Keywords
alpha Gal, immune response, antibody, allergy, tick, coronavirus, COVID-19, Guillain-Barré syndrome, alpha-Gal syndrome
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Coronavirus (COVID-19) collection.
alpha Gal, immune response, antibody, allergy, tick, coronavirus, COVID-19, Guillain-Barré syndrome, alpha-Gal syndrome
The paper was revised in response to reviewer comments by (a) adding information on BSA coated with α-Gal, (b) updating references, (c) adding new information to dataset validation, and (d) including the statement "For the validation of the ELISA with ImmunoCAP Phadia 250 automated platform (Thermo Fisher Scientific, Uppsala, Sweden) with the commercial ImmunoCap α-Gal bovine Thyroglobulin kit according to the manufacturer’s instructions please refer to Pacheco et al. (2021)"
See the authors' detailed response to the review by Jacques Le Pendu
See the authors' detailed response to the review by Tilo Biedermann
The gene coding for α-1,3-galactosyltransferase (α1,3GT) was inactivated in old-world monkeys, an evolutionary adaptation that resulted in the production of high antibody titers against glycan Galα1-3Galβ1-(3)4GlcNAc-R (α-Gal) (Galili, 2015). Previous results showed that up to 1–5% of the circulating IgM/IgG found in healthy individuals are directed against α-Gal (Macher & Galili, 2008). Bacteria in the human gut microbiome express α1,3GT genes to produce α-Gal epitopes (Montassier et al., 2020), suggesting that natural anti-α-Gal antibodies are produced in response to gut microbiota (Bello-Gil et al., 2019; Galili et al., 1988; Mañez et al., 2001; Yilmaz et al., 2014). This evolutionary adaptation has been associated with the protective response of anti-α-Gal IgM/IgG antibodies against pathogens containing this modification on membrane proteins (Galili, 2019; Hodžić et al., 2020a). In contrast, the presence of α-Gal in tick salivary glycoproteins and glycolipids (Araujo et al., 2016; Cabezas-Cruz et al., 2018; Chinuki et al., 2016; Crispell et al., 2019) and tick cement (Villar et al., 2020) induces anti-α-Gal IgE antibodies that mediate delayed anaphylaxis to mammalian meat consumption and immediate anaphylaxis to tick bites, xenotransplantation and certain drugs such as cetuximab (Cabezas-Cruz et al., 2019; Commins et al., 2009; Contreras et al., 2020; de la Fuente et al., 2019a; de la Fuente et al., 2020; Fischer et al., 2016; Levin et al., 2019; Mateos-Hernández et al., 2017; Platts-Mills et al., 2020; Steinke et al., 2015; van Nunen et al., 2007).
Factors that may affect the antibody response to α-Gal include but are not limited to age, repeat consumption of certain food and meats of different origin or innards with higher α-Gal content, exposure to tick bites, ABO blood group, co-occurring disorders and exposure to cats and other pets (Cabezas-Cruz et al., 2017; Cabezas-Cruz et al., 2019; Commins, 2016; Commins et al., 2014; de la Fuente et al., 2020a; Fischer et al., 2014; Fischer et al., 2016; Morisset et al., 2012; Platts-Mills et al., 2020; Wölbing et al., 2013). Additionally, the anti-α-Gal-specific IgE response has been associated with other diseases such as atopy, coronary artery disease and atherosclerosis (Gonzalez-Quintela et al., 2014; Wilson et al., 2017; Wilson et al., 2019). Furthermore, α-Gal-mediated innate and adaptive immune response mechanisms have been associated with protection against pathogen infection in various animal models (Hodžić et al., 2020a). However, little is known about the influence of anti-α-Gal immune response on immune-mediated disorders such as those occurring in patients with COVID-19 and Guillain-Barré syndrome (GBS).
