Keywords
DNA structure, Watson and Crick model, plasmid denaturation, single-stranded circular DNA, non-helical structure, side-by-side model.
At the request of the author, the article titled “Non-intertwined strands of plasmid DNA contradict the Watson and Crick model of DNA structure” [version 3;
... r review: 1 approved, 1 approved with reservations, 1 not approved]. F1000Research 2020, 8:356 (https://doi.org/10.12688/f1000research.18134.3) has been retracted from F1000Research. Previous versions of this article published on F1000Research have also been retracted (https://doi.org/10.12688/f1000research.18134.1; https://doi.org/10.12688/f1000research.18134.2).Since publication, Dr Pawan Kumar has performed additional experiments on the structure of a DNA molecule and was not able to generate further results that support this article. As such, they are no longer confident in the conclusions drawn in this article, and wish to retract. They apologise for any inconvenience caused.
At the request of the author, the article titled “Non-intertwined strands of plasmid DNA contradict the Watson and Crick model of DNA structure” [version 3; peer review: 1 approved, 1 approved with reservations, 1 not approved]. F1000Research 2020, 8:356 (https://doi.org/10.12688/f1000research.18134.3) has been retracted from F1000Research. Previous versions of this article published on F1000Research have also been retracted (https://doi.org/10.12688/f1000research.18134.1; https://doi.org/10.12688/f1000research.18134.2).
Since publication, Dr Pawan Kumar has performed additional experiments on the structure of a DNA molecule and was not able to generate further results that support this article. As such, they are no longer confident in the conclusions drawn in this article, and wish to retract. They apologise for any inconvenience caused.
DNA structure, Watson and Crick model, plasmid denaturation, single-stranded circular DNA, non-helical structure, side-by-side model.
There is no major difference between this and the previous version of the article except for the correction of some typos and Figure 1e.
See the author's detailed response to the review by Dmytro M Hovorun
See the author's detailed response to the review by Bharathwaj Sathyamoorthy and Abhishek Cukkemane
See the author's detailed response to the review by Rebecca Schulman and Yi Li
DNA, Deoxyribonucleic acid
NaOH, Sodium hydroxide
HCl, Hydrochloric acid
HmDNA, Higher electrophoretic mobility DNA.
DNA is the genetic material of all organisms, with the exception of some viruses. The currently accepted model of DNA structure was proposed by James Watson and Francis Crick in 19531. According to this model, a DNA molecule consists of two antiparallel polynucleotide chains, intertwined with each other. Although the Watson and Crick (W/C) model is accepted widely, some researchers have raised questions against it and proposed alternative models for DNA structure2,3. Among these, Rodley’s model which envisaged side-by-side positioning of two strands in a DNA molecule, has generated significant interest in the scientific community2.
In the present study, W/C model of DNA structure was examined with the help of plasmid DNA. It was hypothesized that two strands of a plasmid will remain intertwined and not separate into single-stranded circular DNA molecules under denaturing conditions, if it follows the W/C model. To test this, pUC19 and pBR322 plasmids were denatured using sodium hydroxide (NaOH) and analyzed by gel electrophoresis. Interestingly, addition of NaOH to pUC19 and pBR322 plasmids resulted in new form of DNA showing higher electrophoretic mobility in agarose gel. DNA corresponding to higher electrophoretic mobility band of pUC19 was found to be single-stranded and circular, suggesting the separation of two strands of plasmid DNA. Under suitable conditions, higher electrophoretic mobility DNA (HmDNA) re-annealed to form native pUC19 plasmid. These results demonstrated the reversible separation of plasmid DNA strands and contradicted the W/C model of DNA structure.
pUC19, pBR322 and pCMV6-AN-HA plasmids were isolated from E. coli strains [cultured in Luria Bertani broth (HiMedia, catalog no. M1245) in a shaking incubator at 37°C] by alkaline-lysis method as described previously4. For denaturation, approximately 5 µl plasmid DNA (concentration, ~1.0 µg/µl) was added with 5 µl NaOH solution of indicated concentration. Agarose gel (1%) was prepared in TAE buffer and DNA was visualized using ethidium bromide.
