Keywords
trabeculectomy, mitomycin C, trabeculotomy, exact matching, open-angle glaucoma, iridectomy
This article is included in the Eye Health gateway.
trabeculectomy, mitomycin C, trabeculotomy, exact matching, open-angle glaucoma, iridectomy
We have implemented the changes requested by Reviewer 1 and added a power calculation, effect size calculation. We explain how we selected the eye for the study in patients with two surgical eyes.
See the authors' detailed response to the review by Marc Töteberg-Harms
Since the first description of a guarded filtering procedure in enucleated eyes by Grant in 19581, performance of this surgery in patients in 1961 by Sugar2, popularization of the term “trabeculectomy” in 1968 by Cairns3, and introduction of mitomycin-C as an antifibrotic to make it more effective4, trabeculectomy (TE) has remained a primary surgery in the treatment of glaucoma5. Over the years, multiple modifications of this surgery have been explored to improve its effectiveness, to make outcomes more predictable and to reduce postoperative complications and need for interventions. These modifications include, among others, variations in size, localization, and thickness of the scleral-flap, different suture techniques, variable intra- and postoperative treatment with antifibrotics or a combination of these approaches with one another6.
In this study, we combined elements of deep sclerectomy7,8 and trabeculotomy9 with TE in an attempt to improve conventional outflow as well as subconjunctival aqueous humor drainage. Encouraged by a pilot study of filtering trabeculotomy (FTO) with a complete success rate of 79%10, we hypothesized that FTO had a higher success rate and lower complication rate than TE. We applied advanced statistics, exact matching11,12, to enable a highly balanced comparison of our retrospective data with two-year follow-up.
This retrospective study was approved by the ethics committee of the University of Würzburg, Germany (#2019101601AS). Because of its retrospective nature, informed consent was waived.
All primary open angle glaucoma (POAG) patients at the University Eye Hospital Würzburg who underwent a modified FTO with mitomycin C (MMC) or TE with MMC by a single surgeon (FG) between 2007 and 2014 were analyzed. The indication for surgery was a failure to control intraocular (IOP) despite maximally tolerated medical therapy. Only one eye was included per patient. If two eyes had been operated on, the first eye was chosen to take advantage of a longer history.
Patients were matched by baseline IOP and the number of glaucoma medications.
From patient records, we obtained from 2019 to 2020 the medical history, the best-corrected visual acuity assessed (BCVA [logMAR]), IOP (Goldmann tonometry [mmHg]), topical glaucoma medications (including a glaucoma medication score (GMS)13), as well as postoperative events and complications. These included hypotony with choroidals, hypotony maculopathy, flat or shallow chamber, an IOP relatively high for surgical glaucoma (above 25 mmHg), bleb leakage, a persistently flat bleb indicating absent flow, hyphema, iris incarceration and need for cataract surgery. We computed the complete and qualified success, according to the guidelines set forth by the World Glaucoma Association14. In patients who had either TE or FTO in both eyes, the first eye was chosen to be included in the study. Complete success was defined as a postoperative IOP of ≤18 mmHg with a reduction of ≥30% from baseline without glaucoma medications. Qualified success was a postoperative IOP of ≤18 mmHg with a reduction of ≥30% from the baseline, achieved with or without glaucoma medications14. Follow-up visits occurred 7 days, 1 month, 3 months, 6 months, 12 months, and 24 months after surgery.
The exclusion criteria consisted of patients aged below 18 years, glaucoma types other than open-angle glaucomas, and a history of TE, trabeculotomy or glaucoma drainage device implantation.
The eye was rotated downward with a traction suture. A 5 mm fornix-based peritomy was made at the anatomic 12 o’clock position, and a sub-tenon pocket was fashioned to accommodate a sponge soaked with MMC at a concentration of 0.2 mg/ml and 100 µl volume for 3 minutes.
In TE, a 3 mm × 4 mm half scleral thickness flap was created. A 0.8 mm × 2 mm sclerotrabecular block was excised to enter the anterior chamber, as described before10. A peripheral iridectomy was made. The scleral flap was secured with 10.0 nylon to allow visible percolation of aqueous, and the conjunctiva was closed with an interlocking running suture resulting in a diffusely forming bleb15.
