Keywords
COVID-19, clinical research agenda, hydroxychloroquine
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Coronavirus (COVID-19) collection.
COVID-19, clinical research agenda, hydroxychloroquine
On December 31, 2019, the World Health Organization (WHO) China Country Office was informed of pneumonia cases of unknown etiology1; on January 30, 2020, the WHO declared coronavirus disease 2019 (COVID-19)2 a public health emergency and on March 11 a pandemic3. Radical public health measures, including quarantine, social distancing, school and workplace closures, and others have been implemented worldwide, affecting the lives of billions of people. The pandemic resulted in rapid generation and dissemination of studies and their results4. However, information on trials that are planned, ongoing, finished, or published are spread across trial registries, preprint servers, publication databases and other repositories. In June, 2020, the number of ongoing trials outweighed by far completed trials; however, no overview of COVID-19 trials has followed up on actual enrolment in ongoing trials5–8.
We established the COVID-evidence platform (www.covid-evidence.org) to collect this information in a central database of COVID-19 trials testing any interventions for treatment or prevention. We used COVID-evidence to describe the worldwide clinical research response to COVID-19, its evolution over the first 100 days since the first cases were officially reported, and the expected feasibility and risk of waste of resources. We describe the trials’ characteristics, their place in the research landscape, and how they changed over time.
COVID-evidence includes trials from international registries (ClinicalTrials.gov, WHO International Clinical Trials Registry Platform [ICTRP]), preprint servers (medRxiv, bioRxiv), PubMed, the WHO COVID-19 literature database, and a listing of all trials with ethical approval in Switzerland9.
Our protocol and details on the search strategies and specific definitions used for COVID-evidence are available on the Open Science Framework (OSF)10. We searched the relevant data sources using peer-reviewed search strategies developed by information specialists. Results from literature databases and preprint servers were pre-screened by a single reviewer who excluded unsuitable publications (e.g. opinion papers or observational, non-interventional studies). Cases with unclear eligibility were discussed by at least three reviewers until consensus was reached.
We included any planned, ongoing or completed trial that tested any intervention to treat or prevent COVID-19 in humans that was registered or published within the first 100 days of the COVID-19 outbreak, i.e. after the first cases reported to the WHO to 100 days later (January 1 to April 9, 2020). We considered as a trial any study prospectively assigning an intervention11. This included randomized and non-randomized, controlled or non-controlled trials regardless of language, geographical region, or setting. Epidemiological studies or studies of diagnostic test accuracy (without any health-related outcome) were excluded.
For each trial, we extracted dates of registration and publication, design characteristics and details of the population, intervention, comparison, outcomes, geographic region, funding and setting. We categorized drugs and biologicals according to major pharmacological classes and main clinical indications.
A team of 19 reviewers (including clinicians, clinical researchers, clinical pharmacologists, meta-researchers and systematic reviewers) either manually extracted information or verified information that was obtained with automatic data scraping methods. All details on scraping, variable definitions and extraction/verification procedures are available on OSF10,12. For all trials registered up to April 9, 2020, we extracted data through April 30. The status for each trial was updated on July 6, 2020 (using ClinicalTrials.gov where possible; if not, we used the ICTRP; for trials originally registered in the Chinese Clinical Trial Registry available through ICTRP we used the former if it was more up to date).
When a trial had entries in different data sources, we gave first priority to publications, second to preprints, and third to registries (here, ClinicalTrials.gov was preferred).
We identified 689 trials registered or published over the pandemic’s first 100 days, testing interventions to treat or prevent COVID-19 (see Underlying data)12 with a total planned sample size of 396,366 participants. As of July 6, 2020, 19 trials (including 4,378 participants) had been completed and had published results, and 16 were completed without available results (5,173 participants). Thirty (4.4%) were active but no longer recruiting (59,259 participants), 384 (55.7%) started recruiting (217,357 participants), 174 (25.3%) had not yet started (97,406 participants), 50 (7.3%) were discontinued (12,048 participants), and 4 (0.6%) were terminated (577 participants). The status was unknown for 12 (1.7%; 168 participants).
The 689 trials’ median target sample size was 120 (IQR 60 to 300; Table 1); 40.7% (n=280) planned to enroll fewer than 100 participants, 8.3% (n=57) over 1,000, and 1.6% (n=11) over 5,000 (see Underlying data)12. 75.8% (n=522) trials were randomized and 59.2% (n=408) did not use blinding (Table 1). Randomized trials were on average three times larger than non-randomized trials (median sample size 150 vs. 50).
