Keywords
screening, glaucoma, costs, cost-effectiveness, screening, detection rate
This article is included in the Eye Health gateway.
screening, glaucoma, costs, cost-effectiveness, screening, detection rate
Glaucoma is the second commonest cause of blindness in the world1. There are estimated 12 million people affected by glaucoma in India2. The two main types of glaucoma are angle-closure glaucoma (ACG) and open-angle glaucoma (OAG). It is estimated that about 70% of OAG and 80% of ACG cases occur in developing nations3. In India it is estimated that primary OAG (POAG) affects around 6.48 million people and primary ACG (PACG) affects around 2.54 million2. In India, it is estimated that among the 40+ age group, every eighth person could be at risk of, or, suffering from glaucoma2. As there is no organised screening for glaucoma, opportunistic case finding is the commonest method for case detection. It has been shown that around 90% of the glaucoma remains undiagnosed in both rural and urban populations in India4,5. Another study found that only 50% of people with glaucoma ever visited an ophthalmologist6. Thus, stressing the need of a more effective screening program
The use of intraocular pressure (IOP), field testing and optic disc findings for diagnosis of glaucoma has been stressed for effective screening program for identifying glaucoma in the community. It has been shown that none of them individually have positive predictive value or the sensitivity to be used for community-based screening. These tests when used together have much better sensitivity to diagnose early glaucoma.
In most of the developing countries, there is a shortage of ophthalmologists. In India, the availability of ophthalmologists in rural setting is low7. In order for an effective screening program to function there is need for including other ophthalmic personnel such as optometrists and ophthalmic assistants. It has been shown that trained ophthalmic assistants can be effective in detecting glaucoma in the community8 Thus, the equipment used should be selected according to the cadre involved in screening.
IOP is an important risk factor for developing glaucoma. Although used alone it has a sensitivity of only 47.1%, and specificity of 92.4% if a cut-off of more than 21 mmHg is used for diagnosing POAG9. Tonometers based on rebound technology have been found to have accuracy similar to applanation tonometry10. An optic disc examination for the cup to disc ratio is used to diagnose glaucoma. Direct ophthalmoscope is relatively inexpensive and portable equipment which has been used in the community setting11. It’s sensitivity has been shown to be around 59%12. Frequency doubling technology (FDT) perimetry has emerged as a quick and inexpensive alternative. The sensitivity of the test has been shown to be around 50% in some studies13 as compared to more than 95% for automated perimetry14. Gonioscopy is considered gold standard for identifying eyes at risk of angle closure but it requires clinical expertise to conduct and interpret the test. Thus, it is not an appropriate screening test15. Van Herick test for peripheral anterior chamber depth (ACD) has been shown to have a sensitivity of 91% for detecting shallow chambers16 and of 61.9% in detecting occludable angles17.
WHO Vision 2020 has prioritised interventions for five causes of avoidable blindness18. Cataract being the commonest cause of avoidable blindness in India has been prioritised by NPCB. There are regular cataract screening camps organised by both government and non-government service providers. Within these camps the focus is to examine the maximum number of patients over 50 years of age. This research protocol aims to study the costs, cost-effectiveness and detection rate of glaucoma cases in cataract screening camps in North India.
Dr. Shroff’s Charity Eye Hospital (SCEH), based in New Delhi, is a tertiary referral center providing general and subspeciality services and training. SCEH conducts one-day cataract screening clinics in villages closer to the city of New Delhi. This is made possible through the financial support of private donors. These programs target patients who have not sought out care due to limited or non-existent local eye care facilities, financial constraints, or in most case a lack of awareness regarding eye-care. In anticipation of the one-day screening clinics, local organizers are responsible for advertising the clinic (e.g. through posters, flyers, or door-to-door visits), managing volunteers and procuring an appropriate facility. The cost of transportation of patients to SCEH is borne by hospital These camps are managed by Program Manager (Outreach) based at SCEH with regards to planning, monitoring, and strategy.