In FTO, the scleral flap was sized 4 mm × 4 mm. A smaller, tongue-shaped flap was dissected underneath to unroof and create access ostia to Schlemm’s canal. This second flap was excised similar to deep sclerectomy. The canal was probed with a metal trabeculotomy probe (Mackensen, Geuder Inc., Heidelberg) on both sides while the trabeculo-descemet window at the base of the scleral flap was preserved so that no bulk aqueous outflow could occur and no iridectomy was needed. The remaining steps were identical to those in TE.
Postoperative drops consisted of dexamethasone six times for the first week, which was tapered by one drop per week. Ciprofloxacin eye drops were applied four times a day for one week. All patients received 5 mg of 5-FU (0.1 cc volume) daily for a week unless IOP was below 5 mmHg or a Seidel-positive bleb leak or a corneal erosion was present. 5-FU was also given once a week for the first month during return visits using the same criteria.
Statistical analyses were performed using Statistica 13.1 (StatSoft, Tulsa, Oklahoma, United States) and MedCalc (MedCalc 19.1.3, Ostend, Belgium). A total of 88 patients (1:1, FTO:TE) were matched with exact matching11,12 based on the baseline IOP and glaucoma medications.
Categorical variables were described as the frequency with percentage, whereas continuous and discrete variables as mean with standard deviation (SD) or median with range. The chi-squared test or Fisher’s exact test was used for the analysis of categorical variables. Continuous variables were compared using Student’s t-test or Mann–Whitney U test, whereas discrete variables were compared using Mann-Whitney U test. The distribution of continuous variables was determined by the Shapiro-Wilk test, equality of variances by Levene’s test. Assessment of repeated measures for IOP was performed using repeated measures MANOVA and Tukey’s test, while visual acuity (logMAR) and medications were examined using the Friedman test and Wilcoxon signed-rank test. The success of treatment was expressed by a Kaplan-Meier curve and compared between treated groups using the log-rank test. The success of treatment at particular a given time point was determined by odds ratio (OR) with respective 95% confidence intervals and p-values. P-values below 0.05 were considered statistically significant.
Our power calculation before commencing the study indicated that we would need 39 eyes per group to detect a difference of at least 20% at a power of 80% and alpha of 0.05 (continuous endpoints, two independent sample study). We then performed a post-hoc analysis for IOP at year 1 and 2 and determined the effect size using Cohen’s d (d (.01) = very small, d (.2) = small, d (.5) = medium, d (.8) = large, d (1.2) = very large, and d (2.0) = huge.
A total of 196 patients were included. The unmatched demographic data of FTO and TE had significant differences in preoperative IOP (p=0.017), glaucoma medication score (p=0.001), and pseudophakia (p=0.012). In total 88, eyes (44 in each group) could be matched as exact pairs eliminating key differences in IOP and medications. The IOP at baseline was 22.6±4.7 mmHg in FTO and 22.6±4.7 in TE while on 3.0±0.9 medications in both (Table 1). There were no significant differences between FTO and TE in gender, age, best-corrected visual acuity, type of glaucoma, or surgical side. In FTO, 13 eyes were pseudophakic compared to 3 in TE (p=0.006). Two patients in FTO had a pars plana vitrectomy and one a retinal cryopexy. In TE, there were no prior ocular surgeries other than phacoemulsification.
FTO: filtering trabeculotomy; TE: trabeculectomy; BCVA: best-corrected visual acuity assessed; IOP: intraocular pressure; POAG: primary open angle glaucoma; PXG: pseudoexfoliation glaucoma; PG: pigmentary glaucoma; ALT: argon laser trabeculoplasty; SLT: selective laser trabeculoplasty; CPC: cyclophotocoagulatoin; nd:YAG: neodymium-doped yttrium aluminum garnet
There were no statistically significant inter-group differences in complete or qualified success at any time (p=0.403 for complete success; p=0.204 for qualified success at 24 months; Figure 1 and Figure 2). The complete success rate in FTO ranged from 79% at 6 to 78% at 12 months, and 59% at 24 months. In TE, it was 81%, 85%, and 66%, respectively. Similarly, IOPs of FTO and TE were not significantly different at any time (12 months: p=0.983, 24 months: p=1.000, Figure 3). At one year, the IOP had declined to 12.4±4.3 mmHg in FTO and 11.3±2.2 mmHg in TE with medications (qualified success). At two years, IOP remained at 13.1±4.1 mmHg in FTO and 12.0±3.5 mmHg in TE (qualified success), respectively. The posthoc analysis showed that we could have detected an IOP difference of 16.1% at one year and an IOP difference of 17.6% at two years with a power above 80%. Cohen’s d was 0.32 at one year and 0.29 at two years, respectively, in both cases classifying as small. The postoperative visual acuity was not significantly different in FTO and TE at any time (p=0.894 after 12 months; p=0.443 after 24 months; Figure 4 and Figure 5).