| Trial characteristics | Total (n=689) | For treatmenta (n=607) | For preventiona (n=78) | Not yet recruiting (n=178) | Recruiting (n=385) | China (n=352) | United States (n= 76) | France (n=35) | Spain (n=21) | International (n=21) |
|---|---|---|---|---|---|---|---|---|---|---|
| Randomizedb | 522 (75.8%) | 462 (76.1%) | 56 (71.8%) | 134 (75.3%) | 300 (77.9%) | 258 (73.3%) | 53 (69.7%) | 30 (83.3%) | 21 (100%) | 20 (95.2%) |
| Two arms | 397 (57.6%) | 358 (59%) | 35 (44.3%) | 103 (57.9%) | 231 (60%) | 205 (58.2%) | 40 (52.6%) | 21 (60%) | 17 (80.9%) | 10 (47.6%) |
| Three or more arms | 119 (17.3%) | 99 (16.3%) | 20 (25.6%) | 31 (17.4%) | 67 (17.4%) | 53 (15.1%) | 13 (17.1%) | 9 (25.7%) | 4 (19%) | 9 (42.9%) |
| Number of arms not reported | 6 (0.9%) | 5 (0.8%) | 1(1.2%) | 0 (0%) | 2 (0.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4.8%) |
| Non-randomized | 161 (23.4%) | 139 (22.9%) | 22 (28.2%) | 43 (24.2%) | 85 (22.1%) | 91 (25.9%) | 23 (30.3%) | 5 (16.7%) | 0 (0%) | 1 (4.8%) |
| Single arm | 101 (14.6%) | 88 (14.5%) | 13 (16.2%) | 27 (15.2%) | 52 (13.5%) | 49 (13.9%) | 18 (23.7%) | 4 (11.4%) | 0 (0%) | 1 (4.8%) |
| Two arms | 47 (6.8%) | 40 (6.6%) | 7 (8.7%) | 15 (8.4%) | 23 (6%) | 34 (9.7%) | 3 (3.9%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Three or more arms | 13 (1.9%) | 11 (1.8%) | 2 (2.5%) | 1 (0.6%) | 10 (2.6%) | 8 (2.3%) | 2 (2.6%) | 1 (2.9%) | 0 (0%) | 0 (0%) |
| Blinding | ||||||||||
| None | 408 (59.2%) | 368 (60.6%) | 38 (48.7%) | 103 (57.9%) | 221 (57.4%) | 208 (59.1%) | 41 (53.9%) | 23 (65.7%) | 11 (52.4%) | 10 (47.6%) |
| Double blind | 135 (19.6%) | 110 (18.1%) | 25 (32.1%) | 24 (13.5%) | 84 (21.8%) | 34 (9.7%) | 29 (38.2%) | 10 (28.6%) | 8 (38.1%) | 10 (47.6%) |
| Single blind | 36 (5.2%) | 29 (4.8%) | 7 (9%) | 9 (5.1%) | 24 (6.2%) | 11 (3.1%) | 5 (6.6%) | 2 (5.7%) | 1 (4.8%) | 1 (4.8%) |
| Outcome only | 8 (1.2%) | 3 (0.5%) | 5 (6.4%) | 5 (2.8%) | 2 (0.5%) | 3 (0.9%) | 1 (1.3%) | 0 (0%) | 1 (4.8%) | 0 (0%) |
| Not reported | 102 (14.8%) | 97 (16%) | 3 (3.8%) | 37 (20.8%) | 54 (14%) | 96 (27.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Planned sample sizec | ||||||||||
| Total | 496,450 | 187,293 | 205,841 | 101,932 | 268,398 | 70,435 | 49,855 | 15,476 | 13,005 | 138,120 |
| Median [IQR] | 120 [60-300] | 100 [50-240] | 440 [150-1,200] | 100 [60-204.8] | 131 [60-360.5] | 90 [40-160] | 160 [50-500] | 240 [100-569] | 200 [120-440] | 1,200 [240-4,500] |
| Min-max | 4-55,000 | 4-12,000 | 4-55,000 | 10-55,000 | 5-20,000 | 4-20,000 | 5-15,000 | 11-1,300 | 24-4,000 | 20-55,000 |
| Intervention | ||||||||||
| Drug | 390 (56.6%) | 349 (57.5%) | 39 (50%) | 80 (44.9%) | 234 (60.8%) | 139 (39.5% | 55 (72.4%) | 29 (82.9%) | 19 (90.5%) | 20 (95.2%) |
| Traditional medicine | 108 (15.7%) | 100 (16.5%) | 8 (10.3%) | 41 (23%) | 57 (14.8%) | 101 (28.7% | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Biological | 79 (11.5%) | 77 (12.7%) | 1 (1.3%) | 19 (10.7%) | 40 (10.4%) | 50 (14.2%) | 9 (11.8%) | 1 (2.9%) | 1 (4.8%) | 0 (0%) |
| Procedure | 31 (4.5%) | 30 (4.9%) | 1 (1.3%) | 12 (6.7%) | 15 (3.9%) | 15 (4.3%) | 3 (3.9%) | 4 (11.4%) | 0 (0%) | 0 (0%) |
| Device | 11 (1.6%) | 8 (1.3%) | 2 (2.6%) | 3 (1.7%) | 6 (1.6%) | 2 (0.6%) | 2 (2.6%) | 0 (0%) | 1 (4.8%) | 0 (0%) |
| Vaccine | 13 (1.9%) | 0 (0%) | 13 (16.7%) | 2 (1.1%) | 8 (2.1%) | 6 (1.7%) | 2 (2.6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Multiple interventiond | 18 (2.5%)‡ | 15(2.5%) | 3 (3.9%) | 5 (3.5%) | 11(2.9%) | 13 (3.7%) | 1 (1.3%) | 0 (0%) | 0 (0%) | 1 (4.8%) |
| Other | 36 (5.2%) | 26 (4.3%) | 10 (12.8%) | 15 (8.4%) | 14 (3.6%) | 24 (6.8%) | 4 (5.3%) | 1 (2.9%) | 0 (0%) | 0 (0%) |
| Unclear | 3 (0.4%) | 2 (0.3%) | 1 (0.5%) | 0 (0%) | 0 (0%) | 2 (0.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Control | ||||||||||
| Standard of care / No intervention | 312 (45.3%) | 286 (47.1%) | 23 (29.5%) | 94 (52.8%) | 176 (45.7%) | 187 (53.1%) | 19 (25%) | 15 (42.9%) | 7 (33.3%) | 6 (28.6%) |
