Keywords
urothelial carcinoma, sarcoma, chondrosarcoma, immune checkpoint inhibitor, atezolizumab
This article is included in the Oncology gateway.
urothelial carcinoma, sarcoma, chondrosarcoma, immune checkpoint inhibitor, atezolizumab
We followed the recommended remark of editors and reviewers that a new reference was added and some sentences and phrases were deleted better to understand the case report.
There exist several papers elucidating the importance of genetic profiling in sarcomatoid urothelial carcinoma. One article from Cell Press (Cell Rep. 2019 May 7;27(6):1781-1793.e4. doi: 10.1016/j.celrep.2019.04.048.) introduced Bogdan Czerniak and his research team research about a comprehensive genomic analysis of 28 cases of SARC and 84 cases of conventional urothelial carcinoma (UC), with the TCGA cohort of 408 muscle-invasive bladder cancers serving as the reference. They found out that sarcomtoid urothelial carcinoma showing a distinct mutational landscape, with enrichment of TP53, RB1, and PIK3CA mutations which might give important implications for the development of more effective therapies such as immune check-point inhibitors. We added this reference and their research articles whether it is possible that the genetic profiling might be an important implications for future effective therpies using immune therapy in sarcomatoid urothelial carcinoma. We added some of the sentences to show the genetic profiling research in sarcomatoid urothelial carcinoma in the manuscript.
We also added a new reference (#12) as follows in the reference section.
See the authors' detailed response to the review by Katsuhiro Ito
Ureteral cancer is rare, with a prevalence of less than 10% of all urinary tract urothelial carcinomas. Most (>90%) urothelial carcinomas occurring in the ureter are transitional cell carcinomas, while the others (<10%) include sarcomatoid urothelial carcinoma, squamous cell carcinoma, adenocarcinoma, and small cell carcinoma1. Among the rare histology types, urothelial carcinoma with chondrosarcomatous differentiation is the most rare2. A recent review by Lu et al. showed a poor survival outcome in 25 sarcomatoid ureter cancer cases with chondrosarcomatous differentiation2. In this case study, we present a 68-year-old patient recently diagnosed with sarcomatoid urothelial carcinoma with chondrosarcomatous differentiation of the right ureter discovered during esophageal cancer treatment. We thereby report a case of good response to the immune checkpoint inhibitor with an overall review of the histopathological characteristics of sarcomatoid ureteral cancer with some genetic background.
A 68-year-old male patient was referred to the urology department for right hydronephrosis complicating an incidental distal ureter stricture, which was found during an abdominal computed tomography (CT) scan for distal esophageal cancer. The patient was recently diagnosed with a 1.2 cm round-shaped distal esophageal squamous cell carcinoma, clinically stage T1, and was receiving a two-week 6600 cGy proton therapy in 33 fractions. The patient had a past medical history of restrictive lung disease, alcoholic liver cirrhosis (Child A), and was a heavy ex-smoker.
The CT treatment-planning scan showed right hydronephroureterosis due to distal ureteral stricture and ureter kinking. A CT urography was further performed at the urology department showing an abnormal ureteral kinking lesion with a distal intraureteral enhancing mass, just above the ureterovesical junction of the bladder (Figure 1A). Ureteral cancer was suspected given the findings in the right distal ureter: irregular wall thickening, and hydronephrosis with multiple small stones. Cystoscopy and microscopic urine analysis using the Nuclear Matrix Protein 22 test showed negative findings, except for benign hyperplasia of the prostate and a moderate trabeculated bladder without any voiding symptoms. Further, ureteroscopic biopsy with intraureteral urine cytology under general anesthesia found atypical cells, atypical pleomorphic cell nests, and chondroid tissue, consistent with sarcomatoid urothelial carcinoma (Figure 1B).