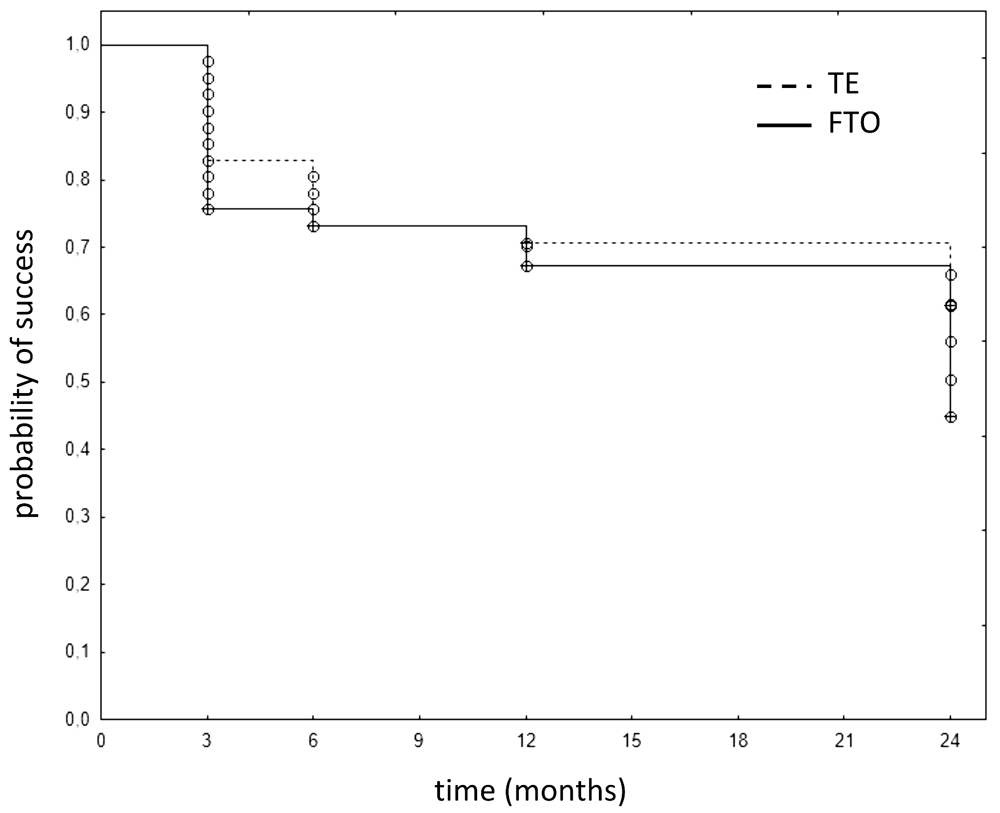
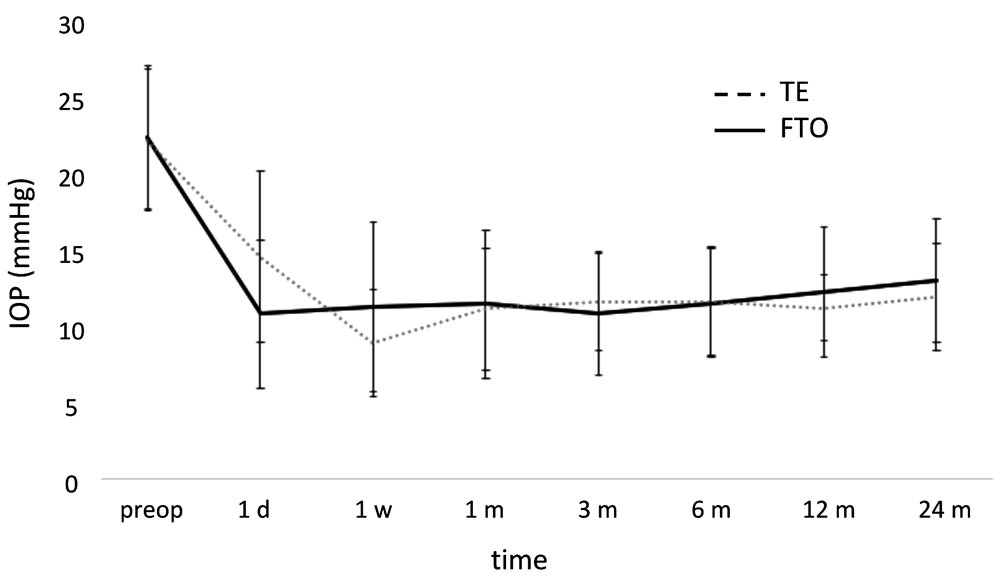

FTO: filtering trabeculotomy; TE: trabeculectomy.
