Keywords
tamoxifen, endometrial, menopausal status, gynecological side effect
This article is included in the Oncology gateway.
tamoxifen, endometrial, menopausal status, gynecological side effect
Breast cancer (BC) is the second most prevalent cancer globally and the top cause of cancer-related deaths in women1. In Iraq, BC is the most prevalent cancer in the population. In 2012, BC occurred in 19.5% of all newly diagnosed cancer cases, and 34% of women with cancer2. Additionally, BC is the primary cause of cancer-related death in Iraqi women, causing 23.6% mortality among all women-related deaths).
Estrogen receptors (ER) have multiple functions that affect the reproductive, musculoskeletal and cardiac systems, and the central nervous system. Additionally, it has been suggested to be involved in the proliferation of BC tumors tissues3; about 70–80% of BC cases are ER-positive. There are two classes of ER, α and β, and their activity is primarily regulated by estradiol (E2) binding. Therefore, E2 plays a vital role in the pathogenesis of BC through these receptors. Patients with these tumors are candidates for endocrine therapy after surgical removal of the primary tumor and treatment with ionizing radiation or cytotoxic chemotherapy4. The major strategy for BC treatment starts with surgery, chemotherapy, ionizing radiation therapy, endocrine (hormonal) therapy, and targeted therapy5.
Tamoxifen (TMX) is a selective ER modulator, which acts as an inhibitor for the effect of estrogen on breast tissues. Its side effects include vaginal dryness, vaginal discharge, flushes, cataracts, night sweats, thrombotic events, stroke, and rarely endometrial cancer and uterine cancer6. Many studies have examined the relationship between TMX use, duration of TMX use, whether female patients are premenopausal (PreM) or postmenopausal (PostM), and the development of gynecological side effects7–14. The present study aimed to identify if there is a relationship between TMX use and changes in the endometrium and ovaries of female breast cancer patients. In addition, the study aimed to classify if there are unreported gynecological side effects with TMX use, and whether menopausal status (PreM or PostM) leads to a different TMX effect on the endometrium and ovaries.
This is an ultrasound (US)-based cohort study, conducted in three oncology centers In Bagdad, Iraq. Female BC patients treated with TMX at dose of 20 mg/day as part of therapeutic regimen for the treatment of BC were included in the study. Abdominal US was used for the assessment of endometrial thickness (ET) and abnormalities in the endometrial cavity and ovaries.
All procedures performed in the study were in accordance with the ethical standards of the Institutional Research Committee at the College of Medicine, Baghdad University, who approved the study protocol (approval date: 3rd December 2019; number: 2019/0234), and with the 1964 Helsinki declaration and its later amendments.
Written informed consent was obtained from all participants to be included in the study.
The study was conducted at the three main oncology centers in Baghdad: Breast Cancer Center of Al-Elwia Teaching Hospital, Oncology Teaching Hospital at Baghdad Medical City, and Al-Amel Oncology Hospital. These oncology centers are teaching hospitals under the supervision of the College of Medicine, Baghdad University. Periods of recruitment were between December 2018 and May 2019. Data about patients (US findings) were obtained from their hospital records (each patient was followed-up for at least 6 months, with maximum duration of follow-up of 2 years).
A sample size of 256 women was calculated considering the power of 80%, the confidence level of 95% and a relative precision 5% and prevalence of ET of 12% in PreM and 10.6% in PostM based on a study by Lee et al. 201815. Therefore, a 20% value was chosen, the following formula were used to calculate the estimated sample size:
Where, p is prevalence, q is (1 – p), and d is relative precision.
A total of 255 patients, 140 premenopausal and 115 postmenopausal female BC patients were included in the study.
We included female patients with ER-positive BC treated with TMX as an adjuvant hormonal treatment at a dose of 20 mg/day for at least three months after surgery and in addition to adjuvant chemo/radiotherapy.
We excluded patients who were on irregular treatment, patients with increased uterine thickness for any reason at baseline assessment, patients who had other gynecological or non-gynecological malignancies, patients with secondary BC and patients with incomplete or lost medical records.
Hormonal levels and menstrual history were used for the assessment of menopausal status. A PreM woman was defined as a woman with regular menstrual cycles with follicular stimulating hormone (FSH) <40.0 mIU/mL. A PostM woman defined as a woman with amenorrhea longer than one year and FSH >40 mIU/mL on two sequential occasions at the time of diagnosis with BC15.