In our data of the camps conducted by the outreach team in Delhi, between June 2016 and May 2017, out of 8283 population of ≥40 years age screened, only 0.25% (n=21) were identified as glaucoma suspects. This is well below the prevalence (1.6-3.5%) found in the rural and urban studies in India19. Model based studies of community screening for glaucoma in rural and urban India has been shown to be cost-effective [20, 21]. The rationale of this study is to analyse the cost and detection rate of detecting glaucoma in cataract camps. If found to be cost effective, this model can be scaled up for detecting glaucoma suspects from cataract screening camps and referring them for further investigations and management.
In addition to the original team for the outreach camp, one of the trained optometrists, a vision technician for handling FDT and a counsellor would travel for the interventional camps.
All people of age ≥40 years, not detected as having a cataract would undergo screening for glaucoma by trained optometrist using additional equipment. Those detected with cataracts are not included in the study as they would have undergone glaucoma screening as a routine before being advised surgery. Those included in the study would have already undergone vision testing and refraction if indicated. The registration number given at the start of the camp would be recorded by the counsellor. There would be two stations for glaucoma screening. The trained optometrist would carry out disc examination, ACD examination and IOP recording. At the second station FDT would be carried out. Any result beyond cut-off and requiring further intervention would be flagged off. After that the person screened would be received by the counsellor. The test used for screening would be disc examination, FDT and IOP using Icare rebound perimetry.
Training. There will be two optometrists trained for the study. The optometrists selected would already be carrying out refractions, basic history taking and slit-lamp examination. The curriculum would pertain to glaucoma-related history-taking and slit-lamp examination. Specifically, for the study the following training would be included for various test to be conducted in the study
The optometrists would have explained to them the need to calibrate before starting measurements and explained the process of calibration. The principles of avoiding injury while recording IOP would be discussed. The cut-offs being used in the study would be stressed upon
The optometrists would be trained to use direct ophthalmoscope and focus on the disc. They would be exposed to patients and photographs with a variety of disc findings.
The concept of the Van Hericks test would be explained. Although they would be already using a slit-lamp, they would be trained to use a hand-held slit lamp. The cut-offs to be used would be explained. The optometrists would be maintaining a log-book to record their findings, and these would be checked and signed by a glaucoma consultant or a senior fellow.
To carry out a field test with FDT, the optometrists would be trained in handling the equipment, giving instructions to the patients and commenting on the printouts keeping the cut-offs of the study in mind.
The training would be conducted in a classroom with visual presentations for theory. A senior glaucoma fellow ophthalmologist would be involved to take classes with a problem-based approach. Theory class would be scheduled for 1 hour in every working day for 2 weeks. A total of 12 hours would be spent in theory classes. The topics to be covered are mentioned in Table 1.
Junior glaucoma consultant would be involved in imparting practical training. In total, 30 h would be taken to develop their practical skills for each trainee. A log book record would be maintained for the patients examined. The criteria used for assessing the level of training are highlighted in Table 2.
This would be carried out after the training is complete. A total of 30 cases each would be given to both optometrists for recording IOP, ACD and C/D ratio. These would be either new cases or they would be masked to previous records. A senior optometrist would independently record IOP and a glaucoma consultant would examine for ACD and C/D ratio. Both the senior optometrist and glaucoma consultant would be masked to the trainee optometrists’ findings. Sensitivity and specificity would be calculated keeping the trainers as gold standard. If not found to be within an acceptable range (85% sensitivity, and 90% specificity), the training would be repeated for the concerned trainee in the identified area of weakness.
Once found acceptable in all aspects, on a separate day, 20 patients would be assigned to both the trainees for calculating inter-observer variation. The cut-off to be used for labelling as glaucoma suspect at the camp site in our study would be based on parameters mentioned in Table 3.