For enzymatic digestion, NaOH in denatured pUC19 solution was neutralized using 5 µl HCl (concentration, 0.5 N) and plasmid was incubated with HindIII (SibEnzyme, catalog no. E073), S1 nuclease (Promega Corporation, catalog E576A) or exonuclease I and alkaline phosphatase (Thermo Scientific, catalog no. EN0581 and EF0651, respectively) at room temperature for 30 min. Samples were immediately run on 1% agarose gel. A mix of forward primer (5’-CTGCTTTCCTGCATGTTC-3’) and reverse primer (5’-AAGCCCTTGCTTCTTATACT-3’) (experimental control) was also digested with exonuclease I and alkaline phosphatase. Digested and undigested primers were used in polymerase chain reaction [initial denaturation, 95°C/3 min followed by 30 cycles of (i) 95°C/30 sec, (ii) 55°C/30 sec, (iii) 72°C/30 sec in the same order] to amplify target sequence in A2780 cell line genomic DNA. PCR product was analyzed on 1% agarose gel.
Individual (bottom and top) strands of pCMV6-AN-HA were prepared by co-digesting the plasmid with bottom (Nb.BbvCI) or top (Nt.BbvCI) strand-specific nicking endonucleases (New England Biolabs, catalog no. R0631 and R0632] and exonuclease III (New England Biolabs, catalog no. M0206S) at 37°C for 30 min. Digested plasmid was analyzed by gel electrophoresis.
HmDNA band was cut with the help of a clean knife. DNA was purified using an extraction kit, as suggested by manufacturer (FairBiotech, catalog no. DE0100). Briefly, gel was dissolved in DE buffer and passed through a column. After washing with buffers, DNA was eluted in 50 μl nuclease free water.
Transformation of E. coli strain DH5-α with gel-extracted plasmid DNA was carried out by heat-shock method (42°C for 30 sec) using water bath. Transformed and non-transformed bacteria were spread on ampicillin-nutrient agar plates supplemented with 25 µl X-Gal (Himedia, catalog no. MB0690. Plates were kept overnight in a 37°C incubator.
Two strands of a DNA molecule are held together by non-covalent interactions, which can be disrupted by alkali5. In the present study, approximately 5 µg of plasmid DNA was denatured using NaOH solution of increasing concentration. It was observed that addition of 0.5 N NaOH to pUC19 resulted in a new form of DNA having higher electrophoretic mobility in agarose gel (Figure 1a). Similar results were obtained with pBR322 added with 0.5 N NaOH (Figure 1b). In keeping with these findings, formation of higher electrophoretic mobility DNA (HmDNA) in NaOH-denatured pBR322 has also been reported previously6.
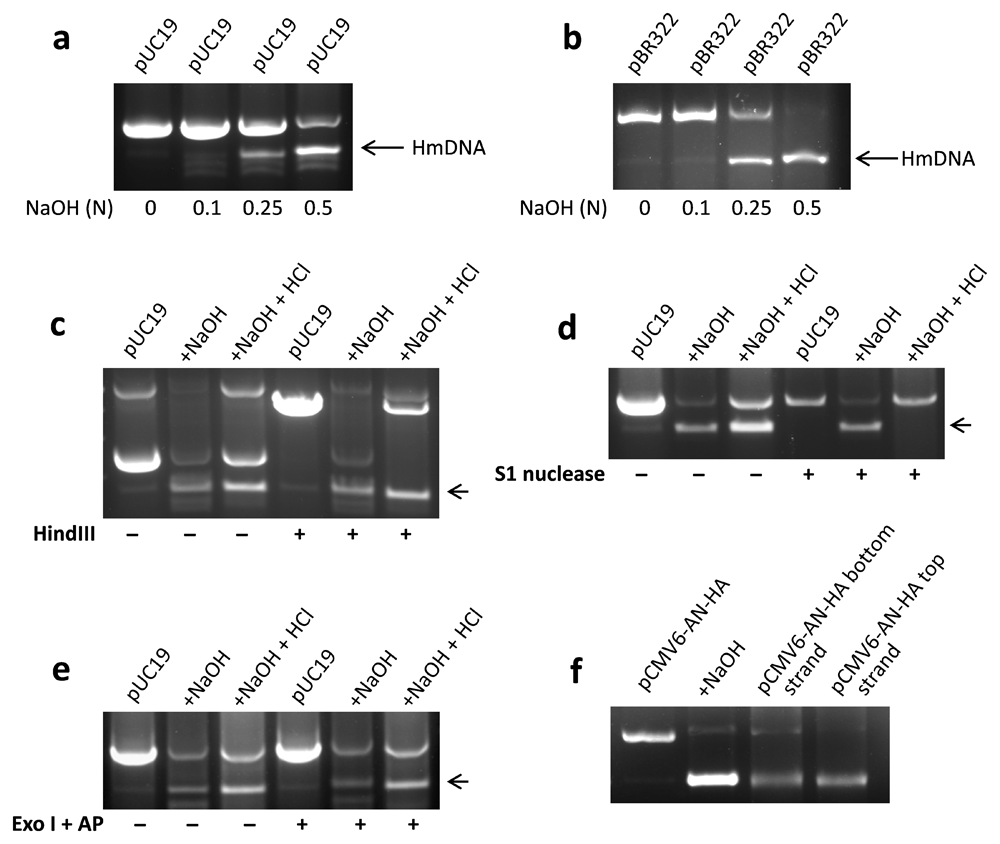