All women that participated in the study were interviewed by the researchers; data were collected from them using a predesigned survey in a personal interview setting and from their medical records. The women were followed-up prospectively, and if the women had previous US reading it will be incorporated in study. Patient characteristics, US findings, and results of histopathological examination of endometrial biopsy whenever available were collected. The survey collected the following patient characteristics: age of the patient, menopausal status, co-morbid chronic illness and past medical history (e.g. hypertension, diabetes mellitus (DM)), duration of TMX use, side effects, and current used medications.
Abdominal US was used for the assessment of ET and abnormalities in the endometrial cavity and ovaries. Endometrial and ovarian assessment by US was performed before the start of hormonal therapy and alterations were periodically measured at an interval of three months. Assessment of the endometrial lining was done by calculating the maximum thickness from the outermost limits of the endometrial-myometrial juncture and the thickening was defined according to menopausal status as follows16:
Patients who were diagnosed with endometrial pathology were followed-up for six months to assess their status and dealt with accordingly. Patients diagnosed with ovarian cyst (> 30 mm on US) were further evaluated by cancer antigen 125 (CA-125); if normal, US examination was commenced every three months, if high CA-125, patients underwent further radiological assessment, including CT scan or MRI15.
Diagnostic hysteroscopy was done for the patients that showed abnormalities on US and biopsy was taken whenever indicated (when the US demonstrated uterine abnormalities or mass, and/or patients with abnormal uterine bleeding).
Chi-square test, Fisher’s exact test, independent t-test, and paired t-test were used to compare between PreM and PostM groups. Logistic regression used to calculate the odds ratio (OR) and 95% confidence intervals. Linear regression analysis was performed to assess the relationship between different variables. SPSS 23.0.0 (Chicago, IL) and GraphPad Prism version 8.0.0 used to perform statistical analysis. P<0.05 was considered significant.
In total, 255 women with BC were included in this study. Age range was 32 to 78 years, with mean age 50.4±9.0 years. The most common age group was 40–49 years (36.1%), followed by 50–59 years (32.5%). In total, 15.3% of the patients had DM, while 3.9% had hypertension (a higher frequency in the PreM compared to PostM women).
There was no significant difference in the history of hypertension, DM, use of medications (angiotensin-converting enzyme/angiotensin receptor blocker, or metformin), cancer stage, and treatment duration of TMX between PreM and PostM women (Table 1).
From US examinations, only the presence of ovarian cyst was significantly higher in the PreM compared to PostM women. Other US findings were not significant between groups (Table 2).
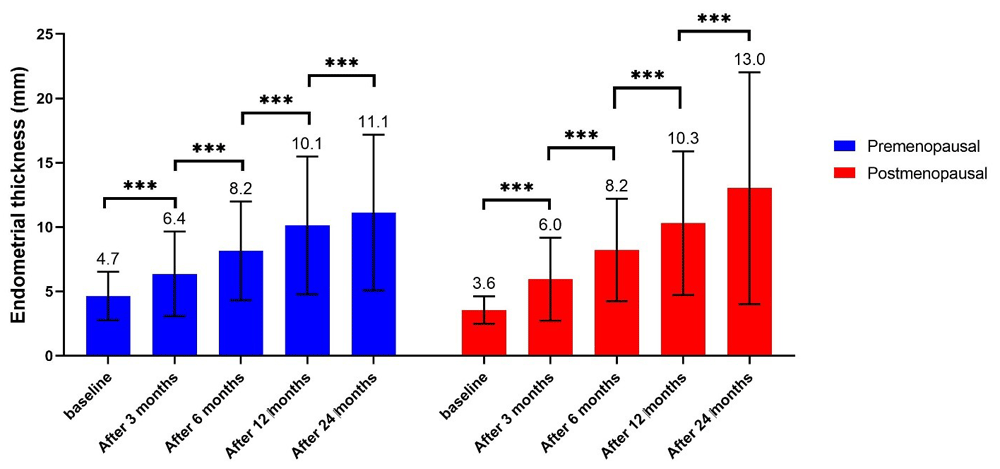
At baseline, ET was significantly higher in the PreM compared to the PostM group. In both groups, women with increased ET became more frequent from baseline to 3 months, from 3 months to 6 months, from 6 months to 12 months, and from 12 months to 24 months. At all time periods, the number of women with increased ET was significantly higher in the PostM compared with PreM group (Table 3; Figure 1).
