Keywords
Co-infection, SARS-CoV-2, Influenza H1N1
This article is included in the Global Public Health gateway.
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Coronavirus (COVID-19) collection.
Co-infection, SARS-CoV-2, Influenza H1N1
Coinfections involving SARS-CoV-2 and respiratory viruses influenza viruses (A or B) have been rarely reported1–6. To date, there is no published case with a co-infection between SARS-CoV2 and influenza H1N1.
A 41-year-old man presented to the hospital’s emergency unit with fever and cough that has been progressing for several days. The SARS-CoV-2 RT-PCR test was positive on March 22nd 2020. On March 24th 2020, the patient had developed a dyspnea aggravation and was taken care of by a medical unity at home. He has a medical history of pulmonary sarcoidosis with a restrictive ventilatory syndrome, which was being treated with Methotrexate (15mg per week) and folinic acid (0,4mg one tablet per day). He also had malaria in 2004 from a trip to Central Africa.
Physical examination on the 24th March revealed a respiratory rate of 41 breaths/minute (normal range (NR) 12–20 breaths/minute) and oxygen saturation SpO2 of 75% (reference range (R-R) 95–100%) on ambient air. The SpO2 became at 96% when given mask flow oxygen at a rate of 12l/minute. The patient was transferred to the emergency room with 97% SpO2, body temperature 37.2°C, and respiratory rate of 30 breaths per minute. The patient presented with superficial polypnea, dyspnea with little effort, difficulty in speaking and bilateral “crackles”. Neurological and cardiovascular examinations were normal.
On supplemental oxygen (12l/min), arterial blood gas analysis revealed pH 7.50 (R-R 7.35–7.45), PCO2 35 mmHg (NR 35–45 mmHg), PO2 88 mmHg (NR 75–100 mmHg), HCO3- 27.3 mmol/l (R-R 22–26 mmol/l), and SaO2 94.4% (R-R 95–100%). The patient was then transferred to intensive care unit (ICU). Respiratory panel tests were negative for adenovirus (subtypes 2, 3, 6, 7.1 and 8), coronaviruses (229E, HKU1, NL63 and OC43), human metapneumovirus, rhinovirus, enterovirus, MERS-CoV, parainfluenza virus (1,2,3 and 4), respiratory syncytial virus, and Bordetella pertussis and parapertussis. However, influenza A, subtype influenza A-H1 variant 2009, was positive.
A chest computed tomography scan revealed a predominant left interstitial lung condensation syndrome. Routine laboratory tests revealed higher parameters during patient hospitalization: creatine phosphokinase 2999 UI/l (R-R 30–200UI/l); gamma glutamyl transferase 119 Ui/l (R-R 12–64UI/l); D-Dimers, which has increased two fold in one week, 3620 ng/ml (April 6th) to 7520 (April 15th ) (R-R 40–500UI/l). Other parameters were also elevated: fibrinogen 8.58g/l (R-R 2–4g/l); aspartate-aminotransferase 55 UI/l (R-R 5–34UI/l); platelets 599x10e9/l (April 7th) (R-R 150–450x10e9/l); leukocytes 15.4x10e9/l (R-R 4–10x10e9/l) (Table 1).
NA: data non available.
Other parameters showed decreased values such as red cells, hemoglobins, hematocrits and mean corpuscular hemoglobin content (Table 1). The number of leukocytes and neutrophils underwent fluctuations with high rates between April 7th and 10th (Figure 1). In this period, bacteriological examination culture revealed infection by additional pathogens, with the presence of yeasts (Candidate albicans) and bacteria (Klebsiella pneumoniae) in bronchial sampling, probably with nosocomial origin.
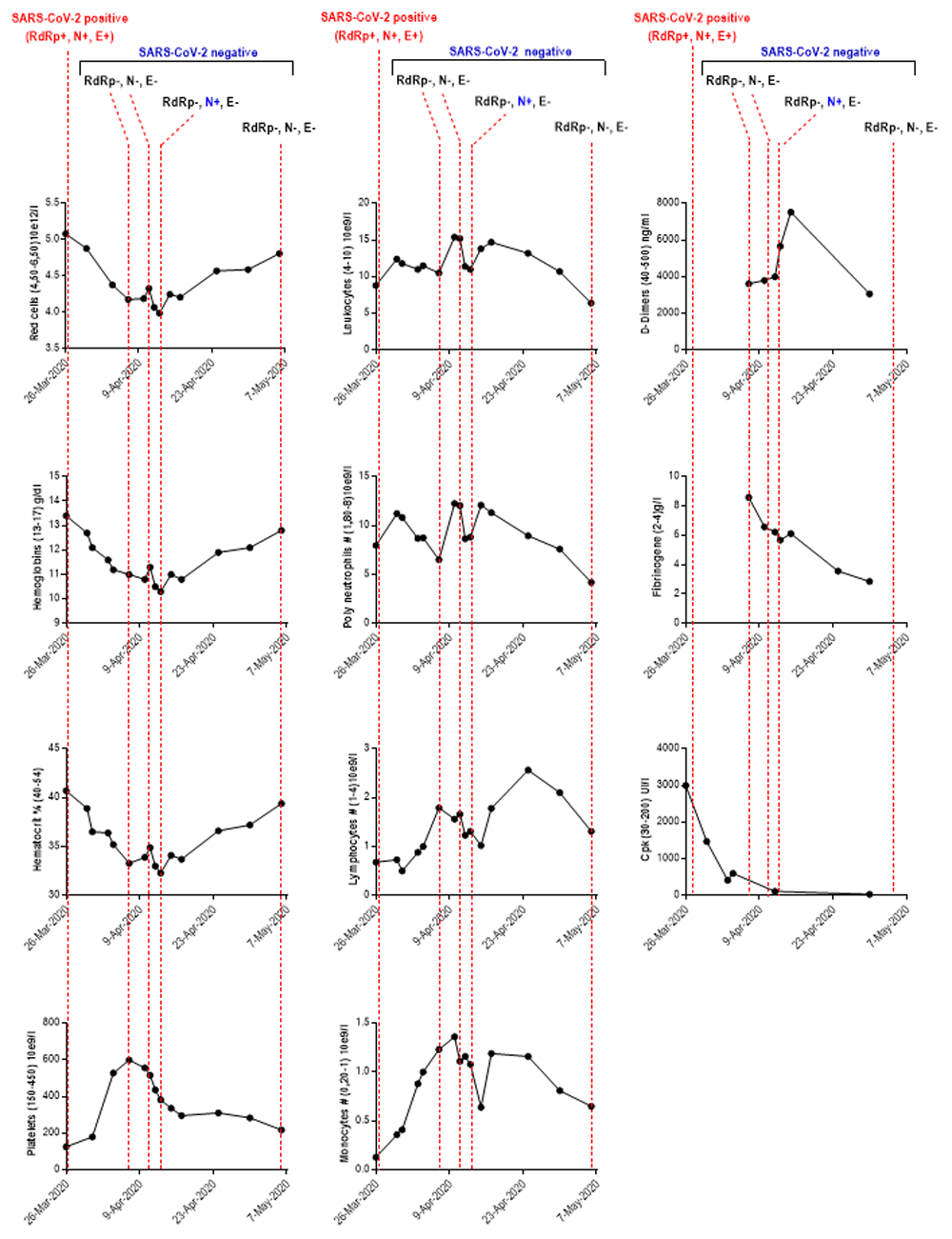
RdRp: RNA-dependent RNA polymerases; N: envelope protein N; S: Spike protein; CpK: creatine phosphokinase; SARS-CoV-2: severe acute respiratory syndrome coronavirus-2; Mar: March; Apr: April; #: Total count. The values between parentheses ‘()’ in the ordinate axis correspond to the reference range values.