Approximately 5 µl plasmid DNA was added with an equal volume of NaOH solution of indicated concentration and after ~5 min, run on 1% agarose gel. A distinct band of HmDNA (marked by arrows) was observed in pUC19 (a) and pBR322 (b) plasmids added with 0.5 N NaOH. For enzymatic digestion, NaOH in denatured plasmid was neutralized using 0.5 N HCl followed by incubation with different enzymes for 30 min. Native pUC19, but not HmDNA was digested by HindIII, which acts on double-stranded DNA (c). S1 nuclease, which recognizes single-stranded DNA, degraded HmDNA but not native pUC19 plasmid (d). Exonuclease I (Exo I) and alkaline phosphatase (AP), which would eliminate single-stranded linear DNA, digested neither of native pUC19 or HmDNA (e). Electrophoretic mobility of top and bottom strands of pCMV6-AN-HA was compared with that of its HmDNA. Both HmDNA and purified strands of pCMV6-AN-HA exhibited comparable migration in agarose gel (f). Representative data of 2–3 independent experiments are shown.
HmDNA in NaOH-denatured pUC19 was characterized using DNA modifying enzymes. HindIII, which acts on double-stranded DNA, digested pUC19 plasmid but not HmDNA (Figure 1c). S1 nuclease, which digests single-stranded DNA, degraded HmDNA but not pUC19 plasmid (Figure 1d). Exonuclease I and alkaline phosphatase, which would digest single-stranded linear DNA, degraded neither of pUC19 or HmDNA (Figure 1e) but a mix of primers (experimental control). Digested primers did not form product in polymerase chain reaction (Supplementary Figure 1). To rule out the possibility that HmDNA might be denatured but unevenly intertwined plasmid DNA, its electrophoretic mobility was compared with those of individual strands of plasmid. Interestingly, top and bottom strands of pMCV6-AN-HA and its HmDNA exhibited comparable electrophoretic mobilities in agarose gel (Figure 1f). These results showed that HmDNA is single-stranded and circular DNA formed with the separation of two strands in NaOH-denatured plasmid.
Next, we examined whether HmDNA from NaOH-denatured pUC19 can reanneal to form the native plasmid. Interestingly, it was observed that neutralization of NaOH in denatured plasmid with 0.25 N HCl resulted in the appearance of pUC19 plasmid (Figure 2a). Similar results were obtained with HmDNA extracted from agarose gel. Gel-extracted HmDNA re-annealed to form pUC19, which exhibited electrophoretic mobility comparable with that of the native plasmid (Figure 2b). Further, we observed that similar to the native plasmid, pUC19 formed by gel-extracted HmDNA was degraded by HindIII but not by S1 nuclease (Figure 2c).