| Premenopausal | Postmenopausal | P-value | |
|---|---|---|---|
| N | 140 | 115 | - |
| ET, mean±SD | |||
| Baseline | 4.7 ±1.9 | 3.6 ±1.1 | <0.001 |
| After 3 months | 6.4 ±3.3 | 6.0 ±3.2 | 0.302 |
| After 6 months | 8.2 ±3.8 | 8.2 ±4.0 | 0.900 |
| After 12 months | 10.1 ±5.4 | 10.3 ±5.6 | 0.811 |
| After 24 months | 11.1 ±6.0 | 13.0 ±9.0 | 0.139 |
| Increase in ET#, n (%) | |||
| Baseline | 2 (1.4) | 19 (16.5) | <0.001 |
| After 3 months | 11 (7.9) | 65 (56.5) | <0.001 |
| After 6 months | 25 (20.0) | 82 (82.8) | <0.001 |
| After 12 months | 42 (38.9) | 71 (80.7) | <0.001 |
| After 24 months | 38 (44.2) | 56 (81.2) | <0.001 |
| Any time | 64 (45.7) | 96 (83.5) | <0.001 |
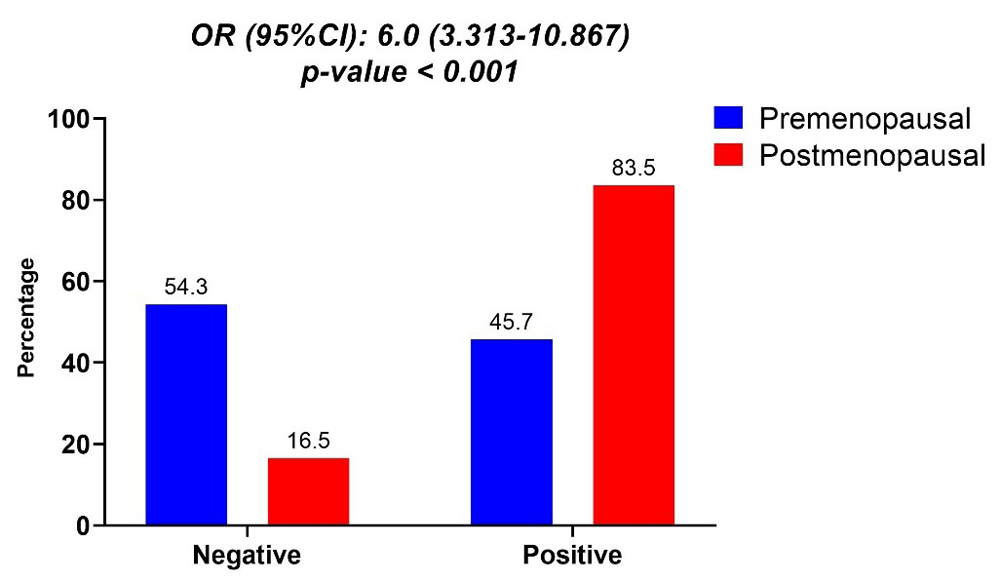
ET was significantly higher in PostM compared to PreM women, resulting in an increased risk of endometrial thickening by 6 fold (OR: 6.000, 95%CI: 3.313 – 10.867, p-value <0.001) in PostM compared to PreM women (Figure 2).
At baseline there was not a significant correlation between duration of TMX with ET; however from 3 months until 24 months after TMX therapy there was significant correlation between duration of TMX with EM thickness (Table 4).
| r | p-value | |
|---|---|---|
| Baseline | 0.029 | 0.646 |
| After 3 months | 0.164 | 0.009 [S] |
| After 6 months | 0.200 | 0.003 [S] |
| After 12 months | 0.221 | 0.002 [S] |
| After 24 months | 0.200 | 0.013 [S] |
There was no significant correlation between TMX treatment duration with any gynecological outcomes (Table 5).
In the present study, mean ET after 24 months was 11.1±6.0 mm in PreM and 13.0±9.0 mm for PostM women (no significant difference between groups). This agreed with other studies17,18. In a retrospective study that examined 614 women with BC, 53 of them had history of TMX usage, and ET was significantly higher in women that received TMX (11 vs 6 mm in those not on TMX therapy). In addition, women with ET ≥5 mm was significantly higher in TMX group (86.8% vs. 52.0%, p-value <0.001), and higher than other endometrial abnormalities (43.4% vs 31.7%, p-value = 0.048), which indicates that the use of TMX increases the risk of ET and abnormalities18.