| Placebo | 130 (18.9%) | 103 (17%) | 27 (34.6%) | 30 (16.9%) | 74 (19.2%) | 46 (13.1%) | 24 (31.6%) | 7 (20%) | 5 (23.8%) | 11 (52.4%) |
| Other active intervention | 119 (17.3%) | 109 (18%) | 9 (11.5%) | 25 (14%) | 70 (18.2%) | 63 (17.9%) | 12 (15.8%) | 7 (20%) | 6 (27.3%) | 2 (9.5%) |
| No control (single arms) | 101 (14.7%) | 88 (14.5%) | 13 (16.7%) | 27 (15.2%) | 52 (13.5%) | 49 (13.9%) | 18 (23.7%) | 4 (11.4%) | 0 (0%) | 1 (4.8%) |
| Other and combinatione | 13 (1.9%) | 9 (1.5%) | 4 (5.1%) | 2 (1.1%) | 11 (2.9%) | 2 (0.6%) | 3 (3.9%) | 2 (5.7%) | 2 (9.5%) | 1 (4.8%) |
| Unclear | 14 (2%) | 12 (2%) | 2 (2.6%) | 0 (0%) | 2 (0.5%) | 5 (1.4%) | 0 (0%) | 0 (0%) | 1 (4.8%) | 1 (0.3%) |
| Mortality outcome | ||||||||||
| Primary outcome | 98 (14.2%) | 95 (15.7%) | 3 (3.8%) | 21 (11.8%) | 63 (16.4%) | 34 (9.7%) | 3 (3.9%) | 16 (45.7%) | 6 (28.6%) | 5 (23.8%) |
| Secondary outcome | 222 (32.2%) | 210 (34.6%) | 10 (12.8%) | 44 (24.7%) | 135 (35.1%) | 80 (22.7%) | 31 (40.8%) | 12 (34.3%) | 9 (42.9%) | 10 (47.6%) |
| Not reported as outcome | 369 (53.6%) | 302 (49.8%) | 65 (83.3%) | 113 (63.5%) | 187 (48.6%) | 238 (67.6%) | 42 (55.3%) | 7 (20%) | 6 (28.6%) | 6 (28.6%) |
| Participants | ||||||||||
| Inpatient | 525 (76.2%) | 506 (83.4%) | 17 (21.8%) | 131 (73.6%) | 297 (77.1%) | 276 (78.4%) | 46 (60.5%) | 30 (85.7%) | 14 (66.7%) | 17 (81%) |
| Outpatient | 56 (8.1%) | 38 (6.3%) | 18 (23.1%) | 19 (10.7%) | 29 (7.5%) | 25 (7.1%) | 10 (13.2%) | 1 (2.9%) | 1 (4.8%) | 2 (9.5%) |
| Healthcare workers | 25 (3.6%) | 0 (0%) | 25 (32.1%) | 5 (2.8%) | 15 (3.9%) | 3 (0.9%) | 7 (9.2%) | 3 (8.3%) | 4 (19%) | 1 (4.8%) |
| Healthy participants | 9 (1.3%) | 1 (0.2%) | 8 (10.3%) | 4 (2.2%) | 4 (1%) | 3 (0.9%) | 3 (3.9%) | 0 (0%) | 1 (4.8%) | 0 (0%) |
| Multiple/mixed | 15 (2.2%) | 8 (1.3%) | 5 (6.4%) | 4 (2.2%) | 9 (2.3%) | 7 (2%) | 4 (5.3%) | 0 (0%) | 1 (4.8%) | 0 (0%) |
| Unclear | 59 (8.6%) | 54 (8.9%) | 5 (6.4%) | 15 (8.4%) | 31 (8.1%) | 38 (10.8%) | 6 (7.9%) | 1 (2.9%) | 0 (0%) | 1 (4.8%) |
| Funding type | ||||||||||
| Public/not-for-profit | 265 (38.5%) | 242 (39.9%) | 22 (28.2%) | 77 (43.3%) | 159 (41.3%) | 178 (50.6%) | 9 (11.8%) | 14 (40%) | 8 (38.1%) | 5 (23.8%) |
| Industry/for-profit | 68 (9.9%) | 63 (10.4%) | 5 (6.4%) | 18 (10.1%) | 37 (9.6%) | 29 (8.2%) | 9 (11.8%) | 1 (2.9%) | 1 (4.8%) | 6 (28.6%) |
| Both | 11 (1.6%) | 11 (1.8%) | 0 (0%) | 2 (1.1%) | 7 (1.8%) | 8 (2.3%) | 0 (0%) | 1 (2.9%) | 0 (0%) | 0 (0%) |
| Reported but unclearf | 288 (41.8%) | 245 (40.4%) | 41 (52.6%) | 64 (36%) | 159 (41.3%) | 105 (29.8%) | 51 (67.1%) | 16 (45.7%) | 10 (47.6%) | 9 (42.9%) |
| Not reported | 57 (8.3%) | 46 (7.6%) | 10 (12.8%) | 17 (9.6%) | 23 (6%) | 32 (9.1%) | 7 (9.2%) | 3 (8.6%) | 2 (9.5%) | 1 (4.8%) |
Additional categories such as other countries can be found in the extended data
a 4 trials assessed interventions for both treatment and intervention
b For 6 trials the randomization was unclear
c The sample size is missing for 17 trials
d Some trials compared different types of interventions: 3 trials biologicals and drugs; 6 trials drugs and traditional medicine; 5 trials other interventions and drugs; 1 trial other interventions and traditional medicine; 2 trials procedures and drugs; 1 trial vaccine and device
e Included for example use trials that used a standard of care arm and an active control arm
f a public/not-for-profit sponsor was reported but the absence of an industrial sponsor does not exclude an industrial funder
Although few trials focused on health-care workers (3.6% [n=25]), they were larger: 96,821 planned health-care workers (median 700 [IQR 400 to 2,486]) versus 155,571 planned patients (median 100 [IQR 50 to 240]) for the inpatient trials (76.2% [n=525]) (Table 1). Overall, 46.4% of the trials intended to use mortality as a primary (n=98) or secondary outcome (n=369; Table 1). Out of the 525 inpatient trials, 55.6% (n=292) planned on reporting mortality as an outcome.