The patient stayed at ICU for 26 days from March 24th to April 19th with orotracheal intubation and using Etomidate (40 mg intravenous dose) for sedation and 120mg of Celocurine for curarization. Enoxaparin (40mg /day) was administered by subcutaneous injection as a preventive anticoagulation up to April 1st and increased to 80 mg/day according to the patient being overweight (Body Mass Index 33.8) and to evolution of biological criteria (D-Dimers 1890ng/l, Fibrinogen 10.82g/l, platelets 528x10e9/l). Unfractionated heparin was used as relay according to the high probability of pulmonary embolism. Mechanical ventilation was used with several sessions of prone position and then oxygen therapy on April 19th. He was treated with hydroxychloroquine for 10 days (Plaquenil, 200mg every eight hours), and by Oseltamivir (4 days, oral suspension 6mg/ml: 75mg twice/day) for influenza H1N1 infection. Klebsiella pneumonia infection was treated using Meropenem (intravenous 1 g every four hours) from 6th to 15th April and Enterococcus faecalis infection was treated using Clamoxyl (intravenous 2 g every eight hours). Venous echodoppler performed on April 14th found no thrombosis and no pulmonary embolism.
On April 19th, the patient was transferred to the pulmonology department where he has a good respiratory evolution allowing oxygen weaning on April 23rd. He was discharged on April 28th, receiving kinesitherapy treatment, and taking a preventive anticoagulant therapy (Enoxaparine 4000 IU /0.4ml once daily by SC injection) for three weeks. The patient was integrated into the post COVID-19 rehabilitation program.
We report, to the best of our knowledge, the first case of coinfection with SARS-CoV-2 and seasonal influenza H1N1. The low incidence rate of this co-infection reported in France may be explained by the late screening for COVID-19, which started in France in March 2020, which corresponds with the tapering off period for H1N17. The prolonged intensive care and detection of SARS-CoV-2 viral RNA on the bronchoalveolar sample for at least three weeks might be explained by patient immunosuppression caused by lung polyinfection (viral, bacterial and fungal) and probably by his medical history of pulmonary sarcoidosis with a restrictive ventilatory syndrome.
Bacterial coinfections in COVID-19 patients have been reported in nine studies with a rate of 8% (62/806) of bacterial/fungal co-infection cases8. A few cases have been reported with co-infection with SARS-CoV-2 and influenza viruses (A or B)1–6. Ding et al. has reported coinfected patients with SARS-CoV-2 and influenza virus and showed similar clinical characteristics as those patients with COVID-19 only, hence not all patients need ICU3. However, in a report by Cuadrado-Payán et al., all COVID-19 patients studied attended the emergency unit but had medical history of hypertension, end-stage kidney disease, or type 2 diabetes5.
The co-detection of SARS-CoV-2 and Influenza H1N1 in our case demonstrates the challenge to screen in the onset of the respiratory illness for a panel of viruses, which have overlapping clinical patterns and might exacerbate clinical symptoms, increase morbidity and prolong ICU stay. Hence, this case highlights the higher risk and poor outcomes caused by co-infection and the importance to achieve a differential diagnosis of respiratory distress syndromes, to limit contamination and adapt therapeutic strategies.
Written informed consent was obtained from the patient for the publication of this article and any associated images.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology and immunogenetics
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
References
1. Stowe J, Tessier E, Zhao H, Guy R, et al.: Interactions between SARS-CoV-2 and influenza, and the impact of coinfection on disease severity: a test-negative design.Int J Epidemiol. 2021. PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Respiratory pathogens
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 03 May 22 |
read | |
|
Version 1 18 Dec 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)