In the current study, increased ET occurred in 45.7% of PreM and 83.5% of PostM women, and these findings were similar to other studies17. However, other studies reported a lower rate of increased ET than the present study; Buijs et al. examined 47 PreM women and found that 7 (17.0%) suffered from increased ET (≥12 mm)19. Jindal et al. reported that 30% of women assessed had EM thickness ≥5 mm which is lower than our study20. Another study of 737 PostM patients with BC who received TMX, revealed that 28% had ET ≥6 mm21, while Lee et al. reported an increased ET in 12% of PreM and 10.6% of PostM women15.
In the current study, there was a significant relationship between TMX treatment duration and ET (the magnitude of this relationship was similar from 3 months to 24 months). In a study by Hann et al., ET increased with the duration of TMX treatment; 73 women treated with TMX for <5 years had a median ET of 5 mm, and 44% of biopsies yielded abnormal results, while 18 women who had received TMX for ≥5 years had a median ET of 14 mm (58% of endometrial biopsies in this group were abnormal)22. This study agrees with our findings. In contrast, Jindal et al. reported no significant correlation between TMX duration and ET, which could be attributed limitations of their study, including a small sample size and short follow-up duration21.
Endometrial polyps are a common pathology and have an increased malignant transformation rate in PostM TMX-treated BC patients17; however, limited studies have investigated the malignant potential of endometrial polyps by hysteroscopy in this population17. In the present study, endometrial polyps were present in 2.1% of PreM women and 6.1% of PostM women. In Jeon et al., the frequency of endometrial polyps was much higher than our findings (41.7%)17. Similarly, Hann et al. found endometrial polyps in 33% cases22 and Deligdisch et al. found a frequency of 23.14%23. Abdaal et al. revealed similar rate of polyps to the present study of 3.9%18. The low incidence of EM polyps in the present study compared with other studies could suggest that the incidence in Iraqi women is lower than other ethnicities. In the present study, endometrial hyperplasia in PreM and PostM women was 5.7% and 11.3%, respectively, while it was 1.7% in Jeon et al.17 and 8% in Deligdisch et al.23.
The use of TMX is associated with two to four fold increased risk of endometrial hyperplasia and polyps24. The increased risk of these complications is related to the effect of TMX on the endometrium, which causes proliferation of the endometrium, particularly in PreM and early PostM women25. In the present study, endometrial cancer occured in 4.3% and 5.3% of PreM and PostM women, respectively, and these findings are similar to other studies18,22,23, apart from Jeon et al.17. In the present study, there was no significant relationship between duration of TMX and risk of endometrial cancer (OR: 0.994, 95%CI: 0.675-1.463), which was in agreement with Katase et al., who concluded that TMX did not increase the risk of endometrial cancer in women with primary BC8. However, another study showed that endometrial cancer was related to TMX and the risk of endometrial cancer is related to the duration of TMX use26. This also confirmed by a meta-analysis7.
Another significant finding in the present study was that the presence of ovarian cyst was significantly higher in PreM (27.9%) compared to PostM women (7.8%), which is in agreement with other studies15,24.
Longer duration of TMX is associated with increased ET in Iraqi women with BC; however, the duration of TMX did not appear to increase the risk of various gynecological outcomes. In addition, endometrial cancer rate was low, and there was a high rate of ET, which appears to be six-folds higher in PostM compared to PreM women.
Zenodo: Effects of Tamoxifen on the Reproductive System of Females with Breast Cancer, http://doi.org/10.5281/zenodo.357622227.
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
References
1. von Elm E, Altman DG, Egger M, Pocock SJ, et al.: Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies.BMJ. 2007; 335 (7624): 806-8 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Surgical oncology (hormonal therapy of endocrine-responsive breast cancer)
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
References
1. Mourits MJ, de Vries EG, ten Hoor KA, van der Zee AG, et al.: Beware of amenorrhea during tamoxifen: it may be a wolf in sheep's clothing.J Clin Oncol. 2007; 25 (24): 3787-8; author reply 3788 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: My field of expertise is gynecological oncology, with special focus on hereditary gynecological cancer. I have a PhD in the field of gynecological side effects of tamoxifen.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 10 Feb 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)