In FDT perimetry reporting, one abnormal spot in the central field or more than one spot in the peripheral field would be considered below the cut-off for normal in this test . Any patient with normal disc examination but abnormal FDT findings will not be labelled as suspected glaucoma but will be advised to undergo detailed visual field analysis in the hospital.
Records of each person screened would be entered Those requiring further investigations would be counselled about glaucoma and importance of early detection. They would be offered a free drop to the base hospital. Those not agreeing to travel on the same day would be given a contact number, in case they would like to report later. Those not reporting within a week would be given a reminder call.
In the case of India, currently no organized community-screening program specifically for glaucoma detection exists. At present, detection of glaucoma is through ‘opportunistic case finding’ of patients who present themselves to various eye clinics in the country for various ophthalmic complaints [22].
The opportunistic case finding of patients presenting in SCEH would be the comparator group for our study. The inclusion criteria would be patients ≥40 years of age and belonging to the low-paid category in the hospital to match the socioeconomic status. People with prior history of glaucoma screening in community or clinic or already diagnosed with glaucoma would be excluded from the study. After presentation to SCEH, patients would undergo glaucoma consultation at the hospital.
For those travelling to the hospital, transport and investigations in the hospitals would be offered free of cost. Overnight stay if required would also be provided free. A record would be made of the tests, number of persons transported and number staying overnight for cost analysis.
In the hospital, a detailed workup would be done for the glaucoma suspects, so as to confirm or rule out the diagnosis of glaucoma with the following tests: refraction, applanation tonometry, gonioscopy, automated visual field analysis and nerve fibre analysis. Patients will be classified as normal or with OH, probable glaucoma, or glaucoma as per the criteria in Table 4. After the investigations, they would be examined by a glaucoma consultant masked to the source of patient sent. Classification will be done using the criteria mentioned in Table 4. During their time at the hospital the patients/care-givers would be administered a questionnaire about glaucoma awareness (Annexure 1, extended data)20. After the investigations, they would be examined by a glaucoma consultant, who would make a diagnosis of glaucoma, glaucoma suspect, angle closure disease or impending angle closure requiring intervention, using the criteria mentioned in Table 4. The patient requiring intervention would be counselled and others would be discharged. Those requiring surgery would be offered surgery at fixed rates meant for camp patients needing non-cataract surgery. The patients staying overnight would be offered a free drop and records would be maintained.
| Category | Criteria |
|---|---|
| Healthy subjects | • IOP <21 mm Hg with no history of elevated IOP • Normal appearing optic disc, intact neuroretinal rim, and RNFL • Minimum of two reliable normal visual fields, defined as pattern standard deviation (PSD) within 95% confidence limits and a glaucoma hemifield test (GHT) result within normal limits • No family history of glaucoma |
| Glaucoma suspects | • Disc suspects: Those who met disc criteria i.e, (structural and functional evidence) Eyes with CDR > 0.6 or CDR asymmetry > 0.2, or neuroretinal rim width reduced to <0.1 CDR between 11 to 1 o’clock or 5 to 7 o’clock, but were not proved to have definite field defects. • Field suspects: Those with definite field defects, but not meeting above disc criteria. • Those with optic disc margin haemorrhages. • Those with an IOP >97.5th percentile(>21 mm Hg)21 • Primary angle closure suspect: An eye in which appositional contact between the peripheral iris and posterior trabecular meshwork is considered possible defined as an angle in which ≥270° of the posterior trabecular meshwork (the part which is often pigmented) cannot be seen • Primary angle closure(PAC): An eye with an occludable drainage angle and features indicating that trabecular obstruction by the peripheral iris has occurred, such as peripheral anterior synechiae, elevated intraocular pressure, iris whorling (distortion of the radially orientated iris fibres), “glaucomfleken” lens opacities, or excessive pigment deposition on the trabecular surface. The optic disc does not have glaucomatous damage22 |