There was a reduction in glaucoma medication from 3±0.9 to 0.6±1.3 in FTO and from 3±0.9 to 0.2±0.5 in TE after 24 months. There was no significant difference between the two groups in glaucoma medications at 24 months with 19% of patients in FTO and 12% of patients in TE using glaucoma drops (p=0.471, Figure 6 and Figure 7).
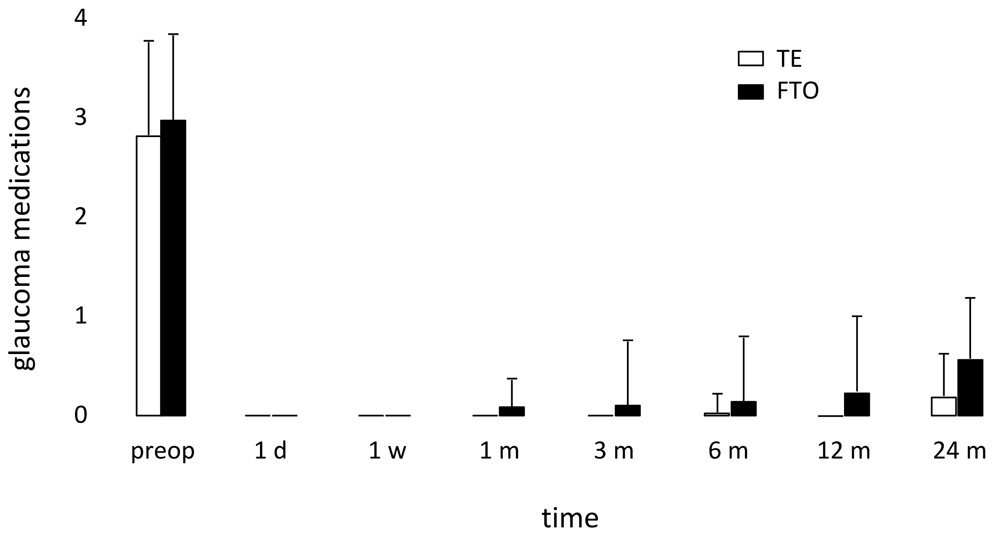
FTO: filtering trabeculotomy; TE: trabeculectomy.
Postoperative complications in FTO included 5 eyes (12%) with a high IOP, hyphema in 5 cases (11%) and hypotony (IOP ≤ 5 mmHg16) with choroidals in 4 eyes (9%). The most common postoperative challenge in TE was a flat bleb in 6 eyes (14%), hyphema in 3 eyes (7%), high IOP in 2 eyes (5%), bleb leakage in 2 eyes (5%), and hypotony in 1 eye (2%). A total of 16, mostly reversible, complications occurred in each group (37%; Table 2).
The number of postoperative interventions was the same in the two groups (p=0.087). Bleb needling, conjunctival suture, and scleral flap suture were the most common interventions. There was no statistically significant difference between both groups in early or late interventions (Table 3), except 5-FU and laser suture lysis, which was performed more often in FTO.