These results raise questions and hypothesis regarding the role of α-Gal-mediated immune responses in disease symptomatology and possible protective mechanisms (de la Fuente et al., 2019b; de la Fuente et al., 2020b; Pacheco et al., 2021; Urra et al., 2021). Consequently, to advance in addressing these questions and hypothesis, here we provide data on the IgE/IgM/IgG/IgA anti-α-Gal antibody response in healthy individuals and patients diagnosed with AGS, tick-borne allergies, GBS and COVID-19. These data contribute to correlative analyses of the anti-α-Gal antibody response with factors such as patient and clinical characteristics, record of tick bites, blood group, age and sex. These analyses could provide insights into the role of anti-α-Gal antibody response in disease symptomatology and protection against immune-mediated disorders.
Essential methods used for the generation of the dataset (de la Fuente et al., 2020) were described in Urra et al. (2021) with additional information in Pacheco et al. (2021) and Doncel-Pérez et al. (2020).
A retrospective case-control study was conducted in patients suffering from COVID-19 admitted to the University General Hospital of Ciudad Real (HGUCR), Spain from March 1 to April 15, 2020. The infection by SARS-CoV-2 was confirmed in all patients included in the study by the real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay from Abbott Laboratories (Abbott RealTime SARS-COV-2 assay, Abbott Park, Illinois, USA) from upper respiratory tract samples after hospital admission. Clinical features, as well as laboratory determinations were obtained from patient's medical records. The patients were grouped as hospital discharge, hospitalized and intensive care unit (Urra et al., 2021). Patients were hospitalized for developing a moderate-severe clinical condition with radiologically demonstrated pneumonia and failure in blood oxygen saturation. Patients with acute respiratory failure who needed mechanical ventilation support were admitted to a hospital ICU. The patients were discharged from the hospital due to the clinical and radiological improvement of pneumonia caused by the SARS-CoV-2, along with the normalization of analytical parameters indicative of inflammation, such as C-reactive protein (CRP), D-Dimer and blood cell count (Urra et al., 2021). Samples from asymptomatic COVID-19 cases with positive anti-SARS-CoV-2 IgG antibody titers but negative by RT-PCR were collected in May 22–29, 2020 and included in the dataset (Urra et al., 2021). Samples from healthy individuals (individuals without record of tick bites and allergic reactions) and patients diagnosed with tick-borne allergic reactions (AGS, anaphylaxis or urticaria) were collected prior to COVID-19 pandemic in April 2019 (Pacheco et al., 2021). The use of human peripheral blood serum samples from healthy individuals and patients diagnosed with tick-borne allergic reactions was done with their written informed consent in compliance with the Helsinki Declaration. Nursing personnel at the General University Hospital of Ciudad Real, Spain, extracted blood samples. Samples and data from patients with GBS included in this dataset were provided by the BioB-HVS, integrated into the Spanish National Biobanks Network. All samples were processed following standard operating procedures with the appropriate approval of the Ethical and Scientific Committees (Toledo Hospitable Complex 29012014-No17, University Hospital of Ciudad Real C-352 and SESCAM C-73).
For the preparation of serum samples, a sterile tube without anticoagulant was used to collect blood samples. The blood from each patient and the healthy individual was maintained in standing position at room temperature (RT) for clotting (20–30 min) and centrifuged at 1,500 × g for 20 min at RT. Serum was collected and conserved at -20°C until used for analysis.