| Primary open angle glaucoma (POAG) | • ONH changes characteristic of glaucoma (focal or diffuse neuroretinal rim thinning, localised notching, RNFL loss), disc based on stereoscopic evaluation by volk 90 D and reviewed by experienced grader. • Characterstic glaucomatous field defects (Hodapp Parish Anderson criteria)23 • Open angles • IOP >21 mm Hg at time of diagnosis. |
| Primary angle closure glaucoma (PACG) | • ONH changes characteristic of glaucoma (focal or diffuse neuroretinal rim thinning, localised notching or RNFL defects) disc based on stereoscopic evaluation by 90 D and reviewed by experienced grader • Characteristic glaucomatous field defects (Hodapp Parish Anderson criteria). • An eye in which appositional contact between the peripheral iris and posterior trabecular meshwork is considered possible defined as an angle in which ≥270° of the posterior trabecular meshwork (the part which is often pigmented) cannot be seen. • IOP>21 mm Hg at the time of diagnosis. |
| Normal tension glaucoma (NTG) | • ONH changes characteristic of glaucoma (focal or diffuse neuroretinal rim thinning, localised notching or RNFL defects). Disc based on stereoscopic evaluation by volk 90 D and reviewed by experienced grader • Characterstic glaucomatous field defects (Hodapp Parish Anderson criteria)21. • Open angles • History of untreated peak IOP of 21 mm Hg or less21. |
| Criteria for Ocular hypertension | • IOP> 21 mm Hg without treatment • Visual Fields normal • Optic disc and retinal nerve fibre layer normal • Gonioscopy: Open anterior chamber angle (exclude intermittent angle closure) • No history or signs of other eye disease or steroid use • Other risk factors: None |
The aim of the study is to measure the cost, cost-effectiveness and detection rate of glaucoma screening in cataract camps in rural India conducted by SCEH. The comparator group would be patients who are undergoing assessment for cataract in other such camps conducted by SCEH and diagnosed with glaucoma on their presentation to the SCEH hospital. The comparator will also include patients reporting directly to SCEH for cataract and/or glaucoma assessment.
The specific objectives of the study are:
a) To measure the detection rate measured by dividing the number of new cases identified during the study by the total number of cases examined.
b) To compare the costs of setting up and implementing glaucoma screening in cataract camps.
c) To estimate the direct and indirect costs of the intervention to the patients undergoing glaucoma screening and subsequent treatment in the camps.
d) To estimate the cost-effectiveness based on cost per case detected of glaucoma screening in comparison with opportunistic case finding in hospitals from non-screened populations.
A prospective cohort study of glaucoma detection and cost assessment of the glaucoma screening conducted in the community. The total and incremental costs of the intervention prospectively from a societal perspective, measuring programme, provider and household costs.
The camps will be held in urban slums in and around the national capital region of Delhi in North India. Figure 1 and Figure 2 depict the flow of patients, that will be maintained in the routine camps and intervention camps, respectively.
Figure 1 shows the patient flow in a routine cataract camp, whereas, in Figure 2, the patient flow in camps which constituted interventional arm is depicted.
A. Intervention arm: From intervention cataract camps
All people examined by ophthalmologist with torch and ophthalmoscope.
Patients aged ≥40 years not having cataract and diagnosed as normal at this step would be included in this arm.
Included patients would undergo ophthalmoscopy, AC depth examination, FDT and IOP measurement with rebound tonometry.
In hospital, these referred cases will undergo the following examinations:
Undergo complete clinical examination with gonioscopy and 90D examination for disc by a glaucoma specialist.
Detailed visual field examination.
If normal, discharged.
If diagnosed with glaucoma (criteria as per Table 4), advised appropriate treatment (YAG PI, surgery offered free or prescribed medication).
B. Non-intervention arm: Routine cataract camps
Those ≥40 year old identified from routine cataract camps without any extra equipment or trained optometrist for glaucoma diagnosis:
People attending camps undergo routine tests by ophthalmologist with torch and ophthalmoscope.
If diagnosed as having cataract by the ophthalmologist, the subject will undergo IOP with Schiötz tonometry.