| Early postoperative interventions | FTO (n, (%)) | TE (n, (%)) | P-Value |
|---|---|---|---|
| No. of interventions 0 1 2 3 4 | 26 (59) 15 (34) 2 (5) 1 (2) 0 (0) | 35 (80) 8 (18) 0 (0) 0 (0) 1 (2) | 0.087 |
| 5-FU (mean±SD) | 2.1±2.4 | 0.8±0.9 | 0.023 |
| Bleb needling | 11 (25) | 6 (14) | 0.280 |
| Conjunctival suture | 4 (9) | 3 (7) | 1.000 |
| Scleral flap revision (high IOP) | 2 (5) | 0 (0) | 0.494 |
| Scleral flap suture (hypotony) | 2 (5) | 2 (5) | 1.000 |
| nd:YAG laser goniopuncture | 1 (2) | 0 (0) | 1.000 |
| Cyclodestruction | 1 (2) | 0 (0) | 1.000 |
| Iris repositioning | 1 (2) | 1 (2) | 1.000 |
| Laser suture lysis (average±SD) | 1.5±1.5 | 0.8±0.9 | 0.026 |
| Late postoperative interventions | |||
| No. of interventions 0 1 2 | 35 (80) 6 (14) 3 (7) | 42 (95) 2 (5) 0 (0) | 0.147 |
| Bleb needling | 1 (2) | 2 (5) | 1.000 |
| Conjunctival suture | 3 (7) | 0 (0) | 0.241 |
| Scleral flap revision (high IOP) | 3 (7) | 0 (0) | 0.241 |
| Scleral flap suture (hypotony) | 0 (0) | 0 (0) | ** |
| nd:YAG laser goniopuncture | 4 (9) | 0 (0) | 0.116 |
| Cyclodestruction | 1 (2) | 0 (0) | 1.000 |
| Re-TE | 0 (0) | 0 (0) | ** |
TE with MMC remains a primary surgery in the management of advanced glaucoma5 despite its potential for serious complications that include choroidal effusions, maculopathy, blebitis, endophthalmitis, and suprachoroidal hemorrhage17. Numerous modifications have been explored over the years to reduce the rate of these. They include a smaller scleral flap18, limbus-versus fornix-based conjunctival closure19, releasable flap sutures20,21, a combination of trabeculectomy with deep sclerectomy22, different concentrations and exposure times of MMC23 or sutureless tunnel trabeculectomy without iridectomy24. The iridectomy, which is part of traditional trabeculectomy with a trabecular block excision, can cause hyphema, inflammation, posterior synechiae, iridodialysis, and cataracts25,26. FTO addresses some of these issues by creating a more spread-out intake of aqueous humor, thereby reducing iris aspiration and avoiding the need for an iridectomy. The trabeculotomy and sclerectomy8,27,28, that are part of the FTO, were meant to remove some of the post-trabecular outflow resistance29–31.
We used matching, a nonparametric method of controlling the confounding influence of pretreatment variables in observational data32. Before matching, FTO and TE had significant differences. We have previously used coarsened exact matching, propensity score matching, and, more recently, exact matching. Coarsened exact matching applies multiple imputations to fill in missing data to not distort any relationships contained in the data while enabling the inclusion of all observed data from moderately uneven groups. Such was the case when we compared patients with a primary IOP indication to patients with a mixed indication for both cataract removal and IOP reduction33–35. Propensity score matching is helpful to compare even more divergent groups, for instance, patients undergoing tube shunt surgery with patients undergoing trabectome surgery36,37. By contrast, exact matching is well suited to compare similar pathological conditions and similar treatments, for instance, phaco-iStent or phaco-trabectome38,39. A downside of exact matching is that a certain number of datasets must be excluded from the analysis because the algorithm accepts only identical primary criteria matches. Overall, our data loss was acceptable because of the high similarity that already existed at baseline. We were able to retain a large number of eyes (about 45%) as identical pairs of preoperative IOP and medication to focus on the preeminent questions of success in IOP and medication reduction. We found that FTO was as successful as TE with a similar reduction of IOP and medications. Both had a similar intervention and complication rate, notwithstanding numerical hypotony within the first six weeks after surgery. We observed a remarkably low rate of hyphema compared to ab interno trabeculectomy40 that occurs when the IOP is at or below episcleral venous pressure allowing blood to reflux into the anterior chamber. This could indicate reduced patency of collector channels in advanced glaucoma that qualifies for filtering surgery, which has been observed ex vivo41.
Trabeculotomy ab externo has been applied to adult POAG before but, compared to TE, was noted to have a lower success rate of 70% at one year, presumably due to a reapproximation or regeneration of the disrupted trabecular meshwork42. A study by Chihara et al. was in agreement with this, finding that a modified trabeculotomy ab externo lowers IOP to an average near 16 mmHg in a safe fashion43, by not as much as TEs. Ogawa et al. compared a nonpenetrating trabeculectomy with or without trabeculotomy44 using a technique that was very similar to the one applied in our study, except without MMC. Despite the absence of this antifibrotic, the authors achieved a two-year IOP of 13 mmHg, not unlike our patients.