For ELISA, high absorption capacity polystyrene microtiter plates were coated with 50 ng of BSA coated with α-Gal (Galα1-3Gal-BSA, 3 atom spacer, product code NGP0203, thereafter named α-Gal; Dextra, Shinfield, UK) per well in carbonate-bicarbonate buffer (Sigma-Aldrich, St. Louis, MO, USA). After an overnight incubation at 4°C, coated plates were washed one time with 100 µl/well PBS with 0.05% Tween 20 (PBST) (Sigma-Aldrich), blocked with 100 µl/well of 1% human serum albumin (HAS) in PBST (Sigma-Aldrich) for 1 h at RT and then washed four times with 100 µl/well of PBST. Human serum samples were diluted 1:100 in PBST with 1% HAS and 100 µl/well were added into the wells of the antigen-coated plates and incubated for 1 h at 37°C. Plates were washed four times with PBST and 100 µl/well of goat anti-human immunoglobulins-peroxidase IgG (FC specific) (Cat. No. I2136), IgM (µ-chain specific) (Cat. No. I1636), and IgE (ɛ-chain specific) (Cat. No. I6284) secondary antibodies (Sigma-Aldrich) diluted 1:1000, v/v in blocking solution were added and incubated for 1 h at RT. Plates were washed four times with 100 µl/well of PBST and 100 µl/well of 3,3,´5,5-tetramethylbenzidine TMB (Promega, Madison, WI, USA) were added and incubated for 20 min at RT. Finally, the reaction was stopped with 50 µl/well of 2 N H2SO4 and the O.D. was measured in a spectrophotometer at 450 nm. The average of two technical replicates per sample was used for analysis after background (coated wells incubated with PBS and secondary antibodies) subtraction.
Anti-α-Gal IgE, IgM and IgG antibody titers (O.D. at 450 nm values) were compared for each Ig by one-way ANOVA test (p < 0.05) (https://www.socscistatistics.com/tests/anova/default2.aspx) (Figure 1A and 1C). A Spearman Rho correlation analysis (p < 0.01; https://www.socscistatistics.com/tests/spearman/default2.aspx) was conducted between anti-α-Gal IgE, IgM and IgG antibody titers and age (Figure 1B).
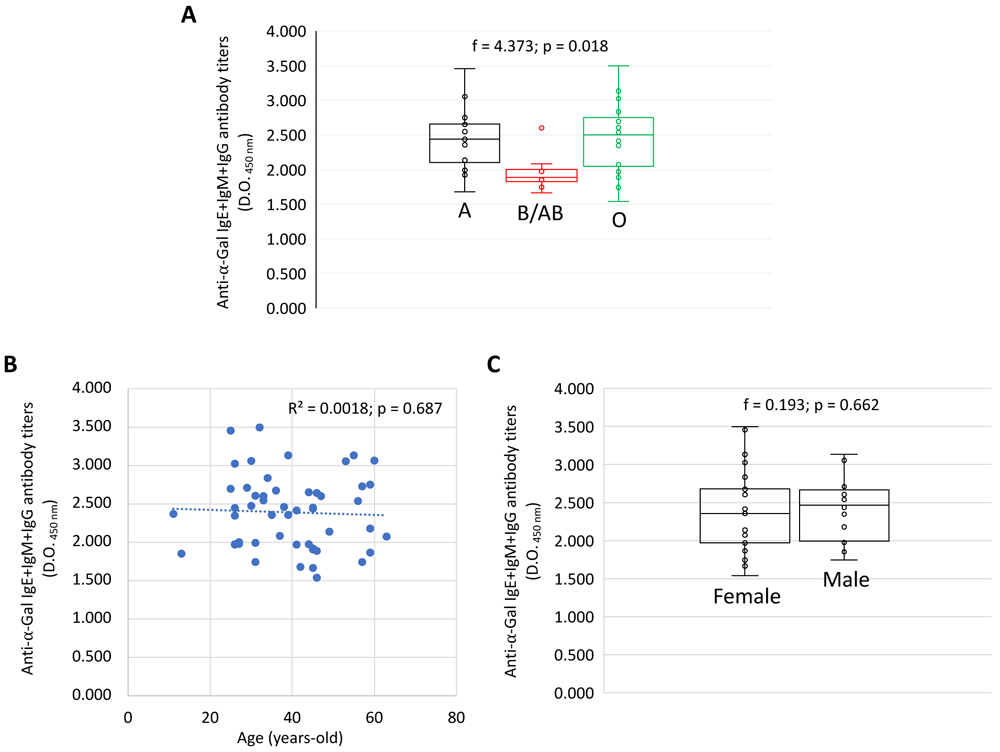
An example of the effect of certain factors such as (A) blood group, (B) age and (C) sex on the antibody response to α-Gal in healthy individuals. Anti-α-Gal IgE, IgM and IgG antibody titers were determined by ELISA. (A, C) The ELISA O.D. at 450 nm values were compared for each Ig by one-way ANOVA test (p < 0.05). (B) A Spearman Rho correlation analysis (p < 0.01) was conducted between anti-α-Gal IgE, IgM and IgG antibody titers and age. Correlation coefficient (R2) is shown. Please refer to Pacheco et al. (2021) for validation of the ELISA with ImmunoCAP Phadia 250 automated platform (Thermo Fisher Scientific, Uppsala, Sweden) with the commercial ImmunoCap α-Gal bovine Thyroglobulin kit according to the manufacturer’s instructions.