Glaucoma suspects (Table 4) in this arm would come from routine cataract camps if
1. detected by ophthalmologist in the camp as suspected glaucoma
2. if picked up as cataract from camps, brought for cataract surgery but diagnosed to be having glaucoma after examination in the hospital
Undergo tests similar as intervention group in hospital:
Undergo complete clinical examination with gonioscopy and 90D examination for disc by a glaucoma specialist
Detailed visual field examination
If normal, the subject is discharged
If diagnosed glaucoma--- advised appropriate treatment
c. Hospital arm: patients presenting to comprehensive semi-private OPD (with similar socio-economic status to camp patients) (Figure 3)
Patients suspected glaucoma for the first time will undergo the following diagnostic tests:
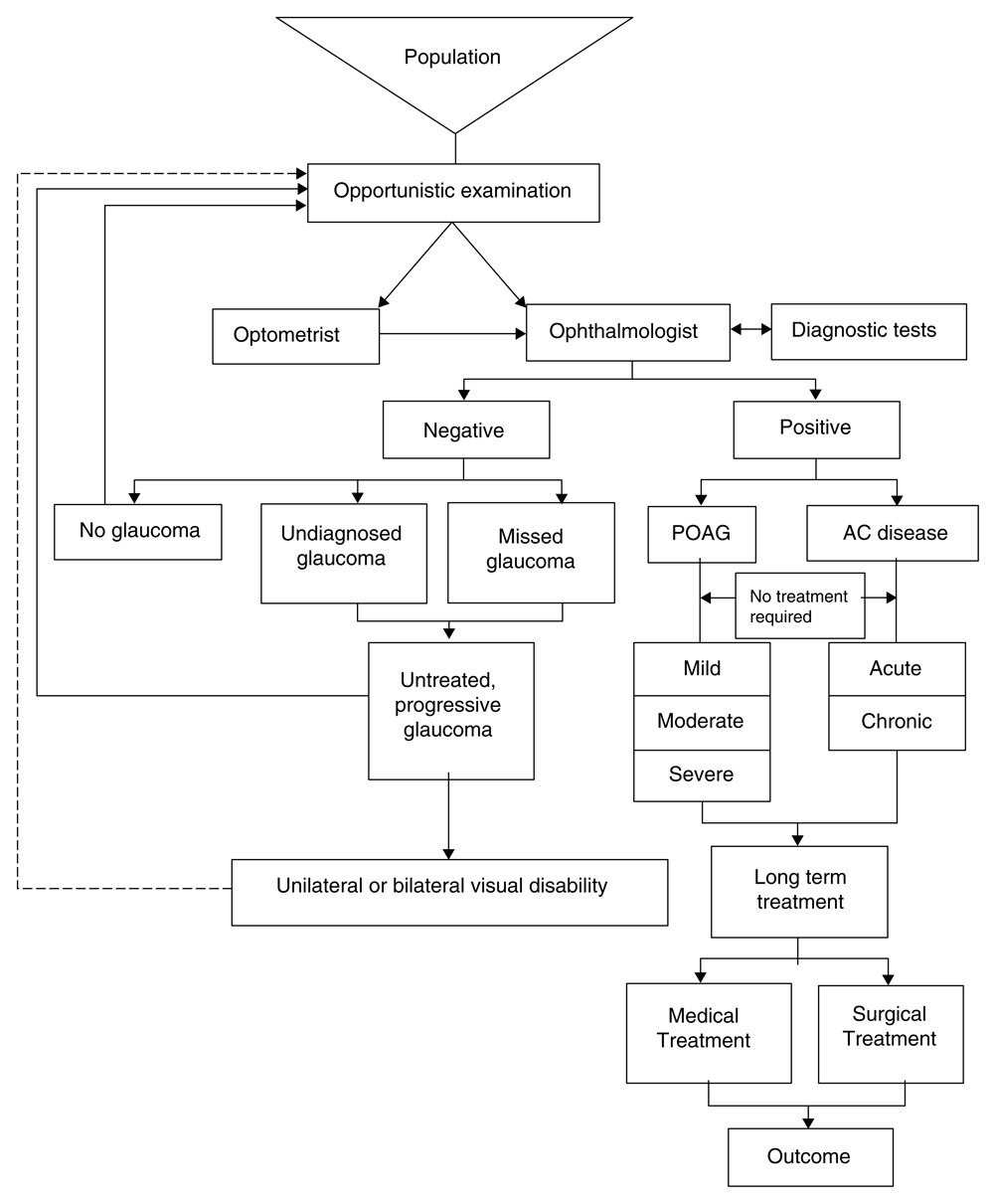
This figure has been reproduced with permission of John (2011)21.