Our two-year TE results match Kirwan et al.’s multicenter study of TE with an iridectomy in 428 eyes well45. These eyes achieved an IOP of 12.4±4 mmHg and 80% did not have to use glaucoma medications anymore, slightly more than in our study population. The authors performed needling in 17% of patients, not unlike our rates, and numerical hypotony during the first six months occurred in 7.2% of patients, which is in a similar range as our 9% in FTO and 2% in TE. Leakage was observed in 14% compared to 9% in our FTO and 2% in our TE. However, Kirwan et al.’s cataract surgery rate was 31%, much higher than our 1% in both FTO and TE, which might represent a difference in practice pattern or simply easier access to this elective procedure, which is done as an outpatient surgery in the UK.
It is interesting to note that TEs in the Tube Versus Trabeculectomy (TVT) study were performed without an iridectomy46. In that study, IOP reduced from 25.6±5.3 mm Hg to 12.7±5.8 mm Hg at one year while the number of glaucoma medications declined from 3.0±1.2 to 0.5±0.947, which is also relatively similar to our results although these investigators included eyes with prior intraocular surgery, including glaucoma procedures. An early complication rate of 37% was observed by these authors, primarily consisting of a shallow or flat anterior chamber in 20% and choroidal effusions in 10%6. In another study of TE without an iridectomy by Jea et al., IOP reduced from 26.3±10.9 mmHg to 10.2±4.1 mmHg at two years48. The number of glaucoma medications decreased from 2.2±1.6 to 0.5±1.0. A complete success occurred in 76.6% at one year and 66.2% at two years, matching ours.
In all of these studies, TE was performed as an outpatient patient procedure, a practice that emerged in the late 1980s49,50 and became a standard for most types of eye surgeries and countries51–54. Even before the now-common use of antifibrotics, there was no significant difference in success or complication rates between inpatients and outpatients50, an observation that has been confirmed with antifibrotics as well53. In the country of our study, TE and FTO are only reimbursable as inpatient procedures. It has been argued that meticulous micromanagement after TE for several days may be associated with better long-term outcomes of TE. However, this hypothesis is challenged by the present study and by the findings of others49,50,53,55. One could argue that the considerably lower cataract surgery rate in our data compared to Kirwan et al.45 might indicate a higher threshold for cataract surgery. These would have typically also been done as inpatient procedures to better handle post-cataract surgery bleb care.
Our study was limited by its retrospective nature and nonrandomized design. In patients with surgery in both eyes, we had selected the first eye undergoing glaucoma surgery for our analysis to take advantage of a longer history with more data. This selection might have favored the eye with more severe glaucoma. However, as this occurred in both groups, a similar bias will have occurred. Certainly, randomized controlled trials are a more sophisticated tool to reduce bias when trying to detect differences. However, given that exact matching already allows for a highly balanced comparison of retrospective data, such an effort might be difficult to justify. The indication for postoperative interventions and length of hospitalization was at the discretion of the treating physicians and was not standardized for both groups. Despite reducing confounding through the exact matching of IOP and medications, other confounding factors might have contributed to small differences in early postoperative patient management, as reflected by the fact that FTO patients received more 5-FU injections. Although these patients had a higher rate of numerical hypotony they were hospitalized for a slightly shorter time and experienced results that were not significantly different.
In conclusion, our results are largely in line with other FTO and TE outpatient studies. Combining elements from both yields reasonable two-year rates of surgical success, postoperative complications, and interventions while avoiding an iridectomy.
Open Science Framework: Retrospective evaluation of 2-year results with a filtering trabeculotomy in comparison to conventional trabeculectomy by exact matching, https://doi.org/10.17605/OSF.IO/KDYF356.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
A previous version of this article is available on medRxiv: https://doi.org/10.1101/2020.01.17.20017913.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Glaucoma
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Glaucoma Clinical Trials, Glaucoma Surgery Outcomes, Glaucoma Imaging, Glaucoma/Cataract Surgery
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Glaucoma, cataract.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 04 Jun 21 |
|||
|
Version 1 15 Oct 20 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)