The dataset (de la Fuente et al., 2020) was validated in studies reported by Urra et al. (2021), Pacheco et al. (2020) and Doncel-Pérez et al. (2020). A recent study correlated blood group with anti-α-Gal antibody response and SARS-CoV-2 infection (Hodžić et al., 2020b). Despite the presence of relatively high anti-α-Gal IgE levels in healthy individuals, factors such as tick bites or allergy correlate with higher IgE antibody titers against α-Gal (Pacheco et al., 2021). Additionally, a comparative analysis was conducted between the IgE+IgM+IgG antibody response to α-Gal and blood groups (Figure 1A), age (Figure 1B) and sex (Figure 1C) in healthy individuals (n = 75) to illustrate lower antibody titers in blood group B/AB individuals as previously reported (Cabezas-Cruz et al., 2017) but no differences regarding age and sex, which have been reported before as factors affecting the antibody response to α-Gal, infection and vaccination (Buonomano et al., 1999; Giefing-Kröll et al., 2015; Wang et al., 1995).
The main limitation of the dataset is sample size for some factors (i.e. age, sex or blood group), which were not disclosed by all individuals, and anti-α-Gal IgA antibody titers that could be considered in the analysis (Mateos-Hernández et al., 2020; Urra et al., 2021).
Harvard Dataverse: A dataset for the analysis of antibody response to glycan alpha-Gal in individuals with immune-mediated disorders. https://doi.org/10.7910/DVN/RBU2VR (de la Fuente et al., 2020).
This dataset contains characteristics and serum antibody levels of the individuals included in the study and was used in analyses reported in publications by Urra et al. (2021), Pacheco et al. (2021) and Doncel-Pérez et al. (2020).
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
We want to particularly acknowledge the patients, healthy volunteers and the Guillain-Barré Syndrome Collection (TOSGB) from BioB-HVS integrated into the Spanish National Biobanks Network for their collaboration in this study. We thank members of our laboratories for fruitful discussions and Almudena González García (IREC, Spain) for technical assistance. We acknowledge UCLM, Spain support to Group SaBio.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for creating the dataset(s) clearly described?
Yes
Are the protocols appropriate and is the work technically sound?
Yes
Are sufficient details of methods and materials provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: immuoparasitology, Molecular biology
References
1. Pacheco I, Fernández de Mera IG, Feo Brito F, Gómez Torrijos E, et al.: Characterization of the anti-α-Gal antibody profile in association with Guillain-Barré syndrome, implications for tick-related allergic reactions.Ticks Tick Borne Dis. 12 (3): 101651 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Glycobiology, Host-pathogens interactions, histo-blood group antigens
Is the rationale for creating the dataset(s) clearly described?
Yes
Are the protocols appropriate and is the work technically sound?
No
Are sufficient details of methods and materials provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Partly
References
1. Urra JM, Ferreras-Colino E, Contreras M, Cabrera CM, et al.: The antibody response to the glycan α-Gal correlates with COVID-19 disease symptoms.J Med Virol. 93 (4): 2065-2075 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology, Allergy, Dermatology.
Is the rationale for creating the dataset(s) clearly described?
Yes
Are the protocols appropriate and is the work technically sound?
Yes
Are sufficient details of methods and materials provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Glycobiology, Host-pathogens interactions, histo-blood group antigens
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 25 May 21 |
read | read | read |
|
Version 1 24 Nov 20 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)