Out of the 689 trials, 607 (88.1%) assessed treatment interventions (187,209 planned patients); drugs were more frequent (349 trials [57.5%]), encompassing a vast range of substances. The two most common pharmacological classes were antiviral drugs (assessed in 144 trials; e.g. lopinavir/ritonavir [n=45]) and antimalarial drugs (112 trials; e.g. hydroxychloroquine [n=83]). There were 106 trials investigating traditional medicine and 70 exploring highly diverse pharmaceuticals of various classes, e.g. bismuth potassium citrate, ebastine, pirfenidone, dipyridamole and hydrogen peroxidase (Figure 1 and see Extended data)12. The comparators were predominantly standard of care or no intervention (47.1% [n=286]), placebo (17% [n=103]) or other interventions (18%; [n=109]) (Table 1).
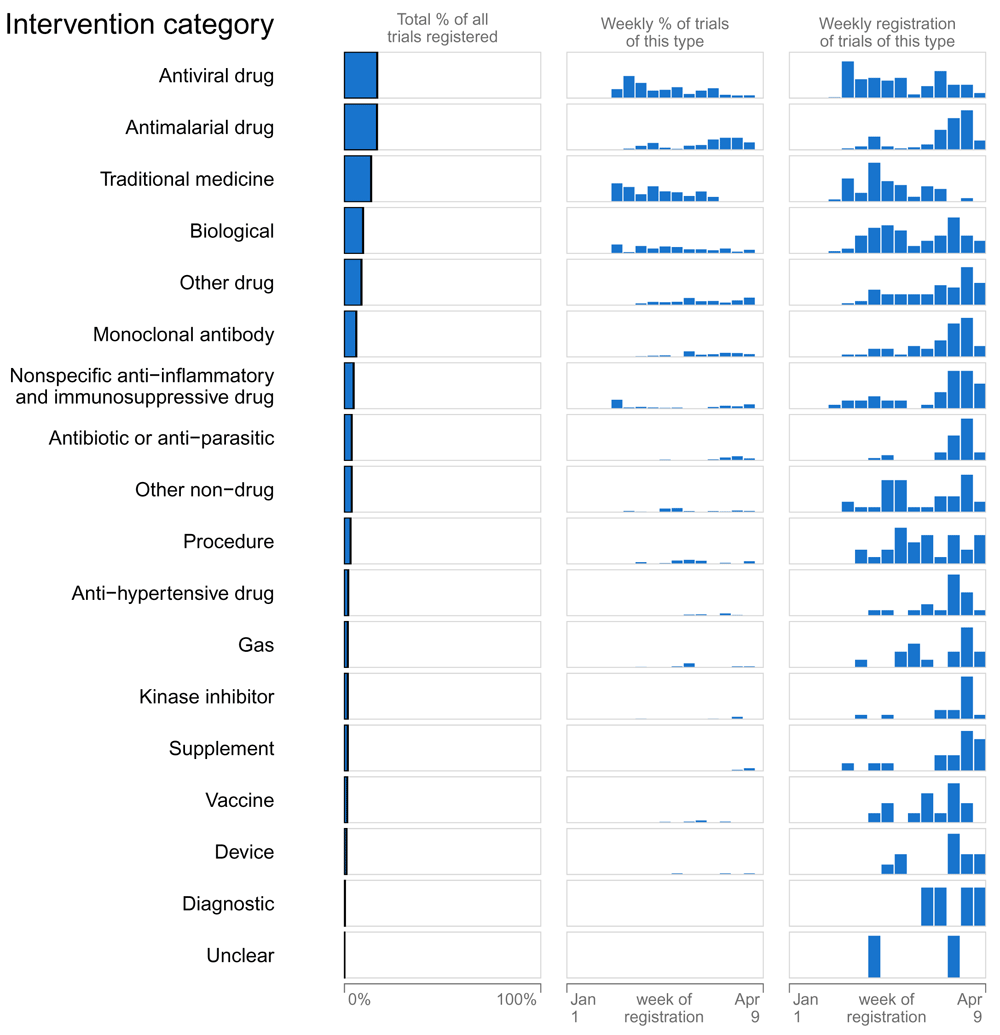
Interventions for treatment assessed in more than 25 trial: antiviral drugs were assessed in 144 trials; (e.g. lopinavir/ritonavir [n=45]), antimalarial drugs in 112 trials; (e.g. hydroxychloroquine [n=83]), monoclonal antibodies in 52 trials (e.g. tocilizumab [n=26]), traditional medicine in 106 trials, other drug intervention in 70 trials, nonspecific anti-inflammatory/immunosuppressive drugs in 42 trials (e.g. colchicine [n=4]), antibiotic/anti-parasitic drugs in 34 trials (e.g. azithromycin [n=28]), biologicals in 80 trials (e.g. convalescent plasma [n=27]), procedures in 28 trials (e.g. renal replacement therapy [n=4]), other non-drug interventions in 27 trials (e.g. physical activity). More information can be found in the Extended data12.
