence of GBS in both travellers and non-travellers with ZIKV infection. Ten publications report on 11 travellers218,220,222–224,227,229,232–234, while 34 publications report GBS due to ZIKV in 402 non travellers4,44,118,219,221,225,226,228,230,231,235–237,239–247,249,251,253–260,262,263.
Study designs: Across the different study designs, the relation between GBS and ZIKV is consistently shown. Table 2 and Extended data, Supplementary File 3 provide an overview of the included study designs17.
Lineages: We still lack evidence on the consistency of the relation between GBS and ZIKV across different lineages from observational studies. The observed cases of GBS were linked to ZIKV of the Asian lineage.
Potential selection bias in case-control studies was introduced by the selection of controls from hospitals rather than from the communities in which the cases arose258,277. Uncertainty about the exposure status due to imperfect tests would tend to result in a bias towards the null. Two case-control studies did not conduct a matched analysis although controls were matched, and no study controlled for potential confounding by factors other than those used for matching. Exclusion criteria and participation rate, especially of the controls, were poorly reported. Extended data, Supplementary File 4 provides the full risk of bias assessment of the studies included in the meta-analysis17.
In this living systematic review, we summarised the evidence from 249 observational studies in humans on four dimensions of the causal relationship between ZIKV infection and adverse congenital outcomes and GBS, published between January 18, 2017 and July 1, 2019.
The strengths of this living systematic review are, first, that we automated much of the workflow10; we searched both international and regional databases daily and we screen papers for eligibility as they became available, so publication bias is unlikely. Second, we have quantified the strength of association between ZIKV infection and congenital abnormalities and GBS and investigated heterogeneity of outcome and exposure assessment within and between studies. Third, for congenital outcomes, we included studies with both microcephaly and other possible adverse outcomes, acknowledging the spectrum of congenital adverse outcomes caused by ZIKV. This work also has several limitations. First, we have not assessed the dimensions of the causality framework that involve laboratory studies, so we have not updated the pathobiology of ZIKV complications, which was addressed in the baseline review8 and the first update to January 201710. Limiting the review to epidemiological domains has allowed more detailed analyses of these studies and we hope that laboratory scientists will continue to review advances in these domains. Second, the rate of publications on ZIKV remains high so, despite the reduced scope and automation, maintenance of the review is time-consuming and data extraction cannot be automated. Third, this review may suffer from continuity bias, which is important for the conduct and interpretation of living systematic reviews and results from changes in the author team. Careful adherence to the protocol will reduce this risk.
ZIKV and congenital abnormalities: Since the earlier versions of the review8,10, evidence on the causal relationship between ZIKV infection and congenital abnormalities has expanded. Unfortunately, the total number of cases investigated in the published cohort or case-control studies remains small. In case-control studies in which infants with microcephaly or other congenital abnormalities are compared with unaffected infants, the strength of association differs according to whether exposure to ZIKV is assessed in in the mother (OR 3.8, 95% CI: 1.7-8.7, tau2=0.18, I2=19.8%) or the foetus/infant (OR 37.4, 95% CI: 11.0-127.1, tau2=0, I2=0%). This large difference in effect size can be attributed to the fact that not all maternal ZIKV infections result in foetal infection. In cohort studies, the risk of congenital abnormalities was 3.5 times higher (95% CI: 0.9-13.5, I2=0%, tau2=0) in mothers with evidence of ZIKV infection than without, which is similar to the OR for maternal exposure to ZIKV estimated from case-control studies. Further research is needed to understand the drivers of mother to child transmission. Higher maternal antibody titres were correlated with a higher incidence of adverse congenital outcomes in one case-control study127. However, amongst ZIKV-infected mothers followed prospectively, severity of ZIKV infection was not associated with more severe congenital abnormalities136. Convincing evidence on a dose-response relation is therefore still lacking.
ZIKV and GBS: Evidence on the causal relation between ZIKV infection and GBS has grown since our last review10. The body of evidence is still smaller than that for congenital abnormalities, possibly because GBS is a rare complication, estimated to occur in 0.24 per 1000 ZIKV infections277. In this review, the strength of association between GBS and ZIKV infection, estimated in case-control studies, tended to be lower than observed in the first case-control study reported by Cao-Lormeau (2016) in French Polynesia277. It is possible that the finding by Cao-Lormeau et al. was a ‘random high’, a chance finding278. Simon et al., however, found a similarly strong association in a case-control study in New Caledonia258. In both these studies, controls were patients in the same hospital. Although matched for place of residence, it is possible that they were less likely to have been exposed to ZIKV than the cases, resulting in an overestimation of the OR. In case-control studies in which controls were enrolled from the same communities as the cases, estimated ORs were lower, presumably because exposure to ZIKV amongst community-enrolled controls is less biased than amongst hospital controls279. Under-ascertainment of ZIKV infection in case-control studies in which enrolment occurred several months after the onset of symptoms255,256 is also likely to have reduced the observed strength of association. There is also possible evidence of a dose-response relationship, with higher levels of neutralising antibodies to both ZIKV and dengue in people with GBS260. However, the level of antibody titre might not be an appropriate measure of viral titre, and merely a reflection of the intensity of the immune response. Taking into account the entire body of evidence, inference to the best explanation280 supports the conclusion that ZIKV is a cause of GBS. The prospect of more precise and robust estimates of the strength of association between ZIKV and GBS is low because outbreaks need to be sufficiently large to enrol enough people with GBS. In the large populations that were exposed during the 2015–2017 outbreak, herd immunity will limit future ZIKV outbreaks.