If diagnosed as glaucoma (Table 4), appropriate treatment is advised.
The detection rate or yield will be measured by dividing the number of new cases identified during the study by the total number of cases examined. However, various other indicators will also be estimated through the study, which are expected to throw light on the efficacies of the FDT setting at the intervention camps in comparison with normal cataract camps and conventional detection setting at the hospital. Therefore, the sample sizes are estimated for different components of the study separately, based on the desired accuracy of the indicators at different level. For an accuracy of ±0.1 with a confidence interval of 95% and assuming a rate of around 50% positive predictive value (PPV) and negative predictive value (NPV), the sample size was estimated to be 97 suspects as diagnosed by the additional setting.
The sample size mentioned above is applicable for both FDT (used in camps and hospital set-up B) and conventional detection setting (triage) at the hospital set-up A. This sample sizes are estimated using the following formula24:
Where
α is such that (1- α) is the probability of confidence interval
Z1-α/2 is defined as probability {|X|< Z1-α/2 | X~ N(0,1)} = (1- α)
d = desired accuracy of the estimate. In other words, length of the confidence interval is 2d.
Since the individuals not suspected of having glaucoma in the intervention camps will not be referred to the hospital for confirmatory diagnosis as per the study protocol, the sample for normal population will be captured by FDT setting at the hospital set-up B. We assume that only 75% post-screening follow-up compliance rates, i.e. people suspected of having glaucoma, referred from the intervention camps turning up for confirmatory diagnosis. However, the confirmatory diagnosis test can be conducted on all those diagnosed as suspected of having glaucoma in the hospital arm as they will be present in the hospital itself. It has been assumed, for the purpose of estimation, that the rate of detection of people suspected of having glaucoma would not exceed 12% in the community. We further assume that it can be, in reality, as low as 10%. The number of screenings in the intervention, non-intervention and hospital arm should be adjusted accordingly in order to capture the required minimum sample sizes for people suspected of having glaucoma (required for estimating PPV) and normal (required for estimating NPV) populations. Although the FDT setting in the non-intervention arm will also capture a few suspected glaucoma cases, its primary objective is to identify normal individuals to be referred for confirmatory diagnosis, the target for capturing suspects at the intervention camps is reduced. Conventional detection setting in the the Hospital arm will identify both suspects and normal for confirmatory diagnosis.
Given the above assumptions on (i) post-screening follow-up compliance rates, (ii) prevalent suspect rates for FDT setting and conventional detection setting and (iii) required minimum sample sizes for estimating PPV and NPV, the number of screenings required for different components are estimated as per Table 5.