The first column represents the proportion of all trials that were of the specified type. The second column represents the proportion of all trials registered that week that were of the specified type (i.e. within week, between trial types). The third column represents the distribution of when trials of this type were registered (i.e. within trial type, between week), and can be interpreted as either a percentage or count (not specified). A trial might assess more than one intervention category and detail for prevention and treatment trials are given in the extended data12.
Overall, 78 trials (11.3%) focused on prevention (205,841 planned participants), mainly prophylactic drug use (n=41), vaccines (n=14; 9 already started recruitment; see Extended data)12 and non-pharmaceutical interventions (n=10) (e.g. masks or the use of media and influencers in people’s compliance to hygienic practices). Four trials (0.6%) assessed interventions both for prevention and for treatment. No trial planned to assess benefits or harms of implementing or de-implementing any social distancing or lockdown measures.
The number of trials increased rapidly; on average 0.5 trials per day were registered in January, 8.1 in February, 8.4 in March, and 17.6 in April 2020.
Trials were conducted in 42 countries and through international collaborations (Table 1; see Extended data)12. Half were from China (51.1% [n=352]), which dominated initially (Figure 2); starting March 2020, more trials came from other countries. Trial characteristics were similar across the five most frequent geographical locations (China, USA, France, Spain and international) contributing to 73.3% (n=505) of the global trial research (Table 1). Traditional medicine was assessed in 30.4% of trials from China (n=107) but rarely in other countries.
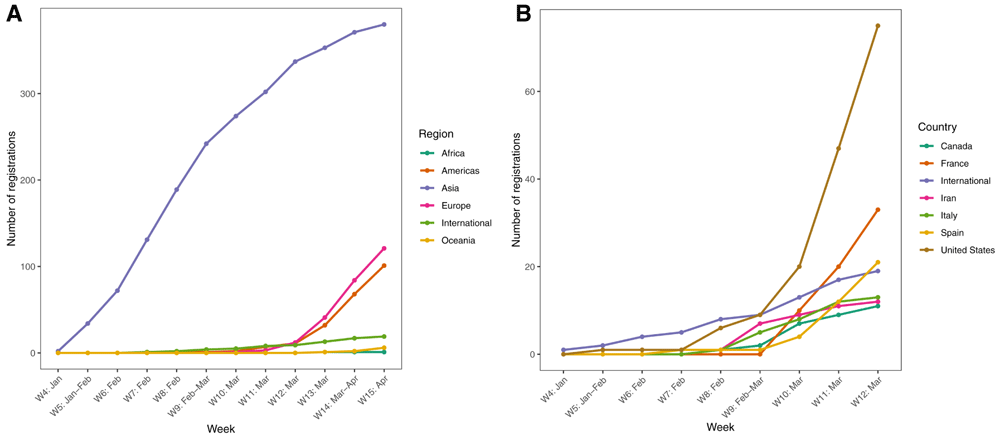
Cumulative number of registered trials over time (a) by continent, and (b) for countries with at least 10 registrations (excluding China). Four trials not shown were registered in 2019 or earlier, with a study design subsequently adapted to address COVID-19 (EUCTR2015-002340-14-NL; NCT03680274; NCT03331445; and NCT03808922). For 18 trials the registration date was unknown.
Larger trials were initiated later. In February, fewer than 8% of trials included more than 500 participants in contrast to 29.6% of trials in March (Figure 3). Later trials more often used blinding, placebo and mortality as primary outcome (Figure 3). Participations of healthcare workers and healthy people also started later. When the proportion of trials from China decreased, so did trials assessing traditional medicine (from 46.9% to 0.9%) while the proportion of trials assessing drugs rose (from 38.1% to 77.2%). Antivirals came under investigation earlier than antimalarials (Figure 1).