The sample sizes of studies published to date are smaller than those recommended by WHO for obtaining precise estimates of associations between ZIKV and adverse outcomes [Harmonization of ZIKV Research Protocols to Address Key Public Health]. Given the absence of large new outbreaks of ZIKV infection in 2017–2019, there is a need for consortia of researchers to analyse their data in meta-analyses based on individual participant data [Individual Participant Data Meta-analysis of Zika-virus related cohorts of pregnant women (ZIKV IPD-MA)]. Future collaborative efforts will help to quantify the absolute risks of different adverse congenital outcomes and allow investigation of heterogeneity between studies136,149,275.
This review highlights additional research gaps. We did not assess the complication rates within the infected group in studies without an unexposed comparison group; the adverse outcomes are not pathognomonic for ZIKV infection, making an appropriate comparison group necessary. Although there are no individual features of ZIKV infection that are completely specific, the growing number of publications on ZIKV will allow better ascertainment of the features of a congenital Zika syndrome281. In this review, we did not take into account the performance of the diagnostic tests in assessing the strength of association. Future research should include robust validation studies, and improved understanding of contextual factors in the performance of diagnostic tests, including the influence of previous circulation of other flaviviruses, the prevalence of ZIKV and the test used.
This living systematic review will continue to follow studies of adverse outcomes originating from ZIKV circulation in the Americas, but research in regions with endemic circulation of ZIKV is expected to increase. Such studies will clarify whether ZIKV circulation in Africa and Asia also results in adverse outcomes, as suggested by the case-control study of GBS from Bangladesh259. Increased awareness might improve the evidence-base in these regions, where misperceptions about the potential risks of ZIKV-associated disease with different virus lineages has been reported282. An important outstanding question remains whether the absence of reported cases of congenital abnormalities or GBS in these regions represent a true absence of complications or is this due to weaker surveillance systems or reporting283. The conclusions that ZIKV infection causes adverse congenital outcomes and GBS are reinforced with the evidence published between January 18, 2017 and July 1, 2019.
All data underlying the results are available as part of the article and no additional source data are required.
Harvard dataverse: Living systematic review on adverse outcomes of Zika - Supplementary Material. https://doi.org/10.7910/DVN/S7USUI17
This project contains the following extended data:
SupplementaryFile1Prisma.docx (PRISMA checklist)
SupplementaryFile2Methods.docx (Supplementary file 2, additional information to the Methods)
SupplementaryFile3Results.docx (Supplementary file 3, additional information to the Results)
SupplementaryFile4ROB.tab (Risk of bias assessment)
Harvard dataverse: Living systematic review on adverse outcomes of Zika - Figures and Table. https://doi.org/10.7910/DVN/DLP5AN16
This project contains the following extended data:
Fig1.pdf (Most recent version of Figure 1)
Fig2.pdf (Most recent version of Figure 3, PRISMA flowchart)
Fig3A.pdf (Most recent version of Figure 4A)
Fig3B.pdf (Most recent version of Figure 4B)
Fig4.pdf (Most recent version of Figure 5)
Fig5.pdf (Most recent version of Figure 6)
Table1.pdf (Most recent version of Table 1)
Table2.pdf (Most recent version of Table 2)
PRISMA checklist and flow diagram for ‘Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barré syndrome: A living systematic review’, https://doi.org/10.7910/DVN/S7USUI and Figure 2.
This work was supported by the Swiss National Science Foundation [320030_170069], end date: 31/10/2021.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The views expressed in this article are those of the authors and do not necessarily represent the official positions of the Centers for Disease Control and Prevention.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the living method justified?
Yes
Have the search and update schedule been clearly defined and justified?
Yes
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular epidemiology; arboviruses; respiratory viruses
Is the living method justified?
Yes
Have the search and update schedule been clearly defined and justified?
Yes
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 14 Aug 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)