After the first two-week proton therapy, the patient underwent a successful right open radical nephroureterectomy from the 11th intercostal incision and pelvic Gibson incision for bladder cuffing without a positive resection margin and intratumoral positive lymph nodes. Macroscopically, the pelvocalyx was enlarged, and the cut surface revealed multiple whitish solid tumors measuring up to 2.1 × 1.7 × 1.3 cm in the pelvis and extending to the cortex. The tumor also involved the distal ureter (Figure 2A). Microscopically, the tumor had a biphasic appearance of a high-grade urothelial carcinoma and a sarcomatous component with chondrosarcomatous differentiation (Figure 2B). Immunohistochemical analysis indicated that the sarcomatous areas were positive for vimentin, and the areas of malignant urothelial cells were diffusely positive for pan-cytokeratin and p63 (Figure 2C). Based on the histological and immunohistochemical findings, a pT3N0 sarcomatoid urothelial carcinoma (heterologous component of chondrosarcoma positive for vimentin, p63, and pan-cytokeratin) located at the right distal ureter and a separate small tumor in the right renal calyx were diagnosed. The tumor in the renal calyx infiltrated the periureteric fat and renal parenchyma of the renal capsule.
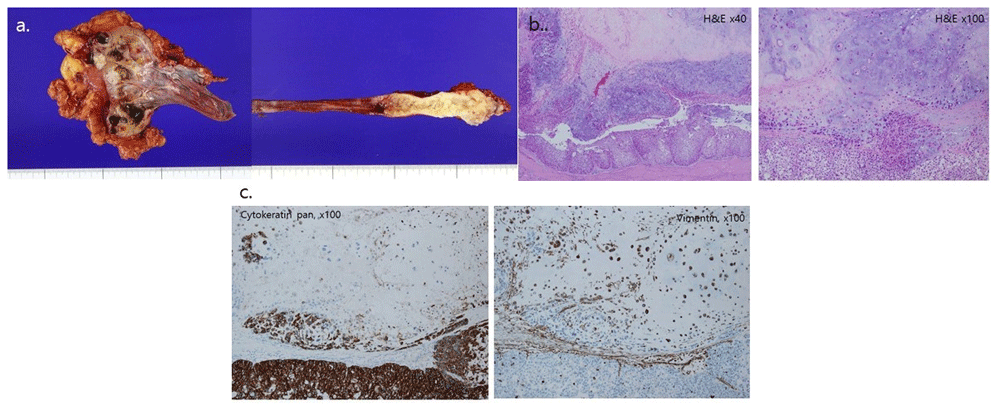
(A) Gross specimen of a right distal ureter mass and of a satellite renal pelvis tumor, (B) microscopic findings, and (C) immunohistochemical findings. (A) The cut surface of the resected kidney shows multiple whitish solid homogenous masses in the pelvic region extending to the renal parenchyma. The tumor in the distal ureter shows an irregular nodularity on the surface, a thickened wall, and periureteric fat invasion. (B) The tumor shows an area of urothelial carcinoma forming an epithelial component and sarcomatous area with abundant chondroid production, and the two components are fused together. (C) Immunohistochemical staining shows strong cytokeratin positivity in the urothelial carcinoma component and vimentin positivity in the cartilaginous sarcoma component.
The patient was discharged within 10 days without any complication including azotemia and resumed the two-week proton therapy for esophageal cancer. On the postoperative one-month follow-up CT scan, an increased size and necrosis of aortocaval lymph nodes and multiple metastatic lung nodules were detected. Three cycles of gemcitabine and carboplatin (gemcitabine 1000mg/m2 D1, 8, and carboplatin AUC 5 D1, every 3 weeks) chemotherapy was administered due to the decreased renal function because of an underlying chronic kidney disease. The follow-up imaging after 3 cycles (9 weeks) of the initial treatment regimen indicated disease progression; thus, a second-line systemic therapy was initiated using an immune checkpoint inhibitor, atezolizumab (1200mg, every 3 weeks). After three cycles of atezolizumab, the multiple enlarged lymph nodes and lung nodules were no longer enlarged, and after five cycles of atezolizumab the overall size of multiple metastatic lesions decreased (Figure 3), indicating a partial response to atezolizumab. The patient has undergone seven cycles of atezolizumab without any grade 3 adverse event, and will continue to be treated.