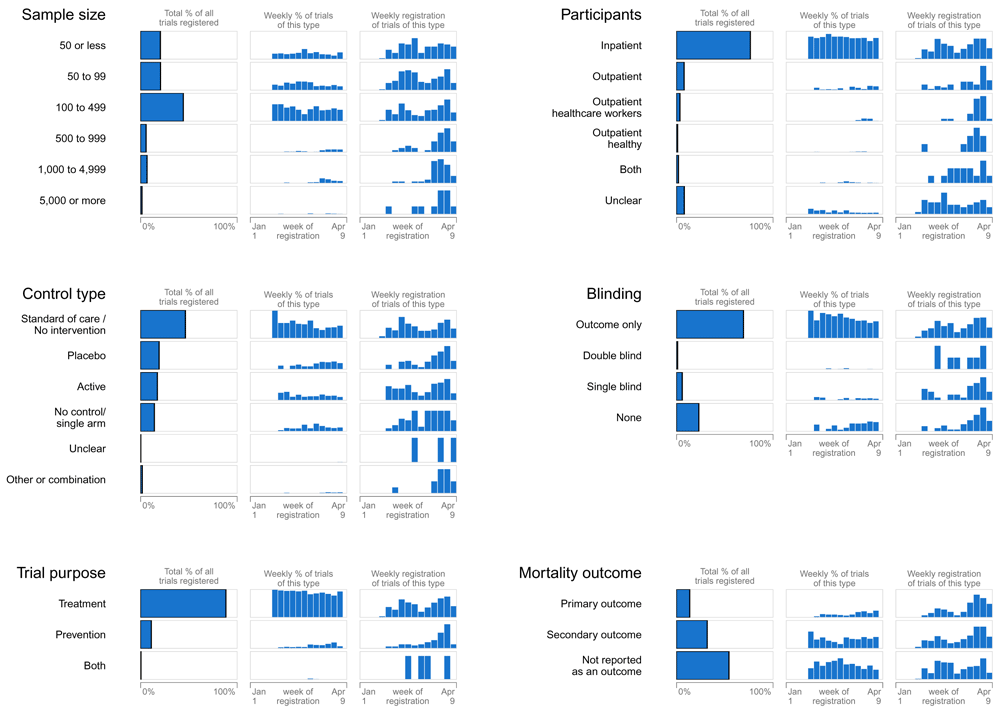
The first column represents the proportion of all trials that were of the specified type. The second column represents the proportion of all trials registered that week that were of the specified type (i.e. within week, between trial types). The third column represents the distribution of when trials of this type were registered (i.e. within trial type, between week), and can be interpreted as either a percentage or count (not specified).
Out of the 689 trials, 6.7% (n=46) planned to enroll 1,000 to 5,000 participants. Most were randomized (89.1% [n=41]), assessed drugs (80.4%; n=37), and many were not blinded (52.2% [n=24]). Five were cluster-randomized. The top three regions were the United States (21.7%; n=10), France (13% [n=6]) and international collaborations (10.9% [n=5]) (see Extended data)12.
Eleven (1.6%) trials, registered between February and April 2020, planned to enroll over 5,000 participants (see Extended data)12. There were 10 randomized (one cluster RCT), eight not blinded and five conducted in multiple countries. These trials tested drugs (n=9), masks (n=1) and traditional medicine (n=1). Three trials are described as platform trials (i.e. WHO Solidarity trial13, RECOVERY trial14 and CROWN-CORONATION trial15) and use an adaptive design.
Six drug interventions tested in these 11 larger trials (seven for treatment and four for prevention) were simultaneously investigated in at least 10 smaller trials (see Extended data)12. Overall, 169 trials (143 for treatment, 24 for prevention and two for treatment and prevention) with fewer than 5,000 participants assessed at least one intervention that was also assessed in a larger trial (median sample size 273 [IQR 90 to 700]; 134 had fewer than 1000 participants). For 107 of those (63.5%) the larger trial was registered before. For example, 106 trials with fewer than 5,000 participants tested hydroxychloroquine and 88 of them (83%) were registered after the first large trial testing this drug and 83 (77.6%) assessed hydroxychloroquine as treatment (Figure 4; see Extended data)12. These 106 trials had a median sample size of 334, but cumulatively, they planned to enroll as many patients as the four larger trials testing hydroxychloroquine (76,617 vs 77,000).
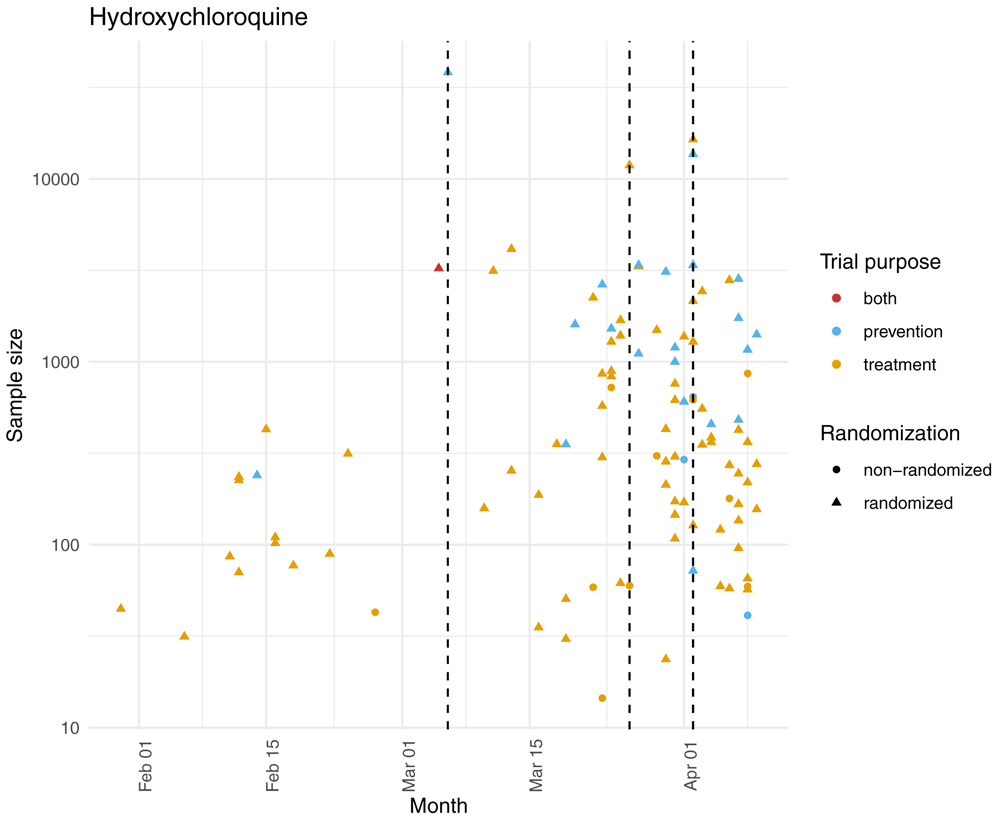
The dashed lines represent the registration of the four trials planning to enroll over 5,000 participants; two were registered on April 2, 2020.