| Detection method | Assumptions | Number of screening required | Expected outcomes of screening | Lost Follow Up | Turn-ups for confirmatory diagnosis | ||||
|---|---|---|---|---|---|---|---|---|---|
| Suspect Rate | Follow-up Compliance Rate | Suspects | Normal | Suspects | Normal | Suspects | Normal | ||
| FDT | |||||||||
| Intervention Camp | 10% | 75% | 1,150 | 115 | 1,035 | 29 | NA | 86 | NA |
| FDT setting at Hospital | 10% | 100% | 108 | 11 | 97 | 0 | 0 | 11 | 97 |
| Total FDT setting | 1,257 | 126 | 1,132 | 29 | 97 | 97 | |||
| Conventional Detection at hospital | 10% | 100% | 1,015 | 102 | 914 | 0 | 0 | 97* | 97* |
All people of age ≥40 years, not detected as having cataract who would consent for screening for glaucoma would be included. A trained optometrist and vision technician would conduct various screening tests, such as disc examination, ACD depth, IOP reading, and FDT. Any result beyond cut-off points as mentioned in Table 3 would be flagged as suspected glaucoma cases and would be received by counselor. For those travelling to the hospital, transport and investigations in the hospitals would be offered free of cost. Overnight stay if required would also be provided free.
In the hospital, a detailed workup would be done for the glaucoma suspects. For glaucoma, they would undergo applanation tonometry, gonioscopy, automated visual field analysis and nerve fibre analysis. After the investigations, they would be examined by a glaucoma consultant, who would be masked to the study arm to which patient belongs. A diagnosis of glaucoma, suspected glaucoma, angle closure disease or impending angle closure requiring intervention will be made. The patient requiring intervention would be counselled and others would be discharged. Those requiring surgery would be offered surgery at fixed rates meant for camp patients needing non-cataract surgery. The patients staying overnight would be offered free transport both to the hospital from the camp and back to the camp site after discharge. Records would be maintained of all patients offered free transport.
A prospective observational study of 1000 adults ≥40 years age attending for glaucoma screening in cataract screening camps will be conducted. These adults would be recruited over an 18-month period across camps being conducted in villages near Delhi. The study is ongoing and recruitment is expected to be completed by December 2019.
The comparator group would be an equal number of adults of similar age who are screened in cataract camps in other nearby similar areas by SCEH and are identified as glaucoma suspects and as glaucoma-positive during their visit to the hospital. Additional comparator would also be patients >40 years age of similar socio-economic backgrounds who visit the hospital for the first time as routine or referral patients for eye check-up and are identified as glaucoma suspects and glaucoma positive.
The primary outcome of the screening algorithm would be the detection rate of the screening tests. The detection rate will be calculated by dividing the number of new cases identified during the study by the total number of cases examined [28].
We will use a step-down costing methodology for capturing costs of screening camps, whereby program costs will be entered into a customized tool created in MS Excel (Annexure 4, extended data)20. Cost data will be regularly entered into the tool for a period of 3 months to reflect any changes in the cost structure of the screening program. Using a step-down method, the main worksheets for entering data allocate costs to one of the following categories: training, pre-screening and screening.
The costs will be calculated by adding the unit costs of all resources used in the different activities occurring the screening process. This process includes all activities and procedures performed to detect cases with glaucoma from the invitation to participate in the screening campaign to the moment when the ophthalmologist establishes the diagnosis in the hospital. We will use the concept of activity-based costing for the screening program, but for the hospital we will use a standardized cost-accounting system. For the activity-based costing, the costs inputs will include screening invitation, screening costs (health professionals, devices, screening venue etc.). Personnel costs will be calculated from the total cost of each professional participating in a certain activity according to the time specifically dedicated to that particular activity. For example, optometrists dedicated 5–6 hours of their daily work time to the screening and only that part of their full salary will be considered as a cost. For ophthalmologists conducting different tasks (training, examination or test interpretation), only the fraction of their work time dedicated to the specific task involved in the glaucoma diagnostic process will be added.
All diagnostic equipment with a usage life of more than a year will be considered as capital equipment. The annual and monthly rental value of the capital equipment, and screening clinic costs per year/month will be calculated as per methods for assessing costs of glaucoma screening in India in other studies [20, 21]. All other diagnostic equipment will be considered as non-capital and will be considered with a depreciation rate of 100% in the first year itself.