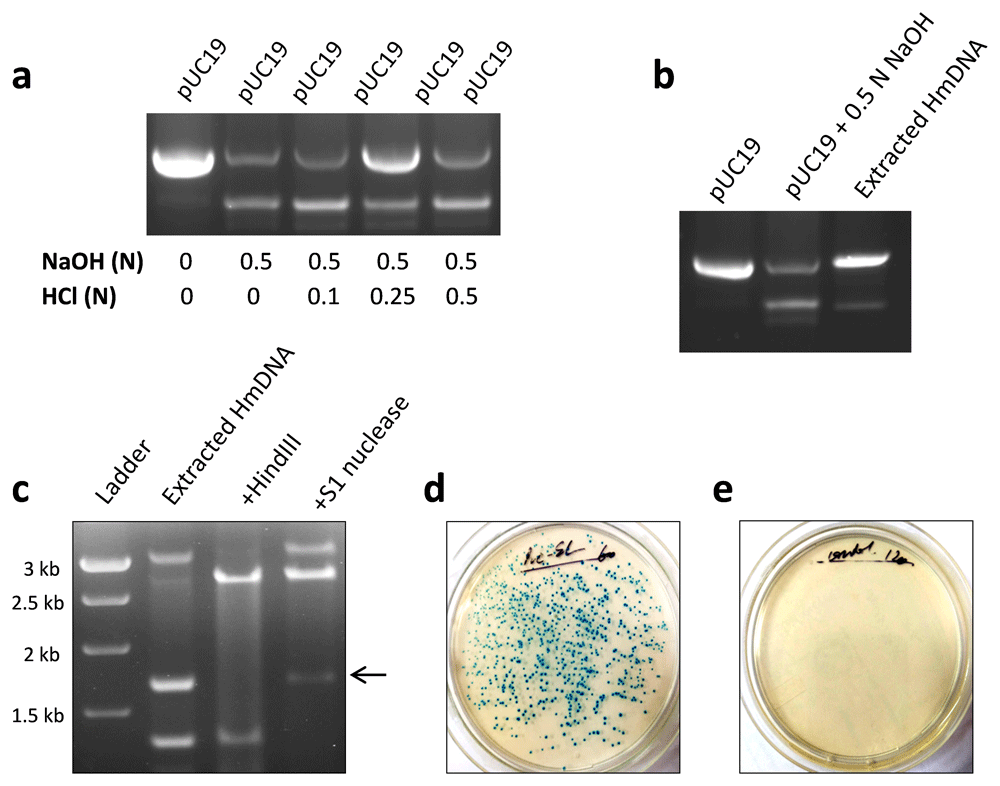
pUC19 plasmid was denatured using 0.5 NaOH as described above. After ~5 min, NaOH in denatured pUC19 was neutralized using hydrochloric acid (HCl) solution of indicated concentration and plasmid DNA was run on agarose gel. Native pUC19 DNA reappeared in denatured plasmid DNA solution added with 0.25 N HCl (a). HmDNA was extracted from agarose gel and approximately 1–3 µg of it was rerun on 1% agarose gel. Gel-extracted HmDNA re-annealed to form pUC19, with its electrophoretic mobility comparable with that of the native plasmid (b). Gel-extracted HmDNA from NaOH-denatured pUC19 was incubated with HindIII and S1 nuclease. pUC19 plasmid formed by HmDNA was degraded by HindIII but not by S1 nuclease (c). E. coli strain DH5-α was transformed with gel-extracted pUC19 HmDNA by heat-shock method. HmDNA-transformed bacteria formed colonies on ampicillin-X-Gal-nutrient agar plates (d). No colonies were formed by non-transformed bacteria (e). Representative data of 2–3 independent experiments are shown.
Functionality of pUC19 formed by gel-extracted HmDNA was demonstrated by its ability to induce bacterial transformation. HmDNA-transformed E. coli acquired ampicillin resistance and formed colonies on ampicillin-nutrient agar plates (Figure 2d). No colonies were formed by non-transformed bacteria (Figure 2e). These results showed that pUC19 plasmid formed by re-annealing of HmDNA is structurally and functionally similar to native plasmid DNA.
Concludingly, reversible separation of two strands of plasmid DNA into single-stranded circular molecules shows that DNA strands are not intertwined with each other. These findings contradict the W/C model of DNA structure and provide evidence for the side-by-side structure of DNA.
Figshare: Formation and characterization of higher electrophoretic mobility DNA (HmDNA) in NaOH-denatured plasmid. https://doi.org/10.6084/m9.figshare.12949280.v17.
This project contains the following underlying data:
Underlying data for figure 1a
Underlying data for figure 1b
Underlying data for figure 1c
Underlying data for figure 1d
Underlying data for figure 1e
Underlying data for figure 1f
Underlying data for figure 2a
Underlying data for figure 2b
Underlying data for figure 2c
Underlying data for figure 2d-e
Underlying data for supplementary figure 1
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 10 Nov 20 |
|||
|
Version 2 (revision) 01 Oct 20 |
read | ||
|
Version 1 01 Apr 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
These findings further corroborate the argument that two strands of plasmid DNA can be reversibly separated from each other, provide insight into DNA structure, and contradict the Watson and Crick model.
These findings further corroborate the argument that two strands of plasmid DNA can be reversibly separated from each other, provide insight into DNA structure, and contradict the Watson and Crick model.