Out of the four trials planning on enrolling over 5,000 participants, two assessed hydroxychloroquine for treatment and two for prevention. Out of the 106 smaller trials, 83 assessed hydroxychloroquine for treatment, 22 for prevention and one for both treatment and prevention
By the end of 2020, 414 trials (60.1%) with a total of 160,107 planned participants were expected to be completed (i.e. last patient, last visit), including 240 drug trials (97,846 participants) and 22 over-1,000 participants trials and five over-5,000 participants trials. For vaccines, five trials expected completion in 2020, five in 2021 and another four between 2022 and 2024 (see Extended data)12. However, of the 270 trials that planned to start recruiting by the end of February, 190 started (70.4%), but 80 had not (as of July 6, 2020) (see Extended data)12.
By July 6, 2020, we received enrolment information for 112 out of the 604 trials listed as planned or ongoing (18.7%). Of the 112 trials, 16 had not started recruiting although their start dates were overdue; one was discontinued. Among the 112 trials, 55 had recruited fewer than 20% of the target sample size, 27 between 20-50%, and 30 more than 50% (median recruitment 14.8% [IQR 2.0 to 62.0%]; median duration of recruitment 72 days [IQR 53.5 to 83 days])). Median recruitment was similar in treatment and prevention trials (15.9% (IQR 2 to 61.1%] vs 14.8% [IQR 4.3 to 62.5%]). For 19 trials, investigators mentioned difficulties in recruitment due to a fortunate decrease in the number of COVID-19 cases.
The global clinical research community has mounted a massive, unprecedented volume of research in response to the COVID-19 pandemic. Almost 700 trials within 100 days planned to include almost 400,000 participants globally. Many treatments were planned for investigation, mostly drugs, often antivirals, and sometimes substances that may seem rather unexpected for an infectious disease (e.g. colchicine or dipyridamole) reflecting the huge heterogeneity of disease manifestations and therapeutic targets16. Few trials focused on prevention, but some were very large and focused on healthcare workers (i.e. 24.4% of planned trial participants are healthcare workers). Trials from China dominated the research agenda before research activities followed the spread of COVID-19 throughout the world. Most trials were planned as randomized, clearly demonstrating that such designs are possible within a pandemic17 and within a very short time, unlike the 2014–2015 Ebola outbreak where only a few therapeutic trials had a randomized design, and none started within 100 days17.
The emergence of 689 trials in a 100-day period is unparalleled. Between 250 to 342 HIV/AIDS trials are registered per year on ClinicalTrials.gov18 and only three were registered for Middle East respiratory syndrome (MERS) coronavirus during 2007–201718. While efforts being put into clinical trials were initially welcome, the vast majority of COVID-19 trials are at risk of being abandoned if they cannot recruit enough patients or if other trials on the same treatment provide conclusive results (favorable or unfavorable).
Thomas Chalmers highlighted in 1977 the need to ‘Randomize the first patient’19, and a reassuring 75% of trials are indeed randomized. However, we identified areas of concern. Most trials are not blinded, and even if placebos may be not available in such short time, blinding of outcome collection would be preferable. Blinding may not be required for mortality outcomes; however, it was rarely a primary outcome. Half of the trials include fewer than 120 patients and many small trials were initiated after public registration of very large trials addressing similar questions. They may have some heterogeneity in design that might be desirable or focus on specific situations, for example early Phase 1 vaccine trials, but it seems unlikely that such small trials would add meaningfully to the overall evidence. The extensive worldwide discussions about limited evidence from small trials reflect the substantial uncertainty patients and decision-makers face about the merits of popular interventions, such as hydroxychloroquine20.
For hydroxychloroquine, over 100 smaller studies with over 76,000 patients were planned in the first 100 days to investigate this single therapeutic option out of many potential options. This case, possibly fueled by media attention relayed by decision-makers and politicians, highlights the urgent need for early evidence-based research and priority setting. Such proliferation may reflect best intentions of clinical researchers to actively contribute to evidence generation and inform timely treatments locally instead of awaiting published evidence or using experimental treatments outside of clinical trials. It may also indicate a lack of research structures allowing them to contribute to larger, synergistic trials. With the emergence of results, the entire agenda may shift. Many hydroxychloroquine and chloroquine trials’ enrolment was temporally halted due to harmful effects in an observational study21, the publication of which was subsequently retracted22. However, the release of the randomized RECOVERY trial results showing no benefit (in fact, a trend for increased mortality)23 with hydroxychloroquine and another “negative” trial on hydroxychloroquine-prophylaxis24 created uncertainties about the feasibility (i.e. inability to recruit planned sample sizes) or futility (i.e. inability to demonstrate treatment effects) of all the ongoing and planned hydroxychloroquine trials.