Key information interviews with Program Manager (Outreach) will be conducted in identifying any donated goods requiring reevaluation and in allocating joint costs between program components. The allocation of joint costs to program components and activities, will also be informed by monthly sheets of screening camps.
The fixed costs at the hospital (electricity, water, gas, maintenance, and security) will be estimated from the annual structural costs of the facility divided by the number of patients seen and multiplied by the number of patients evaluated in the hospital in both screening and conventional arms. The structural costs at the hospital (glaucoma consultation) will be calculated from the hospital cost-accounting system.
All costs captured from accounting statements, such as fixed costs, salaries, etc., will be converted to economic costs. This means that capital costs will be annualized over their expected useful life, and any donated goods or volunteer time appearing as zero costs in the accounting data, or not appearing in the accounting data in any form, will be added to the cost sheets and assigned their current market value. Costs to the community would be captured using a structured questionnaire and will include direct medical (consultation, medicines etc.), direct non-medical (travel), and indirect costs (wage loss of patient) (See Annexure 2, extended data)20.
For the cost-effectiveness analysis, the cost per case detected would be the primary effectiveness outcome measure, in comparison with conventional detection, i.e. opportunistic case finding in hospitals.
The costs for the screening program will be based on the study sample and may vary significantly depending on the size of the target population and other factors. Hence, a sensitivity analysis will be performed using mean of detection rates from other studies in similar developing country settings.
The costs will also be recalculated for the community of 1 million subjects in order to generalize the findings to India as a whole. Costs will be presented in 2019 prices in Indian rupees and United States dollars (USD). Since the study period is less than 12 months, costs will not be adjusted for inflation, nor will discounting be considered for costs and outcomes.
Data entry will be conducted by a single data entry operator at the research unit of SCEH. One of the co-authors (AM) will be reviewing the data entry to check for any discrepancies including any data entry errors from the data entry form. The data will be stored in a desktop computer with access to the data entry operator, and co-auth or (AM). Once the data entry is completed, and cleaned, the data sheet will be transferred to laptops of co-author (AM & DJ) for further analysis. After the analysis these data sheets will be destroyed in these laptops and the data sheet would be available only with the desktop present at research unit of SCEH.
The patients being screened would be undergoing standard investigative procedures and no experimental investigation or procedure would be carried out. Informed consent would be taken before administering the questionnaire regarding cost incurred by patient/relative for screening and hospital examination (Extended data, Annexure 3)20. The identity of the person would not be disclosed, and no identifiable data would be shared or published. The identifiable data stored in database would be password protected.
The study received ethical approval from Dr Shroff’s Charity Eye Hospital-Ethics Committee (study reference number SCEHEC/2018/02) on 13.02.18.
The study results will be submitted to a suitable peer-review publication within 6 months of study period (i.e. patients entering camps and then arriving at hospital) with an acceptable sample size as described in relevant section above. Additionally, the results will also be presented in suitable national/international conferences based on resources available for participation.
This paper constitutes the first published protocol for the cost and cost effectiveness of a glaucoma screening program in a low-middle-income country. The protocol, which will adhere to internationally recognized guidelines for conducting and reporting economic evaluation studies, serves to heighten the transparency of the economic evaluation and planned analyses. The findings from this study will inform funding organizations, eye care institutions and policymakers about the relative value of glaucoma screening in the community. The evidence will contribute significantly to the scarce evidence regarding the cost-effectiveness of community screening for glaucoma in reducing blindness in LMICs.
Extended data for this study, described below, are available on figshare. DOI: https://doi.org/10.6084/m9.figshare.750388720.
Annexure 1. Questionnaire concerning glaucoma awareness. English and Hindi versions are given.
Annexure 2. Patient expenses data form.
Annexure 3. Patient informed consent form.
Annexure 4. Customised Excel tool template.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Health economics, Indian health care system and financing
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 29 Apr 19 |
read | |
|
Version 2 (revision) 25 Feb 19 |
read | read |
|
Version 1 14 Jan 19 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)