According to the 4th edition of the World Health Organization Classification of Tumors, the International Agency for Research on Cancer defined the sarcomatoid variants of urothelial carcinoma as being histologically indistinguishable from those of sarcomas with a prevalence of 0.6% of all bladder tumors3. The most common symptom of SUCCD is gross hematuria with male predominance (ratio 3:1). The aggressive pathologic feature results in mostly nodal and visceral metastases at the time of diagnosis, like in our case. Our patient had an underlying alcoholic liver cirrhosis, a chronic pulmonary disease, and a distal esophageal cancer being treated with radiation therapy. He was diagnosed with pT3NxM0 chondrosarcoma with multiple sites at the distal (pT2) and the proximal ureter (pT3). Our case was similar to a surgical case of an 81-year-old Japanese female with a multifocal, synchronous pT2 sarcomatoid ureteral cancer and pT3 renal pelvic urothelial carcinoma with multiple visceral metastases diagnosed on postoperative month 114. Our patient had no nodal enlargement or metastases at the time of preoperative workups; however, the lung metastases and multiple nodal enlargement observed on postoperative month one strongly support the speculation that micrometastases were already present at the time of diagnosis of the right hydronephroureterosis and surgery. It is also worth noting that our patient presented with multiple gross hematurias during the esophageal cancer workups, and the right atrophic kidney with cystic changes and hydronephrosis were suggestive of a long-existent tumor.
The prognosis of SUCCD is dismal (3–24 months survival time)2 because the majority of urothelial carcinoma cases with sarcomatoid differentiation are known to be high grade, with an increased risk of micrometastases at the time of diagnosis due to late diagnosis, and have challenges associated with diagnosing a rare multifocal ureteral cancer5–8. Therefore, surgical resection is the primary treatment choice especially for chemoradiation-resistant tumors despite diagnosis at advanced stages. However, several retrospective case series and clinical trials have proposed the use of either anti-angiogenic inhibitors or immune therapy in the current genetic era.
Immunotherapy has had enormous success in treating multiple cancer subtypes. Success has been particularly seen with immune checkpoint inhibitors, which are now approved as standard therapy in melanoma, lung cancer, and genitourinary cancers. Immunotherapy agents are increasingly demonstrating success in many cancer subtypes, and there have been preclinical suggestions that they may do the same in chondrosarcoma. The PI3K-Akt-mTOR, SRC, and Hedgehog pathways are the potential oncogenic targets for chondrosarcoma with VEGF2 inhibitors9,10. Other identified targets with next-generation sequencing are recurrent alterations in TP53, ACVR2A, COL2A1, YEATS2, and IDH11. Another research showed a distinct mutational landscape, with enrichment of TP53, RB1, and PIK3CA mutations in sarcomatoid urothelial carcinoma of bladder12. Some downregulation of homotypic adherence genes and dysregulation of the EMT network were also observed with nearly half exhibiting a heavily infiltrated immune phenotype. In addition, immune checkpoint inhibitors have been successful treatments for sarcomas. Atezolizumab, an immune checkpoint inhibitor, has been approved for second-line therapy in ureteral cancer in Korea and in our case we observed a good response. A previous 67-year-old male patient treated with four cycles of nivolumab resulted in a near-complete response in metastatic pulmonary nodules13. Immune checkpoint inhibitors have been suggested to provide tumor-specific immune responses against cancer-specific antigens such as NY-ESO-1 or LAGE-1 in sarcoma patients with dedifferentiated chondrosarcoma, as well as chondrosarcoma cell lines13–15. The expected favorable outcome with the use of a single agent PD1 inhibitor with/without immunomodulatory agents in chondrosarcoma needs to be further investigated.
In conclusion, this recurrent metastatic case of carcinoma is a rare variant of multifocal synchronous ureteral cancers which responded well to atezolizumab. Our findings will help in early diagnosis, treatment planning, and better management with immune checkpoint inhibitors in case the current chemotherapy fails; thus, ensuring improved prognoses. In future, a series of case reports and studies would highlight the clinical benefit of checkpoint inhibitors with/without immunomodulatory agents in this disease clearly.
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient and the caregiver of the patient.
This retrospective study was approved by the Institutional Review Board (IRB) of the National Cancer Center (IRB No. NCC 2020- 0313-0001). This case report was proceeded in accordance with the tenets of the ethical guidelines and regulations of the World Medical Association Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Urology, cancer immunotherapy
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Urology, cancer immunotherapy
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
References
1. Lu W, Wang Y, Li Y, Cao Y, et al.: Sarcomatoid urothelial carcinoma with chondrosarcomatous differentiation of the ureter: A case report and review of the literature.Oncol Lett. 2017; 13 (3): 1331-1337 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Urologic oncology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 31 Mar 22 |
read | |
|
Version 1 15 Dec 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)