There are excellent examples of how efficient structures allow for rapid response to evidence needs, such as the UK RECOVERY trial25. Strongly endorsed and prioritized by authorities and medical representatives26, it is running as streamlined pragmatic platform trial in over 176 hospitals, randomizing over 12,000 patients in just over four months14. It has already provided evidence on the lack of benefit for hydroxychloroquine23 and lopinavir/ritonavir27, and a reduction of mortality with dexamethasone28 (still awaiting results for azithromycin, tocilizumab and convalescent plasma). Such key trials have had major impact on decision-makers such as the FDA revoking the Emergency Use Authorization of hydroxychloroquine29 on June 15.
Conversely, we found other large trials with major recruitment difficulties. The DisCoVeRy trial, for example, was designed as an adaptive trial of 3200 patients, running in 35 countries. However, while DisCoVeRy recruited 758 patients in France, only one was recruited in the rest of Europe30, as of June 17, 2020.
The lack of coordination in the research response created substantial research waste, exposed many patients to unnecessary risks, and harms medical progress by creating competition among trials investigating similarly promising therapeutic alternatives31–33. However, in absence of such desirable research synergies, all these scattered activities can and should be bundled to contribute to rapid evidence generation in living meta-analyses. The COVID-evidence database provides a unique opportunity to surveil the planned, ongoing and completed trials that can then be synthesized – it would only need systematic sharing of trial data.
As many countries are facing restrictions of movement and lifestyle at various severity levels, affecting the physical and mental health of billions of people, it is remarkable that not a single trial was initially planned to evaluate these measures. While the lack of controlled experiments evaluating their implementation may not be unexpected (given the initial urgency, ethical considerations, and organizational challenges), it would now be highly desirable that the de-implementation or re-implementation be subject to systematic evaluation in high-quality trials. The diverse options to ease or reinforce lockdown would be amenable to randomization, such as alternative time points or extents of re-opening schools or kindergartens, of ways to protect elderly in nursing homes, of home office programs, or of contact restrictions. Such evidence would be critical to inform future pandemics or the management of possible second waves of COVID-19, yet it was not on the initial agenda.
Several limitations merit attention. First, unclear reporting in registries might have introduced inaccurate results. Some ambiguously reported items required discussion among several reviewers but were resolved to the best of our ability. Second, we rarely identified protocols or manuscripts, precluding more detailed analyses of trial designs. Third, some control groups receiving “standard of care” interventions were not clearly described, likely some of these included interventions that were or are still under investigation in other trials. Fourth, we may have missed a few cases of duplicate entries across registries or of multiple national parts of an international trial, thus slightly overestimating the number of trials but not affecting the overall interpretation. Fifth, we arbitrarily selected a period of the first 100 days, which is traditionally used to benchmark early outcomes of policies or presidencies. Finally, we do not assess the actual research output from all these early trials. This unprecedentedly fast-moving research body is scattered across data sources and registries without uniform updates. More definitive answers will require more time, but our results allow for the diagnosis of “system cracks”34 that may become symptomatic in this pandemic, such as infrastructure limitations, and also identify best practices.
The incredible volume and speed of trial research observed in the first 100 days of the COVID-19 pandemic should not hide the fact that in its early days the global clinical trial research agenda lacked clear coordination, efficiency and exploitation of synergies. There are excellent examples of very large trials implemented with impressive efficiency, likely providing the clearest evidence. However, early coordination and a unified approach are needed - otherwise futility and waste of resources may be prominent features of such an ambitious research agenda.
All data underlying the results are available as part of the article and no additional source data are required.
Open Science Framework: COVID-evidence: a living database of trials on interventions for COVID-19 / The worldwide clinical trial research response to the COVID-19 pandemic - the first 100 days; https://doi.org/10.17605/OSF.IO/PJEM312.
- 2020-07-06-Dataset_manuscript.xlsx. (Raw trial metadata.)
- 2020-06-03_COVe_Procedures_Variables_manuscript.pdf. (Procedures for screening and extracting data.)
- 2020-09-08-Extended_data_Manuscript.docx. (Extended data Figures 1–5 and Tables 1–5.)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We thank Constantin Sluka (University of Basel) for his help in setting up the COVID-evidence database, Andreas Widmer (University Hospital Basel) for administrative support and Ninox Software GmbH, Berlin, Germany, for freely providing the database. We would also like to thank all the investigators we reached out to and took the time to respond to our inquiries.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Higgins J, Thomas J, et al.: Cochrane Handbook for Systematic Reviews of Interventions (Version 6.1). 2020. Reference SourceCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Research methods, health systems- and policy research, systematic review.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: I have written and broadcast about the failure to trial non drug interventions. My full DOI is at whopaysthisdoctor.org
Reviewer Expertise: I am a general practitioner with an interest in evidence based medicine.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 26 Oct 20 |
||
|
Version 1 02 Oct 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)